Pre-eclampsia onset and SPARC: A possible involvement in placenta development
Abstract
Pre-eclampsia (PE) is a multisystem disorder commonly diagnosed in the latter half of pregnancy and it is a leading cause of intrauterine fetal growth retardation (IUGR). The aim of this study was to investigate the localization and the role of SPARC, secreted protein acidic, and rich in cysteine, in PE and PE–IUGR placentas in comparison with normal placentas. SPARC was mainly expressed in the villous and extravillous cytotrophoblastic cells in first trimester, whereas in PE, PE–IUGR and at term placentas, SPARC immunostaining was visible in both cytotrophoblastic cells and syncytiotrophoblast. SPARC expression significantly decreased in normal placenta from first to third trimester and a further significant reduction was demonstrated in PE and PE–IUGR. The latter downregulation of SPARC depends on hypoxic condition as shown by in vitro models. In conclusion, SPARC can play a pivotal role in PE and PE–IUGR onset and it should be considered as a key molecule for future investigations in such pathologies.
1 INTRODUCTION
Successful pregnancy depends mainly on the precise regulation of extravillous trophoblast (EVT) invasion into the uterine decidua and adequate development of the placental villous tree (Gathiram & Moodley, 2016). Pre-eclampsia (PE) is a multisystem disorder clinically characterized by new onset of proteinuria and hypertension that occurs in 5–8% of pregnancies. It is commonly diagnosed in the second half of pregnancy, but it may involve poor placentation during the earliest stage of pregnancy, determining increased maternal and neonatal morbidity and mortality (Gathiram & Moodley, 2016; Gruslin & Lemyre, 2011; Litwińska, Litwińska, Oszukowski, Szaflik, & Kaczmarek, 2017). PE is a leading cause of premature delivery and intrauterine fetal growth retardation (IUGR), term generally used to describe a fetus that has not reached its growth potential (Kintiraki, Papakatsika, Kotronis, Goulis, & Kotsis, 2015). Moreover, IUGR foetuses have an increased likelihood of neurodevelopmental problems including sensory and motor deficits, cognitive difficulties, and cerebral palsy (Backes et al., 2011; Manokhina, Del Gobbo, Konwar, Wilson, & Robinson, 2017; Temming et al., 2017).
SPARC (secreted protein acidic and rich in cysteine), also called Osteonectin or BM-40, is an extracellular Ca2+-binding matricellular glycoprotein expressed in a variety of mammalian tissues and cell lines (Engel, Taylor, Paulsson, Sage, & Hogan, 1987). It has an important role in modulating interactions between cells and the extracellular environment by regulating extracellular matrix (ECM) assembly and deposition as well as growth factors signaling (Engel et al., 1987; Romberg, Werness, Lollar, Riggs, & Mann, 1985; Termine et al., 1981). SPARC family proteins, such as thrombospondins (TSP) 1 and 2, tenascins (TN)-C and X, Osteopontin (OPN), and the SPARC-related proteins, SC1 and QR1, have three common domains: an acidic N-terminal domain, a cysteine-rich follistatin-like (FS) domain, and an α-helical extracellular (EC) calcium-binding domain with an EF-hand motif (Bradshaw, 2012). Despite SPARC is often found as a secreted glycoprotein, it is also expressed within the intracellular compartment and on the cell surface and it has been shown to act as de-adhesive protein and cell-cycle inhibitor (Bornstein & Sage, 2002). The importance of this protein is demonstrated in SPARC-null mice that experienced osteopenia and decreased bone formation (Delany et al., 2000), as well as defects of dermal wounding (Bradshaw, Reed, & Sage, 2002), and an improper ECM organization (Brekken et al., 2003; Puolakkainen, Brekken, Muneer, & Sage, 2004). In addition, it has been shown that SPARC can induce the expression of matrix metalloproteinases in fibroblasts (Tremble, Lane, Sage, & Werb, 1993).
SPARC expression is elevated during embryogenesis and decreases in normal adult tissues (Yan & Sage, 1999). However, in cancer, diabetes, kidney diseases, and arthritis SPARC expression increases, suggesting an important role in tissue remodeling and repair (Reed & Sage, 1996).
Jiang and colleagues (2013) detected SPARC in first trimester placental villi and they found that SPARC-specific RNA interference significantly reduced the invasion of human extravillous trophoblast HTR8/SVneo cells. These authors, however, did not analyze the expression of SPARC in extravillous trophoblastic cells of cell islands and cell columns that represent the actual sites of placental proliferation and invasion for placental anchorage.
Since it has been shown a shallow invasion by fetal trophoblast in maternal spiral arteries in early pregnancy both in PE and in IUGR (Burton & Jauniaux, 2004; Gathiram & Moodley, 2016; Schoots, Gordijn, Scherjon, van Goor, & Hillebrands, 2018), we hypothesized that SPARC could have a role in modulating trophoblast invasion in normal and pathological conditions. The purpose of this study was to investigate the role of SPARC during the normal placental development and particularly to clarify which could be the possible conditions modulating SPARC expression in PE with or without IUGR.
2 MATERIALS AND METHODS
2.1 Tissue collection
The procedure for this study project complies with the World Medical Association Declaration of Helsinki. All procedures were performed according to relevant national regulations and institutional guidelines. All patients gave their informed consent and the permission of the Human Investigation Committee was granted. To evaluate the expression of SPARC in human placenta we analyzed a total of 36 normal and pathological human placentas. Twenty normal placentas: 10 from first trimester (Obstetrics and Gynaecology of San Severino Hospital, MC, Italy), 10 from third trimester of gestation, and 16 from pathological placentas (Department of Woman and Child Health, A. Gemelli Hospital, Università Cattolica Del Sacro Cuore Roma; Obstetrics and Gynecology, Department of Clinical Sciences, Polytechnic University of Marche, Ancona, Italy): eight from pregnancies complicated by PE and eight from pregnancies complicated by PE–IUGR. The PE was diagnosed on the basis of hypertension (systolic blood pressure > 140 mm Hg or diastolic blood pressure > 90 mm Hg) and proteinuria 300 mg/24 hr, appearing after 20 weeks of gestational age in a previously normotensive subject following the criteria of the American College of Obstetrics and Gynecology (Practice, 2002).
- (1)
Ultrasound measurement of the fetal abdominal circumference below the 10th centile (Nicolini et al., 1986) confirmed by birth weight below the 10th centile according to our birth weight references values (Parazzini, Cortinovis, Bortolus, & Fedele, 1991);
- (2)
Abnormal Doppler flow velocity waveforms (Doppler-FVWs) of the uterine arteries (Bower, Bewley, & Campbell, 1993) and abnormal Doppler-FVWs of the umbilical arteries (Todros et al., 1996).
Gestational age was determined by reference to the last menstrual period and crown rump length measurements between 8 and 12 weeks of gestation, and confirmed by an early second trimester ultrasonography examination. Specific exclusion criteria for the control group included a history of hypertension, renal disease, cardiac disease, diabetes mellitus, thyroid and immunological diseases and congenital or acquired thrombophilic disorders, and the presence of chromosomal and other fetal anomalies. Immediately after delivery, samples of first and third trimester placentas as well as from each PE placenta and PE–IUGR placenta were randomly collected for morphological (immunohistochemistry) and biochemical (western blot analysis) analyses.
Samples for immunohistochemistry were fixed in 4% neutral buffered formalin at 4°C for 12 hr then washed in cold phosphate buffer pH 7,4 for 30 min.
Thereafter the specimens were dehydrated via a graded series of ethyl alcohol (50°C for 30 min, 75°C for 30 min, 2 × 96°C for 75 min, 3 × 100°C for 75 min), and two steps in xylene for 60 min. Then, they were processed for paraffin embedding at 56°C. Paraffin sections (3 µm) were cut and stretched at 45°C, allowed to dry and stored at 4°C until use.
Samples for western blot analysis were put into cryovials and immediately frozen in liquid nitrogen and stored at − 80°C until use as previously described (Lorenzi et al., 2013)
2.2 Immunohistochemistry
Immunohistochemical staining was performed as previously described (Marzioni et al., 2009). After dewaxing, paraffin sections were thoroughly rinsed in phosphate buffered saline (PBS), reacted with 0.3% hydrogen peroxide (in deionized water; 50 min) to block endogenous peroxidase, rinsed again with PBS and incubated with normal horse serum (Vector laboratories, Burlingame, CA) diluted 1:75 in PBS for 1 hr at room temperature.
Sections were then incubated with the primary monoclonal antibody against SPARC (sc-73472; Santa Cruz Biotechnology, Inc., TX) diluted 1:300 in PBS, overnight at 4°C. After a thorough rinse in PBS, sections were incubated in a 1:200 v/v biotinylated secondary antibody solution (in PBS; 30 min). The biotinylated secondary antibody (Vector Laboratories) was horse antimouse IgG. Histochemical reactions were performed using Vectastain ABC Kit (Vector Laboratories) for 1 hr at room temperature and 3′,3′- diaminobenzidine hydrochloride (Sigma- Aldrich, St. Louis, MO) was used as the chromogen. Sections were counterstained with Mayer's haematoxylin, dehydrated and mounted with Eukitt solution (Kindler GmbH and Co., Freiburg, Germany). The immunohistochemistry for SPARC was performed in sections taken from two different parts of placenta. Negative controls were performed by omitting the first or secondary antibody and using an isotype control antibody at the same dilution of primary anti-SPARC antibody for all the immunohistochemical reactions performed in this study.
2.3 Protein extracts and western blot analysis
Samples for protein assay were thawed and washed in PBS 0,1 M, pH 7.4. We homogenized 300 mg of each sample with lysis buffer containing 0.1 M PBS, 0.1% (w/v) SDS, 1% (w/w) NONIDET-P40, 1 mM (w/v) Na orthovanadate, 1 mM (w/w) PMSF (phenyl methane sulfonyl fluoride), 12 mM (w/v) Na deoxycholate, 1.7 µg/ml Aprotinin, pH 7.5. The specimens were centrifuged at 20,000 g for 20 min at 4°C and the supernatants were aliquoted and stored at −80°C. The proteins concentrations were determined by a Bradford protein assay (Bio-rad Laboratories, Milan, Italy).
For western blot analysis assay, all protein samples (50 μg of protein for each sample) were fractionated by 10% sodium dodecyl sulfate-polyacrylamide gels and electrophoreticaly transferred to nitrocellulose membranes. To avoid nonspecific protein binding, membranes were incubated with 5% (w/v) non-fat-dried milk (Bio-rad Laboratories) in Tris-buffered saline (TBS/0.05% Tween 20 (TBS-T)). Blots were incubated with the mouse monoclonal SPARC antibody diluted 1:400 (sc-73472; Santa Cruz Biotechnology, Inc.) in TBS-T. After washing, blots were incubated with a secondary antibody conjugated with horseradish peroxidase (Santa Cruz Biotechnology, Inc.) diluted 1:5,000 in TBS-T. Detection of antibody binding was performed with the Clarity Western ECL Substrate (Bio-rad Laboratories) and images were acquired with Chemidoc (Bio-Rad Laboratories). Bands were analyzed using the ImageJ software (https://imagej.nih.gov/ij/download.html) for quantification, and normalization was completed using β-actin band intensities. MG63 cell line was used as positive control (Maillard, Malaval, & Delmas, 1992).
2.4 Cell culture and hypoxia treatment
Human first trimester extravillous trophoblast HTR8/SVneo cell line (kindly provided by Dr. Charles Graham; Queen's University, Kingston, Ontario, Canada) was routinely cultured in RPMI1640 medium (Life technologies, CA) supplemented with 10% fetal bovine serum (FBS; Gibco, Thermo Fisher Scientific, MA) and 100 U/ml penicillin and streptomycin (Gibco) at 37 °C in incubator with 5% CO2 and it was passaged at a ratio of 1:4 every 3 days. The human choriocarcinoma cell lines JEG3 and BeWo were routinely cultured in DMEM/F12 medium (Gibco) supplemented with 10% FBS (Gibco) and 100 U/ml penicillin and streptomycin (Gibco) in a humidified incubator at 37°C and 5% CO2. For cultures under hypoxic conditions, cells were cultured in incubator with 5% CO2, 3% O2, and 92% N2 for 48 hr. After the treatment, cells were lysed by using lysis buffer, centrifuged at 20,000 g for 20 min at 4°C and the supernatants were aliquoted and stored at −80 °C. Viable counts using the trypan blue dye exclusion test were routinely performed. All experiments were performed in duplicate and were repeated at least three times.
2.5 Statistical analysis
Data represent the mean ± SD, and were analyzed for statistical significance (p < 0.05) using Student's t test.
3 RESULTS
3.1 Evaluation of SPARC expression in normal and pathological human placentas
In first trimester placentas, SPARC was highly expressed in the villous cytotrophoblastic cells, whereas the syncytiotrophoblast was mainly negative, with only few tracts weakly stained for SPARC (Figure 1a). The villous stroma and the endothelium of the fetal vessels were irregularly immunostained for SPARC. In cytotrophoblastic cell columns and cell islands SPARC immunostaining was mainly present in the cytotrophoblastic cells located in the proximal part of the columns (adjacent to the villous), but the syncytiotrophoblast appeared mainly negative (Figure 1b).
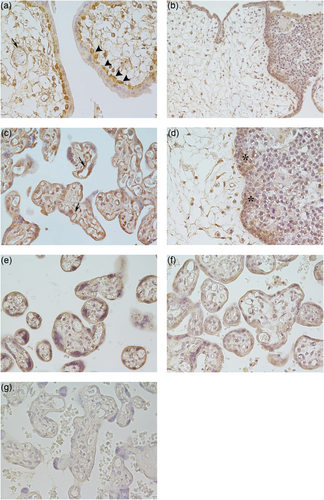
Immunolocalization of SPARC in normal first and third trimester placentas and in placentas affected by PE and PE–IUGR. (a) First trimester of normal placental villi show that SPARC was highly expressed in the villous cytotrophoblastic cells (arrowheads), whereas the syncytiotrophoblast was mainly negative. The endothelium of the fetal vessels (arrow) was irregularly immunostained. (b) The extravillous cytotrophoblastic cells located in the proximal part of the cell columns (adjacent to the villous) were positive for SPARC, see the higher magnification of cell column in d (**). (c) Third trimester normal placental villi show the villous cytotrophoblastic cells and the syncitiotrophoblast as well as the fetal vessels (arrows) immunostained for SPARC. (e) Placental villi from PE and (f) from PE–IUGR show a decreased staining for SPARC in the villous cytotrophoblastic cells and the syncitiotrophoblast as well as in the fetal vessels compared with third trimester placentas (c). (g) Placental villi from PE were used as negative control. a, c, d, e, f, g: ×40; b: ×20. PE: pre-eclampsia; PE–IUGAR: PE–intrauterine fetal growth retardation; SPARC: secreted protein acidic, and rich in cysteine [Color figure can be viewed at wileyonlinelibrary.com]
In the third trimester normal placentas (Figure 1c), in PE (Figure 1d), and in PE–IUGR (Figure 1e) placental samples, SPARC immunostaining was visible in both cytotrophoblastic cells and syncytiotrophoblast. In the villous stroma of third trimester normal placental villi, SPARC was expressed in the endothelium of fetal vessels (Figure 1c, arrows). SPARC immunostaining decreased in PE and PE–IUGR fetal vessels compared to third trimester placentas.
Western blot analysis analysis of SPARC expression in normal and pathological human placentas (i.e., PE and PE–IUGR) evidenced a significant SPARC decrease in normal placentas from first to third trimester (Figure 2a). SPARC expression was significantly reduced in PE and PE–IUGR compared with normal term placentas (Figure 2a). In addition, no significant difference was detected between PE and PE–IUGR placental samples (Figure 2a).
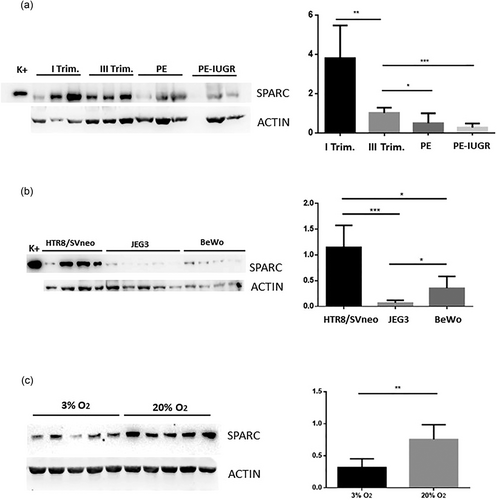
Western blot analysis of SPARC protein expression in normal (first and third trimester placentas) and pathological placentas (PE and PE–IUGR), and western blot analysis of cell lines cultured in normal and hypoxic conditions. Bands were analyzed densitometricaly. Results were calculated in arbitrary units (AU) and reported as bars of a histogram. SPARC quantities were normalized using β-actin expression profile. (a) Western blot analysis of SPARC expression in normal and pathological human placentas. Note the significant decrease of SPARC in normal third trimester placentas compared with normal first trimester placentas (**) as well as in the PE (*) and PE–IUGR (***) placentas compared with third trimester normal placentas. (b) Western blot analysis in three different cell lines (HTR8/SVneo, JEG3, and BeWo) cultured in normal conditions. Note that HTR8/SVneo cell line showed the highest expression of SPARC. (c) SPARC expression in HTR8/SVneo in normal and hypoxic conditions, that is, at 3% and 20% O2 atmosphere. HTR8/SVneo cultured in hypoxic conditions showed a decreased expression of SPARC. K+ = positive control (MG63 cell lines). Data are represented as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001. PE: pre-eclampsia; PE–IUGAR: PE–intrauterine fetal growth retardation; SPARC: secreted protein acidic, and rich in cysteine
3.2 SPARC expression in human placental cell lines
SPARC expression was also evaluated in three different cell lines (HTR8/SVneo, JEG3, and BeWo) normally used as in vitro models of human placenta, by using western blot analysis. As shown in Figure 2b, in normal culture conditions all cell lines expressed SPARC but at different amounts, with the highest level of expression in HTR8/SVneo cells. Significant statistic differences between HTR8/SVneo and JEG3 cell lines (p = 0.0007) as well as between HTR8/SVneo and BeWo cell lines (p = 0.017 Figure 2b) were observed.
To better mimic PE conditions in vitro, we assessed hypoxic effect on SPARC expression in HTR8/SVneo cell line. To this aim cells were maintained in a constant 3% O2 atmosphere. As shown in Figure 2c, HTR8/SVneo cells SPARC expression significantly decreased during hypoxia compared with normoxia.
4 DISCUSSION
Many studies support the idea that SPARC has site-specific functions and that its impaired expression during the development may led to alterations in tissue homeostasis particularly acting on extracellular matrix remodeling (Bornstein & Sage, 2002; Bradshaw et al., 2002; Brekken et al., 2003; Delany et al., 2000; Puolakkainen et al., 2004; Tremble et al., 1993).
It is known that SPARC plays an important role in cancer pathology. In fact, SPARC is differentially expressed in tumors and in surrounding stroma of various types of cancers in comparison to the normal tissues: higher levels of SPARC have been reported in breast cancer (Porter, Sage, Lane, Funk, & Gown, 1995), melanoma (Ledda et al., 1997), and glioblastomas (Rempel et al., 1998).
Similarly to malignant tumors, the trophoblastic cells of human placenta invade the maternal endometrium and its vasculature (Ferretti, Bruni, Dangles-Marie, Pecking, & Bellet, 2007). Therefore, we supposed a possible role of SPARC in regulating trophoblast invasion. Particularly, since an alteration in the invasion of trophoblastic cells into endometrial vessels is responsible for PE, we supposed that SPARC expression may be impaired in this pathology and/or in PE–IUGR.
Our immunohistochemical analysis showed a different expression of SPARC in first and third trimester placentas. Since its expression is high during the first trimester, when the invasive processes predominantly occur, and decreases in the third trimester where the invasive processes are less significant (Aplin, 1991; Tarrade et al., 2001), it could be hypothesized that the presence of this protein is correlated with the invasiveness processes during the placental development.
Particularly, we found SPARC expressed in extravillous trophoblast (EVT) of cell islands and cell columns that represent the implantation sites of the placenta, thus suggesting that SPARC plays a pivotal role in trophoblast invasion during the normal placentation. Our data agree with Jiang et al. (2013) who observed that SPARC-specific RNA interference significantly reduced the invasion of HTR8/SVneo cells.
Interestingly, we found a significant reduction of SPARC in PE and PE–IUGR compared with normal third trimester placentas, indicating that SPARC expression may be compromised in these pathologies. PE is associated with the impairment of the important process of hypoxia-reoxygenation due to a deficient conversion of the distal portions of the spiral arteries supplying the placenta into large-caliber conduits. Failure to complete this process keeps the placenta in hypoxic condition (Gathiram & Moodley, 2016; Jiang et al., 2013; Reed & Sage, 1996). It has been demonstrated that during PE, the functions of EVT are compromised, resulting in an insufficient invasion of EVT into spiral arteries and leading to a reduction in the oxygen supply to the placenta and the fetus. Therefore, PE placentas are subjected to chronic hypoxia (McMaster, Zhou, & Fisher, 2004; Pringle, Kind, Sferruzzi-Perri, Thompson, & Roberts, 2010).
We demonstrated that HTR8/SVneo cells express SPARC in normal conditions but its expression is downregulated in hypoxic conditions, thus strengthening the idea of an involvement of SPARC in PE pathology onset, contributing to the reduction of invasiveness of the cytotrophoblast into maternal side.
In addition, we demonstrated that the endothelium of the fetal villous vessels is irregularly and weakly positive for SPARC during the first trimester of gestation, whereas it is very positive during the third trimester. This modality of SPARC expression can be related to the formation of new capillaries. In fact, it is known that the human placenta develops under hypoxic conditions during the first trimester of gestation supporting the formation of new vessels that mainly occur from Day 32 pc to Week 24. On the contrary, the formation of capillary loops through elongation predominates until to term under hyperoxic conditions (Castellucci, Schepe, Scheffen, Celona, & Kaufmann, 1990; Demir, Kaufmann, Castellucci, Erbengi, & Kotowski, 1989; Jauniaux, Pahal, Gervy, & Gulbis, 2000). Even PE and PE–IUGR develop in hypoxic condition and their main characteristic is the formation of new vessels. Interestingly, PE and PE–IUGR placentas show low levels of SPARC expression in fetal vessels as we have demonstrated during the first trimester of gestation. Collectively, our observations suggest that SPARC is required for angiogenesis during the fetal vessel development and that it is expression is modulated by oxygen concentration. Our data are consistent with previous results indicating that SPARC is expressed in the endothelial cells of newly formed vessels in a manner, which is both temporally and spatially restricted. (Iruela-Arispe et al., 1995). It is known that the rate and mode of angiogenesis are not dictated by one factor alone, but rather by a complex integration of signaling mechanisms of which oxygen and SPARC can be two contributors. In conclusion, SPARC can be considered an important player during the normal human placental development since its dysregulation in the key areas (extravillous cytotrophoblast, fetal vessels) and periods (second half of pregnancy) could cause an altered organ development favouring pathologies such as PE.
ACKNOWLEDGMENTS
We are indebted to Professor Charles Graham for kindly providing the HTR8/SVneo cell line. G.T. is a recipient of a postdoctoral fellowship and S.F. is a recipient of a PhD fellowship from Università Politecnica delle Marche, Italy. This study was supported by grants from Università Politecnica delle Marche (RSA 2017) to D.M., M.M.B., S.R.G., and M.C.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.




