Ferritinophagy/ferroptosis: Iron-related newcomers in human diseases
Abstract
Nuclear receptor coactivator 4 mediated ferritinophagy is an autophagic phenomenon that specifically involves ferritin to release intracellular free iron. Ferritinophagy is implicated in maintaining efficient erythropoiesis. Notably, ferritinophagy also plays a central role in driving some pathological processes, including Parkinson’s disease (PD) and urinary tract infections. Some evidence has demonstrated that ferritinophagy is critical to induce ferroptosis. Ferroptosis is a newly nonapoptotic form of cell death, characterized by the accumulation of iron-based lipid reactive oxygen species. Ferroptosis plays an important role in inhibiting some types of cancers, such as hepatocellular carcinoma, pancreatic carcinoma, prostate cancer, and breast cancer. Conversely, the activation of ferroptosis accelerates neurodegeneration diseases, including PD and Alzheimer’s disease. Therefore, in this review, we summarize the regulatory mechanisms related to ferritinophagy and ferroptosis. Moreover, the distinctive effects of ferritinophagy in human erythropoiesis and some pathologies, coupled with the promotive or inhibitory role of tumorous and neurodegenerative diseases mediated by ferroptosis, are elucidated. Obviously, activating or inhibiting ferroptosis could be exploited to achieve desirable therapeutic effects on diverse cancers and neurodegeneration diseases. Interrupting ferritinophagy to control iron level might provide a potentially therapeutic avenue to suppress urinary tract infections.
Abbreviations
-
- AD
-
- Alzheimer’s disease
-
- APP
-
- amyloid plaques protein
-
- ART
-
- artemisinin
-
- ATF4
-
- activating transcription Factor 4
-
- ATG
-
- autophagy-related
-
- ATG16L1
-
- autophagy-related 16-like 1
-
- Aβ
-
- amyloid-β
-
- FBXL5
-
- F-box and leucine-rich repeat protein 5
-
- FTH1
-
- ferritin heavy chain-1
-
- FTL
-
- ferritin light chain
-
- GPX
-
- glutathione peroxidase
-
- GSH
-
- glutathione
-
- HCC
-
- hepatocellular carcinoma
-
- HERC2
-
- homologous to E6AP carboxy terminus (HECT) E3 ubiquitin ligase 2
-
- HIF-1α
-
- hypoxia-inducible factor-1 alpha
-
- HNC
-
- head and neck cancer
-
- HO-1
-
- heme oxygenase-1
-
- HSPA5
-
- heat shock 70 kDa protein 5
-
- HSPB1
-
- heat shock protein B1
-
- IREs
-
- iron-responsive elements
-
- IRP2
-
- iron-responsive-element-binding protein 2
-
- Keap 1
-
- Kelch-like ECH-associated protein 1
-
- MUC1-C
-
- the mucin 1 (MUC1) C-terminal transmembrane subunit
-
- NCOA4
-
- nuclear receptor coactivator 4
-
- NRF2
-
- nuclear factor (erythroid-derived)-like 2
-
- PCC
-
- pancreatic cancer
-
- PD
-
- Parkinson’s disease
-
- PDAC
-
- pancreatic ductal adenocarcinoma cells
-
- PHDs
-
- prolyl hydroxylases
-
- PKCα
-
- protein kinase Cα
-
- RB
-
- retinoblastoma
-
- RCD
-
- regulated cell death
-
- ROS
-
- reactive oxygen species
-
- SNCA
-
- α-synuclein
-
- Tf
-
- transferrin
-
- TNBC
-
- triple-negative breast cancer
-
- UPEC
-
- uropathogenic Escherichia coli
-
- UTIs
-
- urinary tract infections
-
- xCT (SLC7A11)
-
- cystine/glutamate transporter Xc- light chain
1 INTRODUCTION
Recently, Mancias, Wang, Gygi, Harper, and Kimmelman, (2014) discovered, for the first time, that the nuclear receptor coactivator 4 (NCOA4) is selectively enriched in autophagosomes (Mancias et al., 2014). Moreover, NCOA4, endogenous LC3B, and intracellular iron storage ferritin complexes are extensively colocalized. Therefore, a novel process in which NCOA4 binds ferritin in autophagosome and targets it for autophagic degradation is defined as ferritinophagy NCOA4, recognized as a specific cargo receptor for ferritinophagy, is remarkably different from its original role as an androgen receptor coactivator (Yeh & Chang, 1996). Some evidence supports that the loss of NCOA4 leads to reduced levels of bioavailable iron and inappropriate accumulation of iron-laden ferritin (Bellelli et al., 2016; Mancias et al., 2015). Ferritin, the substrate of ferritinophagy, represents the major form of stored iron via its iron-filled cage structure (Arosio, Ingrassia, & Cavadini, 2009; Harrison & Arosio, 1996). The process of iron liberating from ferritin requires lysosomal proteolysis activity (Kidane, Sauble, & Linder, 2006). Previous research has found that ferritin accumulates and colocalizes with autophagy-related (ATG) proteins at the autophagosome formation sites in ATG-knocked-out cells (Kishi-Itakura, Koyama-Honda, Itakura, & Mizushima, 2014). Therefore, autophagic degradation of ferritin represents a crucial mechanism to maintain iron homeostasis.
In general, ferritin mainly functions in regulating the balance between the reduced states (Fe2+) and the oxidized states (Fe3+). Thus, NCOA4-mediated ferritinophagy is involved in some physiological processes related to cellular iron metabolism, such as erythropoiesis (Mancias et al., 2015). However, abnormal iron balance caused by dysfunctional ferritinophagy triggers toxic oxidative stress (Dixon & Stockwell, 2014), stimulating the occurrence of pathology, including neurodegenerative diseases (Baksi & Singh, 2017), and urinary tract infections (Bauckman & Mysorekar, 2016). Hence, how ferritinophagy influences the physiological process and various diseases has gained intense attention.
Ferroptosis is a new pattern of autophagic and caspase-independent cell death. Importantly, ferroptosis could be initiated by ferritinophagy through promoting iron and reactive oxygen species (ROS) accumulation (Gao et al., 2016; Hou et al., 2016). In 2012, Dixon et al., (2012) demonstrated, for the first time, that an oncogenic RAS-selective lethal compound, erastin, induces an iron-dependent form of cell death, which is termed as ferroptosis. Ferroptosis is significantly distinguished from various regulated cell deaths (RCD), such as apoptosis (Ashkenazi & Dixit, 1998; Nakagawa et al., 2000; Pena-Blanco & Garcia-Saez, 2017; Saraste & Pulkki, 2000), necroptosis (Pasparakis & Vandenabeele, 2015; Wu, Liu, & Li, 2012), pyroptosis (Bergsbaken, Fink, & Cookson, 2009), and autophagy (Mizushima, Yoshimori, & Ohsumi, 2011; Noda & Inagaki, 2015) in terms of morphology, biochemistry, and genetics (Table. 1). In particular, the mitochondria of ferroptotic cells show a smaller volume and disappeared ridge, whereas an increased membrane density (Dixon et al., 2012; Table 1).
| Regulated cell death | Morphological traits | Biochemical traits | Inducing factors | Critical genes | Related diseases | |
|---|---|---|---|---|---|---|
| Ferroptosis | Reduced smaller volume of mitochondria, increased membrane density, disappeared mitochondrial ridge | Accumulation of iron-dependent lipid peroxidation, increased intracellular lipid ROS | Inhibiting antioxidase activities, depressing the cystine/glutamate transporter system Xc-, increasing iron level |
|
Cancer, neurodegeneration | |
| Apoptosis | Death receptors pathway | Cell and nucleus shrinkage, nuclear chromatin condensation, the appearance of membrane-bound apoptotic bodies, neighboring cells, phagocytosis | DNA fragmentation, activation of caspase cascade | Extracellular death signals | Caspase-8/10/3,death receptors (like TNFa, FasL) | Neurodegeneration, cardiovascular diseases, cancers |
| Mitochondrial pathway | Intracellular death signals | Bcl-2,Apaf-1,caspase-9/3 | ||||
| Endoplasmic reticulum pathway | High level of Ca2+, endoplasmic reticulum stress | Caspase-12/3 | ||||
| Necroptosis | Cell membrane damage, organelles swelling and disintegration, autophagy as the downstream response | The formation of Necrosome complex | The activation of death receptors ligands under apoptotic deficient conditions. |
|
Neuronal and immune diseases, cancer | |
| Pyroptosis | Karyopyknosis, plasma-membrane rupture, DNA damage, intracellular proinflammatory contents releasing | Dependent on caspase-1 activation and proinflammatory cytokine releasing | Pathological stimuli | Caspase-1/4/5/11,IL-1β, and IL-18, | Microbial infection, inflammation | |
| Autophagy | Formation of autophagosome and autolysosome, degradation of substrate in lysosome | Increased lysosomal activity | Nutrient starvation, oxidative stress | LC3,Atg5/7/12/13/16, Beclin1,p62 | Inflammation, cardiomyopathy, neurodegeneration, cancer | |
- Note. GPX4: glutathione peroxidas; GSH: glutathione; RB: Retinoblastoma; ROS: reactive oxygen species.
Some specific mechanisms are involved in regulating ferroptosis, including inhibition of the cystine/glutamate transporter system Xc- and depletion of antioxidase activities (Friedmann Angeli et al., 2014; W. S. Yang et al., 2015; Wu & Chen, 2014). Notably, glutathione peroxidase (GPX4), an antioxidant enzyme and an inhibitor of selective ferroptosis, is highly expressed in NCOA4-null mice (Bellelli et al., 2016). The observation supports that NCOA4-mediated ferritinophagy plays an important role in the initiation of ferroptosis. GPX4, as a critical inhibitor of ferroptotic cell death, is a beneficial and therapeutic target in cancer progression. In contrast, ferroptosis suppressed by GPX4 provides a protective mechanism against neurodegeneration (Cardoso, Hare, Bush, & Roberts, 2017). Thus, triggering or inhibiting ferroptosis might present a novel therapeutic strategy for cancer and neuronal diseases.
Here, the current overview summarizes the specific involvements of ferroptosis in cancer and neuronal diseases, and also illustrates the importance of ferritinophagy in erythropoiesis, some neurodegenerative diseases, and urinary tract infections. Taken together, modulation of ferroptosis or ferritinophagy can be a very attractive approach to future treatment of such kinds of diseases.
2 THE REGULATORY MECHANISMS RELATED TO FERRITINOPHAGY AND FERROPTOSIS
NCOA4-mediated ferritinophagy for degrading ferritin is a responsive mechanism to dynamic iron states (Mancias et al., 2015). When ferritinophagy is impaired via NCOA4 depletion, iron availability is reduced, whereas iron-responsive-element-binding protein 2 (IRP2) activity is induced to promote the translation of transferrin (Tf; Mancias et al., 2014). IRP2, a protein of the iron regulatory network, is controlled by cellular iron levels by binding to iron-responsive elements (IREs) for modulating Tf translation (Pantopoulos, Porwal, Tartakoff, & Devireddy, 2012). The regulation of IRE-IRP2 in Tf is also closely linked to the initiation of ferroptosis (Dixon et al., 2012). In addition, upon lower iron concentration, the recycling of Tf is significantly inhibited by heat shock protein B1 (HSPB1; Chen et al., 2006), which represents an inhibitory mechanism of ferroptosis (Sun et al., 2015). In addition, HERC2, a large multi-domain homologous to E6AP carboxy terminus (HECT) E3 ubiquitin ligase 2 (HERC2) and a NCOA4-interacting protein with ubiquitin ligase activity, plays a considerable role in the ferritinophagy process in response to altered intracellular iron status (Mancias et al., 2015; Bekker-Jensen et al., 2010). In response to the high iron level, HERC2 mediates NCOA4 turnover in ubiquitin-dependent manners through CUL7-homology domain in HERC2 and a C-terminal domain in NCOA4, thereby preventing ferritinophagy and blocking excess iron liberating ferritin. Besides, HERC2 regulates the proteasomal degradation of F-box/LRR-repeat protein 5 (FBXL5; Moroishi, Yamauchi, Nishiyama, & Nakayama, 2014), which could target IRP2 for its degradation under iron repletion (Salahudeen et al., 2009). Thus, HERC2 ubiquitination and the FBXL5-IRP2 axis play an integral role in the NCOA4-ferritinophagy process and maintain iron metabolism.
To date, it remains unclear whether there are other existing regulatory pathways related to ferritinophagy. Specifically, NCOA4 selectively interacts with the ferritin heavy chain-1 (FTH1) subunit of ferritin, not ferritin light chain (FTL), via a conserved C-terminal domain on NCOA4 and a key residue on FTH1. So, further research to determine the molecular mechanisms of the cooperation between FTH1 and FTL in ferritin, is greatly needed.
3 THE INHIBITORY ROLE OF FERROPTOSIS IN CANCERS
Cancer, one of most threatening diseases worldwide, is attributable to the unlimited proliferation of cancer cells. Cancer cells exhibit more dependence on iron and enhanced sensitivity to ferroptosis than normal cells (Manz, Blanchette, Paul, Torti, & Torti, 2016). For example, erastin, as a ferroptosis-inducing agent, further promotes the therapeutic effects of cisplatin on nonsmall cell lung cancer cells (Yamaguchi et al., 2013). Furthermore, ferroptosis-dependent cell death is considered as a novel therapeutic mechanism of artemisinin derivatives for treating cancers (Ooko et al., 2015). These findings suggest the pivotal role of ferroptosis in tumor suppression. Then, what are the critical involvements of ferroptosis in inhibiting the progression of cancers?
3.1 Ferroptosis suppresses hepatocellular carcinoma
Hepatocellular carcinoma (HCC), the most frequent type of liver cancer, has been ranked as the leading cause of tumorous deaths worldwide (Torre et al., 2015). For decades, the multikinase inhibitor sorafenib has already been used as an effective agent for treating HCC (Zhai & Sun, 2013). Mechanically, Louandre et al. (2013) found that deferoxamine, an iron chelator, strikingly protects HCC cells against iron-dependent oxidative stress caused by sorafenib. Ferroptosis is induced by sorafenib and plays an inhibitory role in the growth of liver cancer cells (Lachaier et al., 2014).
Some evidence supports that ferroptosis plays an inhibitory role against HCC via p62-Keap1-NRF2 pathway. Mice with genetic depletion of GPX4, a ferroptosis suppressor, display extensive hepatocytes degeneration by upregulating the nuclear factor (erythroid-derived)-like 2 (NRF2; Carlson et al., 2016). The inhibition of NRF2 significantly increases the anticancer effects of some ferroptosis-inducing compounds on HCC cell lines (Sun et al., 2016), such as erastin and sorafenib. Importantly, p62 effectively prevents the degradation of NRF2 by inactivation of Kelch-like ECH-associated protein 1 (Keap1), resulting in NRF2 accumulation and increased expression of heme oxygenase-1 (HO-1). HO-1 is also a well-characterized ferroptosis-inhibiting gene. Thus, the activation of the p62-Keap1-NRF2 pathway is involved in the suppression effects of ferroptosis on HCC.
Besides, retinoblastoma (RB) protein is an important modulator of ferroptosis in sorafenib-treated HCC cells. RB is mainly responsible for cell-cycle progression and liver tumorigenesis (Knudsen & Knudsen, 2008). It is revealed that HCC cells with lower RB levels promotes the occurrence of ferroptosis upon exposure to sorafenib (Louandre et al., 2015). In response to RB depletion, sorafenib induces greater production of mitochondrial oxidative stress. Since mitochondrial-targeted antioxidants are identified as a kind of ferroptosis inhibitors, RB protein might play a significant role in sorafenib-induced ferroptosis in HCC cells (Krainz et al., 2016).
In addition, the proliferation of HCC cells is stimulated by the activation of the Ras/Raf/MEK pathway (Yagoda et al., 2007). The mutation of such pathway is involved in ferroptotic cell death through erastin selectively binding to mitochondrial voltage-dependent anion channels, thereby attenuating the growth of HCC cells (Zhang et al., 2016). Together, the p62-Keap1-NRF2 pathway, RB protein, together with the Ras/Raf/MEK pathway may represent some important targets for ferroptosis in treating HCC (Figure 1).
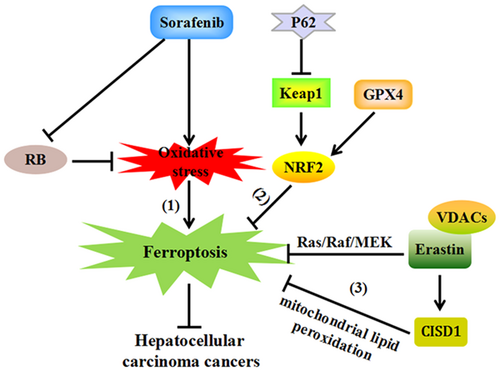
Ferroptosis inhibits the progression of Hepatocellular carcinoma cancers (1) Ferroptosis occurs as a result of the oxidative stress and suppressed RB protein levels upon exposure to sorafenib, playing inhibitory effects in hepatocellular carcinoma cancers. (2) GPX4 upregulates the expression of NRF2 response genes, which is promoted by p62 inactivating Keap1, resulting in cellular resistance against ferroptosis in HCC. (3) Erastin selectively bind to VDACs to inhibit ferroptosis by activating the Ras/Raf/MEK pathway. Meanwhile, erastin also elevates the expression of CISD1, protecting against mitochondrial lipid peroxidation, negatively regulating ferroptosis and promoting the proliferation of HCC. CISD1: CDGSH iron sulfur domain 1; GPX4: glutathione peroxidase 4; HCC: hepatocellular carcinoma; Keap 1: Kelch-like ECH-associated protein 1; NRF2: nuclear factor (erythroid-derived)-like 2; RB: retinoblastoma; VDACs: voltage-dependent anion channels [Color figure can be viewed at wileyonlinelibrary.com]
3.2 Ferroptosis attenuates pancreatic carcinoma
Pancreatic carcinoma (PCC), as the most deadly tumor, has a lower survival rate due to a limited number of available treatment measures (Gupta, Amanam, & Chung, 2017). Artemisinin (ART), as an antimalarial compound, induces ferroptosis-dependent pancreatic ductal adenocarcinoma (PDAC) cell death (Eling, Reuter, Hazin, Hamacher-Brady, & Brady, 2015; Ooko et al., 2015). Thus, ferroptosis may represent a new mechanistic pathway underlying ART-induced death of PCC cells (Figure 2).

Ferritinophagy promotes the development of erythropoiesis and urinary tract infections (1) Upon iron-depletion, NCOA4 selectively interacts with the FTH1 subunit of ferritin, via its C-terminal domain and a key surface arginine residue on FTH1. NCOA4, coupled with PCBP1, mediating the flux of iron out and into of ferritin respectively, are critical for regulating heme synthesis and further erythropoiesis differentiation. Meanwhile, NCOA4-dependent ferritinophagy promotes the persistence of UPEC in host cells by increasing iron availability, accelerating urinary tract infections. (2) In the presence of iron-repletion, HERC2 mediates NCOA4 degradation in ubiquitin-dependent manners through CUL7-homology domain in HERC2 and a C-terminal domain in NCOA4, preventing ferritinophagy. Arg: arginine; FTH1: ferritin heavy chain-1; HERC2: homologous to E6AP carboxy terminus (HECT) E3 ubiquitin ligase 2; NCOA4: nuclear receptor coactivator 4; PCBP1: iron chaperones poly rC–binding protein 1; UTIs: urinary tract infections; UPEC: uropathogenic Escherichia coli [Color figure can be viewed at wileyonlinelibrary.com]
Interestingly, ART induces the autophagic degradation of ferritin via activating the lysosomal function, which is reversed by the genetic depletion of NCOA4 (N. D. Yang et al., 2014). Lysosomal activity is indispensable for lipid ROS-mediated ferroptotic cell death. The excess cellular labile iron liberated by the lysosomal degradation of ferritin, is the prerequisite for iron-dependent ROS generation and ferroptosis occurrence. Then, it is inferred that ART activates ferroptosis via enhancing the ferritinophagy process.
In addition, pancreatic cancer (PCC) cells exhibit an elevated expression of xCT (SLC7A11) protein (Lo, Ling, Wang, & Gout, 2008), a membrane cysteine–glutamate exchange transporter (Sato, Tamba, Ishii, & Bannai, 1999). SLC7A11, a downstream metabolic target of p53, is repressed by p53. P53 regarded to act as a tumor suppressor through mediating oxidative stress responses and ferroptosis (Jiang, Hickman, Wang, & Gu, 2015). In detail, p53′ acetylation at lysine K98 plays a determined role in ferroptotic responses and tumor suppression activity in some mouse xenograft models (Wang et al., 2016). SLC7A11 is mainly responsible for transporting intracellular glutamate out or extracellular cystine in cells for glutathione (GSH) synthesis (Bridges, Natale, & Patel, 2012). GSH, as a pivotal factor to maintain oxidation–reduction balance via GPX4, is a potent inhibitor of ferroptosis via the activity of cysteine dioxygenase 1 (Hao et al., 2017; W. S. Yang et al., 2014). Upon cystine deprivation, erastin-induced ferroptosis is suppressed by the loss of cysteinyl-tRNA synthetase through upregulating sulfur-transfer-related genes in cancer cells (Hayano, Yang, Corn, Pagano, & Stockwell, 2016). In addition, GPX4 degradation and lipid peroxidation are blocked by heat shock 70 kDa protein 5 (HSPA5), which is induced by activating transcription Factor 4 (ATF4), thereby resisting against ferroptosis in PDAC (Zhu et al., 2017). Therefore, genetic inhibition of the ATF4-HSPA5-GPX4 pathway may enhance the activity of ferroptosis, attenuating the proliferation of PCC. Collectively, ferroptosis represents a potential therapeutic target to inhibit PCC through ferritinophagy and the p53-SLC7A11 axis.
3.3 The interruptive effects of ferroptosis on other cancers
Ferroptosis also closely correlates with other kind of cancers. Some findings demonstrate that suppressing HSF1-HSPB1 pathway facilitates erastin-induced ferroptosis in human cervical cancer cells, osteosarcoma cells, and prostate cancer cells (Sun et al., 2015). In triple-negative breast cancer (TNBC) cells, the MUC1-C/xCT/CD44 variant pathway is important to inhibit ferroptosis via controlling GSH levels (Hasegawa et al., 2016). HO-1 as a substrate for metabolizing heme to generate free iron and necessary enzymes to accelerate ferroptotic cell death, is identified to have antitumoral effects in prostate cancer cells and colorectal cancer (Andres et al., 2014; Gueron et al., 2014; Kwon, Park, Lee, & Chung, 2015; Yin, Fang, Liao, Maeda, & Su, 2014). Dihydroartemisinin, a kind of artemisinin semi-synthetic derivative, causes ferroptosis-dependent head and neck cancer (HNC) cell death (Lin et al., 2016). Similar to HCC, activating Keap1-NRF2-ARE pathway is also proposed as a promotive mechanism to enhance the effectiveness of artesunate-induced ferroptosis in HNC cells (Roh, Kim, Jang, & Shin, 2017). Ferroptosis is suppressed by N-ethylmaleimide-sensitive fusion protein 1, an iron–sulfur cluster biosynthetic enzyme, thereby resisting against high oxygen tension and inducing lung tumor growth (Alvarez et al., 2017). Ferrostatin-1, as another ferroptosis inhibitor, remarkably impedes ART-induced ROS production and ferroptosis-dependent cell death in ovarian cancer (Basuli et al., 2017; Greenshields, Shepherd, & Hoskin, 2017). These findings point out the essential contributions of ferroptosis in inhibiting the growth of diverse types of cancer cells. The novel involvement of ferroptosis in these cancer cells may be beneficial therapeutically to the progression of cancers.
4 THE AGGRAVATED IMPACTS OF FERROPTOSIS ON NEURODEGENERATIVE DISEASES
Neurodegenerative diseases, such as Parkinson’s disease (PD) and Alzheimer’s disease (AD), share several common processes in neural cell death, including iron accumulation and excess oxidative stress (Jenner, Dexter, Sian, Schapira, & Marsden, 1992; Ward, Zucca, Duyn, Crichton, & Zecca, 2014). Iron-dependent oxidative stress is a hallmark of ferroptosis cell death (Yang & Stockwell, 2016). Emerging studies show the critical involvement of ferroptosis in the progression of neurodegenerative diseases. Do Van et al., (2016), for the first time, highlighted the implication of ferroptosis in dopaminergic neurons and PD development via the activation of protein kinase Cα (PKCα). In addition, the mice with conditional deletion of GPX4 display obvious neural function deficits and cognitive impairment, which are ameliorated by treatment with an ferroptosis inhibitor (Hambright, Fonseca, Chen, Na, & Ran, 2017). Thus, ferroptosis might be an important driving mechanism to the progression of neurodegenerative diseases. Surprisingly, the complete loss of HERC2 is responsible for severe neurodevelopmental abnormalities, contributing to the pathogenesis of neurogenetic diseases (Morice-Picard et al., 2016). HERC2 is a key modulator in ferritinophagy, particularly with high levels of cellular iron (Mancias et al., 2015). So, it is presumed that HERC2 deficiency might result in defective response to increased iron levels, inducing abundant ferritinophagy, promoting the release of free iron and damaging neuronal cells. Thus, ferritinophagy might also be a potent force to induce neurodegenerative diseases due to the functional deficits in HERC2.
4.1 Ferroptosis accelerates PD
PD is a progressive neurodegenerative disorder characterized by depletion of dopamine neurons in substantia nigra pars compacta and aberrant aggregation of cellular inclusions, known as Lewy bodies (Hornykiewicz, 2008). The α-synuclein (SNCA) in Lewy bodies is modulated by iron at the translational level (Febbraro, Giorgi, Caldarola, Loreni, & Romero-Ramos, 2012).
Importantly, iron accumulation contributes to the pathogenesis of PD by inducing oxidative stress and SNCA aggregation (Jiang, Wang, Rogers, & Xie, 2017). The abnormal aggregation of SNCA and its neurotoxicity are mediated by the downregulation of the NRF2/HO-1 pathway (He et al., 2013), which plays an inhibitory role against ferroptosis (Sun et al., 2016). Ferroptosis is verified to promote the progression of PD via the activation of PKCα and MEK in a RAS-independent manner (Do Van et al., 2016). The marked characteristics of ferroptosis (Dixon et al., 2012), such as excessive iron-induced oxidative stress and lipid peroxidation, could cause oxidative injury to the substantia nigral neurons (Mohanakumar et al., 1994). In addition, iron accumulation is also closely related to the decreased concentration of FTL, which serves as an important modulator underlying neurodegeneration through inhibiting glutamate uptake (Alekseenko, Waseem, & Fedorovich, 2008; Friedman, Arosio, Finazzi, Koziorowski, & Galazka-Friedman, 2011). Reduced levels of GSH are recognized as indicators of PD diseases (Jenner et al., 1992) by increasing the availability of cellular iron for catalyzing ferroptosis. GPX4, a key factor for maintaining GSH function and an inhibitor of ferroptosis, provides protective mechanisms against neurodegeneration (Cardoso et al., 2017). Hambright et al., (2017) support the promotive role of ferrotosis in the pathological process of PD diseases via the alteration in GPX4 function. A recent research showed that the administration of ferrostatin-1, an inhibitor of ferroptosis, could largely attenuate iron deposition and ferroptosis-induced neuronal death (Li et al., 2017). Thus, ferroptosis accelerates the progression of PD via disturbing the NRF2/HO-1 pathway and GPX4 function.
Besides, a recent study demonstrated that α-syn impairs ferritinophagy by disrupting lysosomal activity, resulting in the emergence of PD (Baksi & Singh, 2017), implying that ferritinophagy might play a significant role in the progression of PD.
4.2 Ferroptosis may exacerbate AD
AD is a well-known neurodegenerative disease, pathologically marked by the deposition of amyloid plaques protein (APP) aggregates. APP aggregation consists of amyloid-β (Roberts, Ryan, Bush, Masters, & Duce, 2012) and closely interacts with iron accumulations in the progression of AD (Everett et al., 2014). Similar to PD, iron accumulation extensively deposited in brain regions is considered as a pathogenic factor underlying AD by inducing oxidative stress and neuronal cell death (Mena, Urrutia, Lourido, Carrasco, & Nunez, 2015).
Some evidence reveals the significantly decreased levels of hepcidin and ferroportin in brains of AD patients (Raha, Vaishnav, Friedland, Bomford, & Raha-Chowdhury, 2013). Hepcidin and its target-ferroportin are systemic iron-regulatory proteins, which are mainly responsible for iron homeostasis and iron exporting, and are modulated by iron stores and hypoxia (Ganz, 2011; Papanikolaou et al., 2005). Interestingly, the administration of iron chelator provides a novel neuroprotective mechanism against AD through maintaining the level of hypoxia-inducible factor-1 alpha (HIF-1α) in nerves and inhibiting neuronal cell death (Ashok, Ajith, & Sivanesan, 2017). HIF prolyl hydroxylase (HIF), a component of the HIF pathway, is regarded as an inhibitory target to ferroptosis and plays a neuroprotective role against neurological diseases (Speer et al., 2013). Furthermore, iron chelators promote the expression of HIF-1α-dependent target genes, such as HO-1 (Kupershmidt et al., 2011). The upregulation of the NRF2/HO-1 pathway is involved in interrupting ferroptotic cell death and induced a neuroprotective effect (Adedoyin et al., 2018; Kim et al., 2017; Roh et al., 2017). In particular, the ferroptosis inhibitor of ferrostatin-1 prevents mitochondrial dysfunction and oxidative cell death, mediating neuroprotective effects (Neitemeier et al., 2017). Therefore, ferroptosis is likely to exacerbate AD via targeting the HIF-1α and HO-1 pathways.
Overall, it is promising that inhibiting ferroptosis may attenuate neurodegenerative disorders, such as PD and AD.
5 THE CRITICAL INVOLVEMENT OF FERRITINOPHAGY IN ERYTHROPOIESIS
Erythropoiesis is facilitated by the process of hematopoietic stem and erythroid progenitor cells differentiating into mature red cells (An et al., 2014). Mature red cells are rich in hemoglobin, which shows strong iron dependence. Iron is essential for sulfur cluster synthesis and mostly presents as heme in the hemoglobin of maturing erythrocytes (Camaschella, Pagani, Nai, & Silvestri, 2016). Due to the significance of ferritinophagy in regulating cellular iron availability, NCOA4-mediated ferritinophagy is required for the survival process of erythropoiesis (Mancias et al., 2015).
A series of research suggest the implication of NCOA4 in erythropoiesis. Weber et al., (2005) observe the high expression of NCOA4 at sites of erythropoiesis in zebrafish. Furthermore, NCOA4 messenger RNA is upregulated at the orthochromatic erythroblast stage during erythroid differentiation (An et al., 2014). By contrast, mice with genetic knockdown NCOA4 induce ferritin accumulation in primary embryonic fibroblasts, developing severe hypochromic microcytic anemia and ineffective erythropoiesis. All evidence reveal the specific involvement of NCOA4-mediated ferritinophagy in regulating organismal iron metabolism and its biological function in erythropoiesis.
Moreover, poly rC–binding protein 1 (PCBP1), an iron chaperones, has recently been reported to be critical for the development of erythroid differentiation (Ryu, Zhang, Protchenko, Shakoury-Elizeh, & Philpott, 2017). NCOA4 and PCBP1 are implicated in mediating the flux of iron into and out of ferritin through a direct protein-protein interaction. The dynamic process of NCOA4-PCBP1 interaction is induced by iron deprivation and suppressed by iron excess (Ryu, Duck, & Philpott, 2018). Consequently, the depletion of PCBP1 and NCOA4 impairs iron trafficking from ferritin and perturbs erythroid regulatory systems. Therefore, NCOA4-mediated ferritinophagy and PCBP1 represent novel mechanisms of iron-dependent regulation in erythropoiesis.
In conclusion, NCOA4-mediated ferritinophagy functions in maintaining efficient erythropoiesis via balancing iron levels.
6 FERRITINOPHAGY PROMOTES THE PROGRESSION OF URINARY TRACT INFECTIONS
Urinary tract infections (UTIs), bacterial infectious diseases (Foxman, 2010), are caused by Uropathogenic Escherichia coli (UPEC) persisting in bladder epithelial cells (Flores-Mireles, Walker, Caparon, & Hultgren, 2015). Iron is indispensable for UPEC surviving in host cells, since free iron supplements could significantly promote the growth rate of UPEC (Gao et al., 2012; Garcia, Brumbaugh, & Mobley, 2011; Khasheii, Anvari, & Jamalli, 2016). Therefore, the great dependence of UPEC on iron should be a central target to defend against pathogens. Then, whether ferritinophagy, a novel pathway to release iron in the form of shuttling iron-bound ferritin, might contribute to the proliferation of UPEC and exacerbation of UTIs?
As expected, Bauckman and Mysorekar, (2016) reported, for the first time, that NCOA4-dependent ferritinophagy triggers the overproliferation and persistence of UPEC in host cells, caused by increased intracellular iron availability. In general, UPEC transports ferritin-bound iron into the autophagosomal and lysosomal compartments in urothelium. However, when administered with an autophagy inhibitor or an iron chelating agent, bacterial prevalence, and host cell death are significantly impended. Interestingly, ferritinophagy is designated as a kind of xenophagy, which involves pathogens and foreign entities, such as bacteria and virus for autophagic responses (Bauckman, Owusu-Boaitey, & Mysorekar, 2015; Knodler & Celli, 2011). The new recognition promotes the understanding of the relationships between symbiotic bacterial and autophagy since UPEC (as a primary pathogen involved in UTIs) prolongs its intracellular survival through the autophagic pathway.
Although autophagy is considered to be antipathogenic via resisting pathogens, autophagy-related 16-like 1 (ATG16L1)-deficient mice are revealed to have host-protective effects against UPEC infection by getting rid of bacteria thoroughly (Wang et al., 2012; Wang, Symington, & Mysorekar, 2012). ATG16L1 is an autophagy protein, hinting the involvement of autophagy. Furthermore, UPEC also develops some iron chelating mechanisms to gain host iron stores in the pathogenesis of UTIs (Subashchandrabose & Mobley, 2015; Wiles, Kulesus, & Mulvey, 2008). When acquired iron is prevented, the growth rate of UPEC is effectively inhibited (Brumbaugh, Smith, & Mobley, 2013; Yep, McQuade, Kirchhoff, Larsen, & Mobley, 2014). Thus, ferritinophagy might be a potent driving force to induce UPEC persistence. Inhibition of the selective autophagy pathway might provide a therapeutic target to reverse host cell death and impede the development of UTIs.
7 SUMMARY
In brief, the repressed functions of ferroptosis in diverse cancer cells and stimulated role in neuronal cells are great advancements in the research of RCD. The inhibitory or promoted mechanism, as well as multiple pathways related to ferroptosis, support the potential involvements of ferroptosis in neurodegeneration diseases and cancers. Besides, NCOA4-dependent ferritinophagy, autophagic transferring of iron out of ferritin, is a critical pathway for erythropoiesis, although it exacerbates the progression of urinary tract infections. Thus, ferritinophagy and ferroptosis, as the two iron-related newcomers in diverse human diseases, should be considered for the design and assessment of nutritional and pharmacologic intervention for treating cancer, neurodegeneration, erythropoiesis, urinary tract infections diseases, which might be a promising disease-reversing strategy for patients.
7.1 Prospection
To date, the ferroptosis pathway has only been reported to aggravate PD definitely, but iron accumulation is also an evident hallmark of other neurodegeneration diseases, such as Huntington’s diseases and Friedreich ataxia (Martelli & Puccio, 2014; Muller & Leavitt, 2014). Then, whether ferroptosis inhibitors show promise for other neurodegenerative diseases and exhibit broad-acting benefits to the therapies? In addition, what is the definitive role of iron in ferroptosis to suppress various tumorous diseases? And the detailed mechanisms of lipid oxidation and iron involved in ferroptosis remain unclarified. Therefore, future studies would be expected to deliberately identify the precise iron-derived ROS target sites in ferroptosis, which is significant to treat ferroptosis-related diseases beneficially.
Ferritinophagy, as a recently discovered selective autophagy, is remarkably different from some other selective autophagy, such as mitophagy (Liu et al., 2012; Novak et al., 2010; Okamoto, Kondo-Okamoto, & Ohsumi, 2009; Quinsay, Thomas, Lee, & Gustafsson, 2010), reticulophagy (Khaminets et al., 2015; Mochida et al., 2015), glycophagy (Jiang, Wells, & Roach, 2011), xenophagy (Thurston, Wandel, von Muhlinen, Foeglein, & Randow, 2012), aggrephagy (Liu et al., 2017; Rogov, Dotsch, Johansen, & Kirkin, 2014), pexophagy (Farre, Manjithaya, Mathewson, & Subramani, 2008; Motley, Nuttall, & Hettema, 2012), nucleophagy (Luo, Zhao, Song, Cheng, & Zhou, 2016; Mochida et al., 2015), ribophagy (Kraft, Deplazes, Sohrmann, & Peter, 2008), DNautophagy (Aizawa et al., 2017; Fujiwara, Hase, Wada, & Kabuta, 2015), and RNautophagy (Fujiwara et al., 2015; Hase et al., 2015; Table 2). Although NCOA4-mediated ferritinophagy has been certified to be responsible for erythropoiesis via releasing iron, the specific transferred way of ferritinophagy-derived iron delivering to mitochondria warrants further investigation. Further research should be focused on the integrated functions and regulatory mechanism about ferritinophagy, such as ascertaining the reliable upstream and downstream molecules as well as specific tissue compartments that NCOA4-mediated ferritinophagy relies on. Importantly, it is possible that ferritinophagy or ferroptosis might contribute to other iron-related diseases, such as cardiomyopathy, since ferroptosis suppressed by rapamycin, a target of autophagy, is reported to play a protective role in cardiomyocytes (Baba et al., 2018).
| Selective autophagy | Substrate | Receptor | Consequence | References |
|---|---|---|---|---|
| Ferritinophagy | Ferritin | NCOA4 | Degradation of ferritin and release free iron | Mancias et al., 2014; Mancias et al., 2015 |
| Mitophagy | Mitochondria | BNIP3, PINK1-parkin, Nix, Atg32, FUNDC1, Optineurin TAX1BP1, p62 | Degradation damaged mitochondria | Liu et al., 2012; Novak et al., 2010; Quinsay et al., 2010; Okamoto et al., 2009 |
| Reticulophagy | Endoplasmic reticulum | FAM134B, Atg40 | Endoplasmic reticulum turnover | Khaminets et al., 2015; Mochida et al., 2015 |
| Glycophagy | Glycogen | STBD1, Atg7 | Degradation of glycogen | Jiang et al., 2011 |
| Xenophagy | Intracellular pathogens | Galectin8, NDP52 | Targeting and removing pathogens | Thurston et al., 2012 |
| Aggrephagy | Protein aggregates | NBR1, p62,HDAC6, WDR81 | Selective clearance of aggregated ubiquitinated proteins | Rogov et al., 2014; Liu et al., 2017 |
| Pexophagy | Peroxisomes | Atg30, Atg36 | Autophagic degradation of peroxisomes | Farré et al., 2008; Motley et al., 2012 |
| Nucleophagy | Nuclear envelope | Atg39 | Removal of nucleus-derived material | Mochida et al., 2015; Luo et al., 2016 |
| Ribophagy | Ribosomes | Ubp3p, Bre5p, Atg7 | Specific degradation of ribosomes | Kraft et al., 2008 |
| DNautophagy | DNA | SIDT2, LAMP2C, Dnase II | Degradation of DNA | Fujiwara et al., 2015; Aizawa et al., 2017 |
| RNautophagy | RNA | SIDT2, LAMP2C, Rnase T2 | Degradation of RNA | Fujiwara et al., 2015; Hase et al., 2015 |
ACKNOWLEDGMENTS
The study was supported by grants from the National Natural Science Foundation of China (81703515, 81670265 and 81503074), the Construct Program of the Key Discipline in Hunan Province (2014M560647), and Hunan Province Cooperative Innovation Center for Molecular Target New Drug Study (Hunan Provincial Education Department document) (2014-405).
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interests.