BMAL1 and CLOCK proteins in regulating UVB-induced apoptosis and DNA damage responses in human keratinocytes
Abstract
A diverse array of biological processes are under circadian controls. In mouse skin, ultraviolet ray (UVR)-induced apoptosis and DNA damage responses are time-of-day dependent, which are controlled by core clock proteins. This study investigates the roles of clock proteins in regulating UVB responses in human keratinocytes (HKCs). We found that the messenger RNA expression of brain and muscle ARNT-like 1 (BMAL1) and circadian locomotor output cycles kaput (CLOCK) genes is altered by low doses (5 mJ/cm2) of UVB in the immortalized HaCat HKCs cell line. Although depletion of BMAL1 or CLOCK has no effect on the activation of Rad3-related protein kinases–checkpoint kinase 1–p53 mediated DNA damage checkpoints, it leads to suppression of UVB-stimulated apoptotic responses, and downregulation of UVB-elevated expression of DNA damage marker γ-H2AX and cell cycle inhibitor p21. Diminished apoptotic responses are also observed in primary HKCs depleted of BMAL1 or CLOCK after UVB irradiation. While CLOCK depletion shows a suppressive effect on UVB-induced p53 protein accumulation, depletion of either clock gene triggers early keratinocyte differentiation of HKCs at their steady state. These results suggest that UVB-induced apoptosis and DNA damage responses are controlled by clock proteins, but via different mechanisms in the immortalized human adult low calcium temperature and primary HKCs. Given the implication of UVB in photoaging and photocarcinogenesis, mechanistic elucidation of circadian controls on UVB effects in human skin will be critical and beneficial for prevention and treatment of skin cancers and other skin-related diseases.
1 INTRODUCTION
A diverse array of biochemical and physiological processes, such as metabolisms, hormonal levels, body temperatures, and sleep-wake cycles, have been known to chart a 24-hr rhythmicity that depends on the coordination between external signals and the internal timing system—referred to as the circadian clock (Dibner, Schibler, & Albrecht, 2010; Reppert & Weaver, 2002). In mammals, the 24-hr light/dark cycle is sensed by the retina and transmitted as electronic signals to the suprachiasmatic nucleus (SCN) of the brain. The master clock in the SCN would then in turn relay either neural or hormonal signals to synchronize downstream oscillators in other parts of the brain as well as peripheral organs (Hastings, Reddy, & Maywood, 2003; Tanioka et al., 2009). At a molecular level, the clock mechanism is driven by four transcription factors, and their expression autonomously oscillates through the transcription-translation feedback loop (Reppert & Weaver, 2002). Among the four core clock proteins, BMAL1–CLOCK heterodimers, encoded by the respective genes of brain and muscle ARNT-like 1(BMAL1) and circadian locomotor output cycles kaput (CLOCK), constitute the positive arm by activating transcription via binding to the E-box (CACGTG) in the promoter regions of numerous target genes (Kondratov et al., 2003). The negative arm of the core clock is composed of CRY and PER proteins encoded by cryptochrome (CRY1 and CRY2) and period (PER1, PER2, and PER3) genes, respectively, which are the target genes of the BMAL1-CLOCK transcription activity (Kume et al., 1999; Zylka, Shearman, Weaver, & Reppert, 1998). Translated PER-CRY dimers can move from the cytoplasm to the nucleus and repress transcriptional activities of BMAL1-CLOCK after a time lapse, leading to negative feedback. BMAL1 oscillation is also fine tuned by an additional feedback loop that contains two nuclear receptors, retinoic acid receptor-related orphan receptors (RORs) and REV-ERBs (Guillaumond, Dardente, Giguere, & Cermakian, 2005; Preitner et al., 2002).
Consequently, with such a far-reaching physiological impact, disruptions of the circadian clock machinery can be detrimental and lead to various diseases related to obesity, aging, asthma, neurological disorders, heart attack, and cancers (Hastings et al., 2003; Janich, Meng, & Benitah, 2014; Sahar & Sassone-Corsi, 2009; Sancar et al., 2015). More significant, this machinery is also imperative to human skins. Skin is an organ that is under constant exposure to an environment with daily fluctuations in temperatures, humidity levels, and ultraviolet (UV) solar irradiation intensities. Recently, local circadian oscillators have been identified in different skin compartments of multiple cell types, including keratinocytes, dermal fibroblasts, and melanocytes (Brown et al., 2005; Hardman et al., 2015; Janich et al., 2013; Kawara et al., 2002; Sandu et al., 2012). Studies using genetic mouse models have demonstrated the involvement of circadian clocks in numerous aspects of skin homeostasis such as hair cycle, cell proliferation, stem cell functions, carcinogenesis, aging, immunity, and UV-induced DNA damage responses (Brown, 2014; Dakup & Gaddameedhi, 2017; Geyfman et al., 2012; Plikus et al., 2015; Sancar et al., 2015).
Based on their wavelengths, UV rays are divided into three categories: UVA (320–400 nm), UVB (290–320 nm), and UVC (200–290 nm; Diffey, 2002). A small portion of UVB, at longer wavelengths, can reach the earth and is the major cause for photoaging and photocarcinogenesis (Matsumura & Ananthaswamy, 2004). UVB carries enough energy to penetrate both the epidermis and dermis, thereby triggering DNA damage responses in keratinocytes, melanocytes, and other cell types in the human skin (Bowden, 2004). The absorbance of UVB by DNA molecules produces two unique DNA adducts: pyrimidine-pyrimidine (6-4) photoproducts and cyclobutane pyrimidine dimers (CPDs; de Gruijl, 2000). CPDs-associated C-to-T or CC-to-TT conversions can generate UV-signature mutations, which have been found in oncogenes or tumor suppressor genes associated with skin cancers (Brash et al., 1991; de Gruijl, 2002). In mammals, CPDs are removed exclusively by the nuclear excision repair (NER) mechanism (Reardon & Sancar, 2005). UV-induced DNA damages are sensed by the ataxia telangiectasia and Rad3-related protein kinases (ATR), which phosphorylates the checkpoint kinase 1 (CHK1). CHK1 further transduces signals by phosphorylating its downstream effector proteins, such as cdc25 and p53 (Heffernan et al., 2009, 2002; Sancar, Lindsey-Boltz, Unsal-Kacmaz, & Linn, 2004). Such DNA damage responses in keratinocytes are critical in the prevention of skin carcinogenesis. P53 is the critical transcription factor responsible for UVB-induced cell cycle arrest, apoptosis, and DNA damage repair (Decraene, Agostinis, Pupe, de Haes, & Garmyn, 2001; Latonen & Laiho, 2005). UV-signature mutations of p53 can be found in patches of normal human skin, especially at the sun-exposed areas (Jonason et al., 1996). These mutations are also associated with initiation of most nonmelanoma skin cancers, such as squamous cell carcinoma (Brash et al., 1991; Ziegler et al., 1994).
The circadian control of UVB-induced DNA damage responses has been well studied in the mouse skin. Gaddameedhi, Selby, Kemp, Ye, and Sancar (2015) found that UVB-triggered sunburn apoptosis and erythema are more robust in the morning than in the afternoon for wild-type mice. In contrast, such time-of-day-dependence is lost in mice with double knockout of Cry1/2 genes or Per1/2 genes, or with Bmal1 deletion (Geyfman et al., 2012). Moreover, the clock-dependent severity of apoptotic responses in UVB-irradiated wild-type mice appears to correlate with the magnitude of DNA damage repair, p53 expression, and activity, and the activation of the ATR-Chk1-p53 mediated DNA damage checkpoint pathway (Gaddameedhi et al., 2015). In response to UVB irradiation, mouse epidermis also shows a circadian rhythmicity of excision repair rate that correlates with the time-of-day dependent expression level of XPA, one of the six excision repair factors (Gaddameedhi, Selby, Kaufmann, Smart, & Sancar, 2011). Such circadian control of UVB-induced DNA damage repair is lost in Cry1/2 double knockout mice. More important, mice exposed to UV radiation in the morning tend to display a higher predisposition to develop invasive squamous cell carcinoma, which is consistent with the minimum excision repair rate at this time (Gaddameedhi et al., 2011).
A recent study by Nikkola et al. reveals that UVB irradiation in the evening triggers more severe sunburn erythematic responses than irradiation in the morning in human skin (Nikkola et al., 2018; Sarkar & Gaddameedhi, 2018). This finding confirms the circadian control of UVB initiated responses in human skin, although the exact molecular mechanism remains unclear. Clock genes have been detected in primary human keratinocytes (HKCs) and the immortalized human adult low calcium temperature (HaCaT) keratinocytes (Sporl et al., 2011). In addition, expressions of core clock genes could also be altered in response to low doses of UVB radiation in cultured primary HKCs (Kawara et al., 2002). In this study, we aim to investigate the functions and mechanisms of BMAL1 and CLOCK proteins in regulating UVB-induced apoptosis and DNA damage responses in both types of HKCs; and we report our findings here.
2 MATERIALS AND METHODS
2.1 Cell culture
The HaCat cell line was obtained from Cobioer Biosciences Co. Ltd. (Nanjing, China). As described previously (Li, Zhou, & Dai, 2018), HaCat cells were cultured in the Dulbecco modified Eagle medium (Corning, Shanghai, China) supplemented with 10% fetal bovine serum (Capricorn, Germany) and 1% penicillin/streptomycin (Solarbio, China). Primary HKCs were cultured as described previously (Dai, Brooks, Lefort, Getsios, & Dotto, 2013) in serum-free keratinocyte-SFM medium (Gibco-Thermo Fisher Scientific, Waltham, MA) supplemented with 30 mg/ml bovine pituitary extract and 0.2 ng/ml rEGF on plates coated with type I collagen (Sigma-Aldrich, St. Louis, MO). Both types of keratinocytes were maintained at 37°C with 5% CO2.
2.2 RNA interference
Predesigned human Bmal1 small interference RNA (siRNA; s100702), CLOCK siRNAs (s114292, s114293, s114294), and negative control siRNA (#4613) were purchased from Ambion-Invitrogen/Thermo Fisher Scientific (Pittsburg, PA). HaCat or HKCs were reversely transfected with 20 nM of siRNA duplexes using lipidoid (Li, Zhou, & Dai, 2018). CLOCK siRNAs contained a mixture of three siRNA duplexes in equal parts. The cells were exposed to 5 mJ/cm2 UVB radiation at 48 hr after transfection.
2.3 UVB irradiation
HaCat cells or HKCs were washed with phosphate buffered saline (PBS) and irradiated with double-bank UV lamps (UVP XX-15M; Analytik Jena/Thermo Fisher Scientific, Pittsburg, PA), which emit UVB wavelengths with the peak intensity at 302 nm. The dose of UVB (5 mJ/cm2) was measured by the ILT2400 radiometer equipped with a SED005/UVF/A313 UV detector (International Light Technologies, Peabody, MA).
2.4 Cell viability assay
HaCat or HKCs cells were reversely transfected with different siRNAs and plated in 24-well plates. Two days later, after washing with PBS, one group of cells was exposed to 5 mJ/cm2 UVB, whereas the control group was not irradiated. The HaCat cells were fixed with 4% paraformaldehyde (PFA)/PBS at 24 hr after UVB and stained with Hoechst 33342 (Pierce Biotechnology-Thermo Fisher Scientific, Rockland, IL) at 1 μg/ml for 10 min. Cell images with fluorescent nuclear staining were taken with a Nikon Eclipse Ti-S inverted fluorescence microscope. Three different fields per experiment were counted with the ImageJ software to get the average number of cells/field. For HKCs, cell viability was measured using an AlamarBlue Assay Kit (G-Biosciences, St. Louis, MO) according to the manufacturer’s instructions. The absorbance was measured at 570 nm and 600 nm using a Synergy HTX Multi-Mode Microplate Reader (BioTek, Winooski, VT).
2.5 Real-time reverse transcription polymerase chain reaction
Total RNA was isolated from cells using the Eastep® Super Total RNA Extraction kit (Promega, Madison, WI) and was reverse-transcribed into complementary DNA (cDNA) using a HiFiScript cDNA Synthesis Kit (Cwbiotech, China). Real-time reverse transcription polymerase chain reaction (RT-PCR) with UltraSYBR Mixture (Cwbiotech) was performed on an ABI QuantStudio™ 6 Flex Real-Time PCR System (Foster City, CA) according to the manufacturer’s instructions. The messenger RNA (mRNA) levels of target genes were normalized to the expression of the housekeeping 36B4 gene.
| Primers for RT-PCR (human) | ||
|---|---|---|
| Gene | Forward | Reverse |
| BMAL1 | ACGGAGGTGCCTGTTTACC | CATTGGAAAAGTTAAGCTTGGG |
| CLOCK | GGCTGAAAGACGACGAGAAC | GGTGTTGAGGAAGGGTCTGA |
| p21 | GATTAGCAGCGGAACAAGGA | CAACTACTCCCAGCCCCATA |
| PUMA (BBC3) | GACCTCAACGCACAGTACGA | CACCTAATTGGGCTCCATCT |
| KRT1 | GTTCCAGCGTGAGGTTTGTT | TAAGGCTGGGACAAATCGAC |
| KRT10 | GAAAAGCATGGGCAACTCACA | TGTCGATCTGAAGCAGGATG |
| 36B4 | GCAATGTTGCCAGTGTCTGT | GCCTTGACCTTTTCAGCAAG |
2.6 Western blot analysis
Whole cell lysates for western blot were prepared with the SDS-sample buffer. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto the polyvinylidene fluoride membrane (Millipore/Thermo Fisher Scientific, Darmstadt, Germany/Pittsburgh, PA). Antibodies for immunoblotting from Cell Signaling Technology (Dancers, MA) included rabbit anti-Bmal1 (#14020), rabbit anti-CLOCK (#5157), rabbit anti-phospho-p53 (Ser15) [#9284], rabbit anti-p21 Waf1/Cip1 (#2947), rabbit anti-Histone H2AX (#2595), rabbit anti-cleaved poly ADP-ribose polymerase (PARP; #9541), and rabbit anti-phospho-CHK1 (Ser 345) (#2348). Other antibodies included mouse anti-p53 (sc-126; Santa Cruz Biotechnology Inc., Santa Cruz, CA), rabbit anti-Keratin 10 (Poly19054; Biolegend, San Diego, CA), mouse anti-phospho-Histone H2AX (Ser139) (05-636; Millipore/Thermo Fisher Scientific), and mouse anti-α-tubulin (T9026; Sigma-Aldrich). horseradish peroxidase (HRP)-conjugated secondary antibodies were from Santa Cruz Biotechnology Inc. Chemiluminescence images were acquired using an Amersham Imager 600 from GE Healthcare Life Sciences (Pittsburgh, PA) or iBright FL1000 (Invitrogen-Life Technology/Thermo Fisher Scientific, Pittsburgh, PA). The level of target proteins was quantified by densitometry scanning with the ImageJ software and normalized to the amount of α-tubulin.
2.7 Fluorescence microscopy
HaCat cells transfected with siRNAs were plated on coverslips. At 48 hr after siRNA transfection, the cells were exposed to 5 mJ/cm2 UVB and fixed at 2 hr or 24 hr later, with 2% PFA/PBS at room temperature for 10 min. After 5-min of permeabilization with 0.5% Triton X-100/PBS, the cells were stained with the antibody against phospho-Histone H2AX (Ser139) followed by staining with Alexa Fluor 488-conjugated donkey anti-mouse IgG (Invitrogen/Thermo Fisher Scientific). DNA was counterstained with propidium iodide (PI). Fluorescence images were visualized with a Nikon Eclipse Ti-S inverted fluorescence microscope.
2.8 Annexin V staining-based apoptosis analysis
HaCat cells transfected with different siRNAs were plated in a 60-mm dish, followed by UVB irradiation 48 hr later. At 24 hr after UVB irradiation, the cells were detached with 0.25% trypsin and stained with Annexin V-FITC and PI using an Annexin V-FITC Apoptosis Detection Kit (#A211-02; Vazyme, China). The percentages of live and apoptotic cells were analyzed using flow cytometry on a FACSCalibur flow cytometer (Becton Dickinson, San Diego, CA).
2.9 Statistics
Statistical evaluations were carried out using the GraphPad Prism 7.0 software. Real-time RT-PCR analysis was performed in duplicates. All experiments were repeated at least three times. Data were analyzed by the Student t test for comparison between two groups or two-way analysis of variance for comparison between multiple groups. Combined data were presented as mean-fold over control ± standard error of the mean. P-values < 0.05 were considered significant.
3 RESULT
3.1 Expression of BMAL1 and CLOCK genes is altered in HaCat HKCs in response to low-dose UVB irradiation
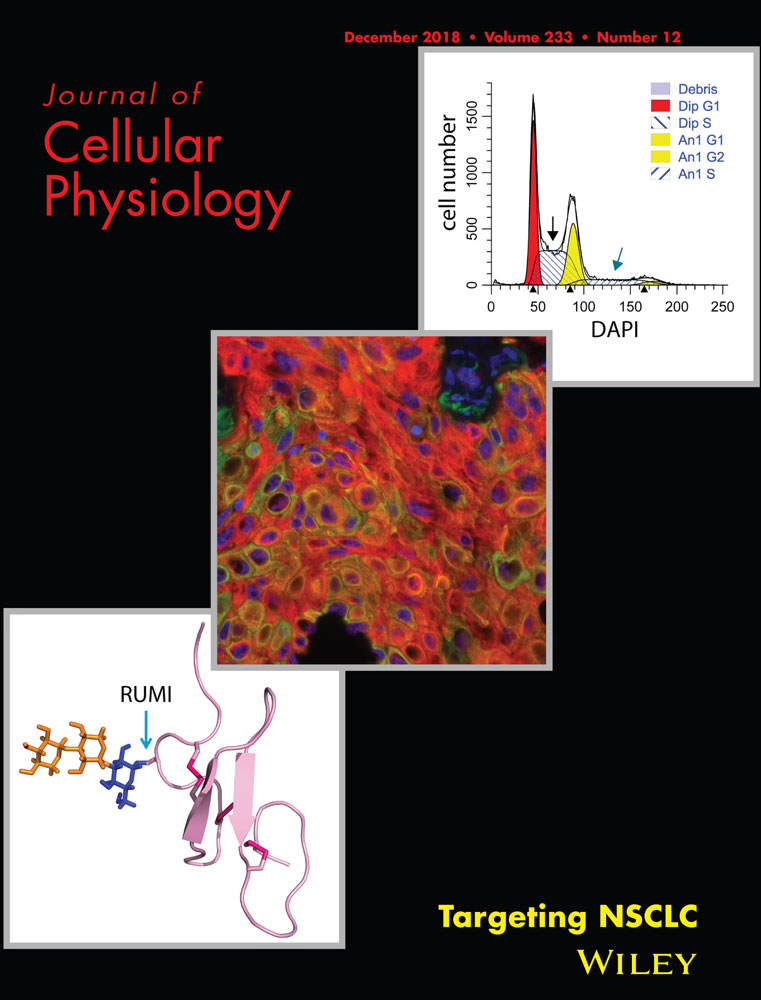
Kawara et al. (2002) have shown that UVB in low doses can trigger oscillating mRNA expressions of several clock genes in cultured HKCs. We observed a similar pattern of gene expression in immortalized HaCaT keratinocytes (Figure 1a). Following a low dose (5 mJ/cm2) of UVB irradiation, the mRNA levels of BMAL1 and CLOCK genes were decreased at 4 hr and increased later at 8 hr. At 24 hr, the level of CLOCK mRNA went back to the basal level, whereas the BMAL1 expression was elevated further to 3.8 folds of the original level. In contrast, the magnitude of change in both BMAL1 and CLOCK protein levels was marginal within 24 hr post UVB irradiation (Figure 1b).
Low-dose UVB resets the expression of BMAL1 and CLOCK genes in HaCat cells, and clock gene depletion increases the viability of normal and UVB-irradiated cells. (a,b) Human HaCat keratinocytes were exposed to 5 mJ/cm2 UVB irradiation and harvested at indicated time points for (a) real-time RT-PCR; (b) western blot analysis of BMAL1 and CLOCK genes and proteins. (c) HaCat cells were reversely transfected with indicated siRNAs and exposed to UVB radiation 48 hr later. Cells were harvested at 24 hr after UVB for real-time RT-PCR or western blot analysis of BMAL1 or CLOCK. (d) Cells transfected with different siRNAs were exposed to UVB, as described in c. The cells were fixed at 24 hr after UVB and stained with Hoechst 33342. Images of nuclei were taken from three different fields per experiment for cell counting. The average cell number/field was presented as mean-percentage over control ± S.E.M. *P < 0.05, **P < 0.01, ns, not significant, N = 3 independent experiments. The mRNA level of each gene is normalized to 36B4 and presented as mean-fold over control ± S.E.M. ***P < 0.001, N = 3. The relative protein levels were quantified by densitometry scanning with ImageJ, and normalized to α-tubulin. Data were presented as mean-fold over control ± S.E.M., N = 3. BMAL1, brain and muscle ARNT-like 1; CLOCK, circadian locomotor output cycles kaput; HaCat, human adult low calcium temperature; RT-PCR, reverse transcription polymerase chain reaction; S.E.M., standard error of the mean; siRNA, small interference RNA
3.2 BMAL1 controls cell growth and apoptosis in HaCat keratinocytes in response to UVB irradiation
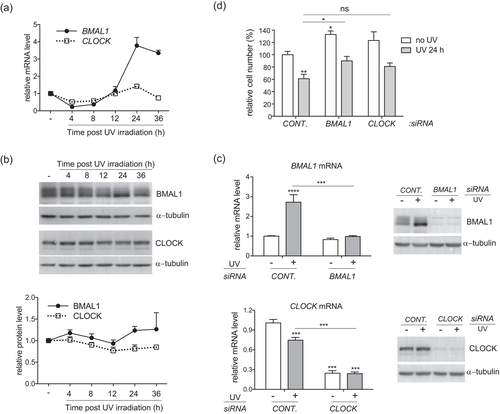
We used the loss-of-function approach to determine the roles of BMAL1 and CLOCK in regulating UVB responses in keratinocytes. Specific siRNAs were able to efficiently knock down the basal level and increased expressions of BMAL1 and CLOCK at 24 hr after UVB irradiation in HaCat cells at both mRNA and protein levels (Figure 1c). While depletion of BMAL1 significantly increased the viability of HaCat cells both at the steady state and after UVB exposure, depletion of CLOCK only showed a moderate effect (Figure 1d). We then investigated whether the enhanced survival in clock-disrupted HaCat cells was associated with diminished apoptotic responses to UVB stimulation. Annexin V staining assay revealed that both early and late apoptotic populations were significantly increased in control cells following UVB irradiation. Despite the lack of effect on UVB-induced early apoptosis, BMAL1 or CLOCK depletion markedly reduced the number of late apoptotic cells from 21% (in control cells) to 11% and 12%, respectively (Figure 2a). Apoptosis was also evaluated through the protease activity of caspase 3 in cleaving its target protein PARP (Figure 2b). UVB-induced elevation of cleaved PARP protein level was significantly suppressed by clock gene deficiency (Figure 2b), which is consistent with the results of Annexin V staining. These results indicate that BMAL1 and CLOCK play a positive role in promoting apoptosis in HaCat cells in response to a low dose of UVB irradiation.
BMAL1 and CLOCK are required for UVB-induced apoptosis in HaCat keratinocytes. HaCat cells were transfected with control siRNA or siRNAs against BMAL1 or CLOCK and subjected to UVB irradiation as described in Figure 1c. (a) At 24 hr after UVB irradiation, live cells were double stained with Annexin V-FITC and propidium iodide (PI) and analyzed by flow cytometry. Cells that stained positive for Annexin V and negative for PI were categorized as in the early apoptotic stage. Cells that stained positive for both Annexin V and PI were categorized as in the late apoptosis. Data were presented as average percentage of cells ± S.E.M. *P < 0.05, **P < 0.01, ***P < 0.001, N = 3. (b) At 24 hr after UVB, the cells were harvested for western blot analysis using an antibody against the cleaved form of PARP. Relative protein levels were quantified by densitometry scanning with ImageJ, normalized to α-tubulin, and presented as mean-fold over control ± S.E.M. ***P < 0.001, N = 3. BMAL1, brain and muscle ARNT-like 1; CLOCK, circadian locomotor output cycles kaput; HaCat, human adult low calcium temperature; PARP, poly ADP-ribose polymerase; S.E.M., standard error of the mean; siRNA, small interference RNA
3.3 Effects of BMAL1 and CLOCK depletion on UVB-induced DNA damage responses in HaCat keratinocytes
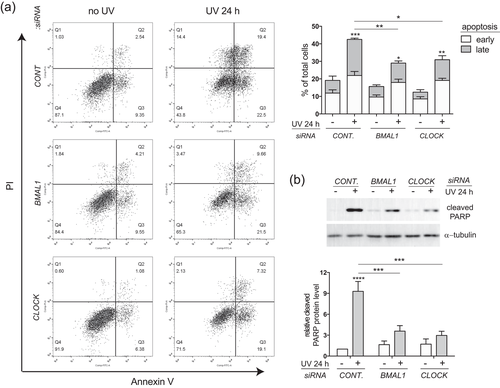
The reduced sensitivity of cells to UVB-induced apoptosis upon BMAL1 or CLOCK depletion could be related to lower degrees of DNA damage and DNA damage checkpoint activation. The phosphorylation of histone H2AX (γ-H2AX) at Ser139 has been considered as a biomarker of UVB-induced DNA damage responses related to NER, replication arrest, and apoptosis (Cleaver, 2011). Both immunostaining and western blot analysis showed that the observed robust induction of γ-H2AX expression in HaCat cells induced by low-dose UVB was significantly decreased through depletion of either BMAL1 or CLOCK at 24 hr, albeit not at any earlier time points (2 hr or 3 hr) after UVB irradiation (Figure 3a,b). These results imply that clock proteins may be involved in later stages of DNA damage responses stimulated by UVB irradiation.
UVB-induced expression of γ-H2AX is suppressed by BMAL1 or CLOCK depletion in HaCat keratinocytes. HaCat cells were transfected with control siRNA or siRNAs against BMAL1 or CLOCK, and subjected to UVB irradiation as described in Figure 1c. (a) At 3 hr and 24 hr after UVB irradiation, the cells were fixed and subjected to immunostaining with an antibody against γ-H2AX (green). DNA was counterstained with propidium iodide (PI; red). Bar = 50 μm. (b) At 24 hr after UVB, the cells were collected for western blot analysis of γ-H2AX or H2AX. The relative protein levels were quantified by densitometry scanning with ImageJ and normalized to α-tubulin. Data were presented as mean-fold over control ± S.E.M, *P < 0.05, **P < 0.01, ***P < 0.001, ns, not significant, N = 3. BMAL1, brain and muscle ARNT-like 1; CLOCK, circadian locomotor output cycles kaput; HaCat, human adult low calcium temperature; S.E.M., standard error of the mean; siRNA, small interference RNA [Color figure can be viewed at wileyonlinelibrary.com]
UV rays have been shown to activate DNA damage checkpoints via the ATR-CHK1-p53 pathway (Heffernan et al., 2002). This pathway was strongly activated in control HaCat cells at 2 hr after UVB irradiation, as reflected by the robust increase in the phosphorylation levels of CHK1 and p53 (Figure 4a). Knockdown of BMAL1 or CLOCK did not affect early phosphorylation of CHK1 and p53 (Figure 4a), thereby suggesting that clock proteins are dispensable for UVB-triggered activation of DNA damage checkpoint response. Ser15-phosphorylation has been associated with increased protein stability of the wild-type p53 (Shieh, Ikeda, Taya, & Prives, 1997). However, the persisting p53 phosphorylation following UVB irradiation was not accompanied by an increase in its total protein level (Figure 4b), which is associated with its mutational status in HaCat cells (Chouinard, Valerie, Rouabhia, & Huot, 2002). Although depletion of BMAL1 or CLOCK did not affect the p53 phosphorylation (Figure 4b), it showed a strong suppressive effect on the induction of p53 target genes, such as the cell cycle inhibitor p21 and the pro-apoptosis factor PUMA at 24 hr after UVB irradiation (Figure 4c). Reduced expression levels of p21 and PUMA are consistent with increased cell growth and decreased apoptosis by clock disruption (Figures 1d and 2a). These results seem to indicate that BMAL1 and CLOCK may bypass early activation of the ATR-CHK1-p53 pathway and control UVB-induced apoptosis and DNA damage responses at a later stage in HaCat cells.
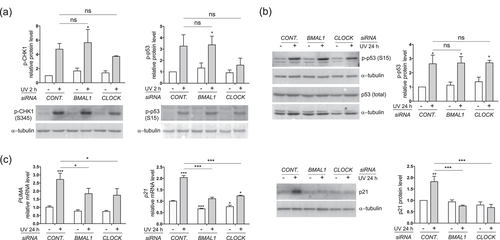
Effects of BMAL1 or CLOCK depletion on UVB-induced DNA damage checkpoint responses in HaCat keratinocytes. HaCat cells were transfected with control siRNA or siRNAs against BMAL1 or CLOCK and subjected to UVB irradiation as described in Figure 1c. (a) Protein lysates were collected at 2 hr after UVB for western blot analysis of phospho-CHK1 (p-CHK1) or phosphor-p53 (p-p53). (b) Protein lysates were collected at 24 hr after UVB for western blot analysis of p-p53 and total p53 protein. (c) Cells were harvested at 24 hr after UVB for RT-PCR analysis of PUMA and p21 genes, or for western blot analysis of p21 protein. The mRNA level of each gene is normalized to 36B4 and presented as mean-fold over control ± S.E.M. *P < 0.01, ***P < 0.001, ns, not significant, N = 3. Relative protein levels were quantified by densitometry scanning with ImageJ, normalized to α-tubulin, and presented as mean-fold over control ± S.E.M., *P < 0.05, **P < 0.01, ***P < 0.001, ns, not significant, N = 3. BMAL1, brain and muscle ARNT-like 1; CLOCK, circadian locomotor output cycles kaput; HaCat, human adult low calcium temperature; mRNA, messenger RNA; RT-PCR, reverse transcription-polymerase chain reaction; S.E.M., standard error of the mean; siRNA, small interference RNA
3.4 BMAL1 and CLOCK control UVB-induced apoptosis in primary HKCs
Because of the existing molecular differences between the immortalized HaCat cell line and primary HKCs, we set out to compare the functional impacts of BMAL1 and CLOCK proteins on UV responses between the two types of cultured HKCs. 5 mJ/cm2 UVB rays initiated a similar alternating pattern of BMAL1 and CLOCK mRNA expression in primary HKCs under our own experimental conditions (Figure 5a), which is consistent with the observation made in HaCat cells. The siRNAs were able to efficiently knockdown the protein expression of BMAL1 and CLOCK in HKCs (Figure 5b). In contrast to its effects in HaCat cells, depletion of either BMAL1 or CLOCK downregulated the viability of HKCs at their steady state (Figure 5c). In response to UVB irradiations, the cell viability dropped from 100% to 60% in control HKCs in comparison with reductions from 66% to 50% and from 76% to 54% in BMAL1 depleted cells and CLOCK depleted cells, respectively (Figure 5c).
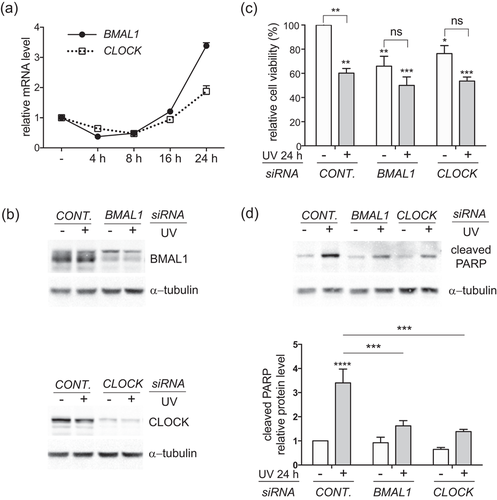
Depletion of BMAL1 or CLOCK attenuates UVB-induced apoptosis in primary human keratinocytes (HKCs). (a) HKCs were exposed to 5 mJ/cm2 UVB radiation and harvested at the indicated time points for real-time RT-PCR analysis of BMAL1 and CLOCK mRNA expression. (b–d) HKCs were transfected with control siRNA or siRNAs against BMAL1 or CLOCK. At 48 hr after transfection, the cells were exposed to 5 mJ/cm2 UVB radiation, as described in FIGURE 1c. The cells were harvested at 24 hr after UVB irradiation for (b) western blot analysis of BMAL1 or CLOCK proteins; (c) cell viability analysis using the Alamar Blue assay; (d) Western blot analysis of cleaved PARP. Relative cell viability was presented as the mean-percentage over control ± S.E.M. *P < 0.05, **P < 0.01, ***P < 0.001, ns, not significant, N = 3. The relative protein level of cleaved PARP was measured by densitometry scanning with ImageJ, normalized to α-tubulin, and presented as mean-fold over control ± S.E.M. ***P < 0.001, N = 3. BMAL1, brain and muscle ARNT-like 1; CLOCK, circadian locomotor output cycles kaput; mRNA, messenger RNA; PARP, poly ADP-ribose polymerase; RT-PCR, reverse transcription-polymerase chain reaction; S.E.M., standard error of the mean; siRNA, small interference RNA
Subsequently, we examined whether the smaller scale of growth reduction after clock disruption was associated with weakened apoptotic responses. As anticipated, UVB-induced increase in the cleaved PARP protein level was markedly reduced in HKCs with depletion of either clock gene (Figure 5d). These results suggest that BMAL1 and CLOCK play a positive role in promoting apoptosis in response to low-doses of UVB irradiation in both HaCat and HKCs.
3.5 Effects of BMAL1 and CLOCK depletion on UVB-induced DNA damage responses in HKCs
In HKCs, an upregulation of γ-H2AX protein was detected between 6 hr and 24 hr after UVB (Figure 6a). Similar to the observation made in HaCat cells, depletion of BMAL1 or CLOCK showed the tendency to suppress γ-H2AX induced by UVB at 24 hr, but not at 6 hr after UVB irradiations (Figure 6a). In contrast to that observed for HaCat cells, the UVB-induced Ser15-phosphorylation of p53 was accompanied with an increase in the total p53 protein level in control HKCs (Figure 6b). Moreover, depletion of CLOCK showed a significant suppressive effect on the phosphorylation and accumulation of the p53 protein (Figure 6b). These results indicate that CLOCK could control the UVB-induced apoptosis and DNA damage responses in part through regulating the p53 induction in HKCs.
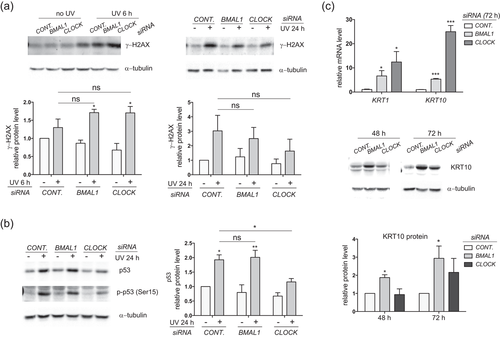
Depletion of BMAL1 or CLOCK alters UVB-induced DNA damage responses and induces terminal differentiation in HKCs. (a) HKCs were transfected with control siRNA or siRNAs against BMAL1 or CLOCK and exposed to 5 mJ/cm2 UVB radiation. Cells were harvested at 6 hr or 24 hr post UVB irradiation for western blot analysis of γ-H2AX. (b) Protein lysates collected at 24 hr post UVB irradiation were analyzed for p-p53 and total p53 protein expressions. (c) Cells were collected at 48 hr or 72 hr after siRNAs transfection (without UVB irradiation) and subjected to RT-PCR analysis or western blot analysis of keratin 1 (KRT1) or keratin 10 (KRT10). The mRNA level of each gene was normalized to 36B4 and presented as mean-fold over control ± S.E.M. *P < 0.05, ***P < 0.001, ****P < 0.0001, N = 3. Relative protein levels were quantified by densitometry scanning with ImageJ and normalized to α-tubulin. Data were presented as mean-fold over control ± S.E.M. *P < 0.05, **P < 0.01, ***P < 0.001, ns, not significant, N = 3. BMAL1, brain and muscle ARNT-like 1; CLOCK, circadian locomotor output cycles kaput; HKCs, human keratinocytes; mRNA, messenger RNA; RT-PCR, reverse transcription-polymerase chain reaction; S.E.M., standard error of the mean
3.6 Depletion of BMAL1 or CLOCK triggers terminal differentiation in HKCs
HKCs depleted of BMAL1 or CLOCK began to exhibit morphological changes compared with control cells as early as 48 hr after siRNA transfection, even before UVB exposure. Such morphological deviation can be associated with altered cell fates in keratinocytes. Real-time RT-PCR analysis showed that knockdown of BMAL1 or CLOCK significantly increased the expressions of early differentiation markers keratin 1 (KRT1) and keratin 10 (KRT10) at both mRNA and protein levels (Figure 6c). It has been documented that keratinocytes at the proliferative stage are more susceptible to DNA damage in response to UVB irradiations (Pantazis, 1980). We speculate that the reduction in apoptosis post UVB exposure is in part due to the differentiation state in BMAL1 and CLOCK depleted HKCs.
4 DISCUSSION
Studies have demonstrated that the severity level of UVB-induced apoptosis and DNA damage responses in mouse skin is regulated in a time-of-day-dependent manner through functional core clock proteins (Gaddameedhi et al., 2015; Geyfman et al., 2012). In this study, we investigated the functions of two clock genes, BMAL1 and CLOCK, in controlling UVB responses in HKCs. We found that a low dose (5 mJ/cm2) of UVB triggered a rhythmic expression of BMAL1 and CLOCK in the immortalized HaCat HKCs, which is consistent with previous observations reported in primary normal HKCs (Kawara et al., 2002). In HaCat cells, UVB-induced apoptosis was alleviated by the knockdown of either clock gene. The reduced apoptotic response was accompanied with a diminished expression of the cell cycle inhibitor p21 and the DNA damage marker γ-H2AX. Similar to the observation made in HaCat cells, UVB-triggered apoptosis and γ-H2AX expression were downregulated in HKCs with the clock disruption. CLOCK depletion also showed suppressive effects on p53 phosphorylation and protein accumulation stimulated by UVB irradiation. In addition, clock disruption was found to trigger terminal differentiation of HKCs at their steady state, which could contribute to better survival after the UVB irradiation. Overall, our findings indicate that circadian clocks play an important role in promoting apoptosis and DNA damage responses in HKCs exposed to low-dose UVB irradiation, and that the mechanistic mode of action for immortalized HaCat cells is likely different from the one for HKCs.
Reports have shown that UVB irradiation in low dose can trigger a rhythmic mRNA expression of core clock genes in primary HKCs (Kawara et al., 2002). In this study, we observed a similar expression pattern of BMAL1 and CLOCK genes stimulated by low-dose UVB in HaCat cells. HaCat cells have been shown to possess a robust and functional circadian clock, which responds to temperature changes and initiates circadian expression of genes involved in keratinocyte differentiation and cholesterol homeostasis (Sporl et al., 2011). Our results suggest that UVB could act as another time cue to reset the oscillation of core clock genes and clock output genes in this type of cultured keratinocytes.
In both HaCat and HKCs, UVB-induced apoptosis was attenuated by depletion of BMAL1 or CLOCK (Figures 2 and 5d), which is similar to that observed in mouse skin with double knockouts of Cry1/2 genes (Gaddameedhi et al., 2015). Our results verify the activities of clock proteins in controlling UVB-initiated apoptotic responses in cultured HKCs.
It is noteworthy that clock disruption led to different cell fates between two types of keratinocytes. In HaCat cells, cell proliferation was amplified after depletion of BMAL1 (Figure 1d). This outcome is comparable with what has been found in Bmal1−/− mouse epidermis, which has shown constantly accelerated cell proliferation due to the loss of time-of-day dependence (Geyfman et al., 2012). In contrast, depletion of BMAL1 seemed to hinder cell proliferation and promote terminal differentiation in HKCs (Figure 5c and 6c). Similar effects have been reported in HKCs with either overexpression of PER1/PER2 or knockdown of CRY1/CRY2 (Janich et al., 2013). Our findings confirm critical roles of normal circadian oscillation in maintaining the balance between proliferation and differentiation in normal human epidermal stem cells (Brown, 2014; Janich et al., 2011). These results also indicate that the functional impacts of clock proteins on keratinocyte life cycle may vary between normal keratinocytes and transformed keratinocytes at the precancerous or cancerous stage.
Many differences exist between HaCat and primary keratinocytes at the molecular level (Chaturvedi et al., 1999; Lewis, Hengeltraub, Gao, Leivant, & Spandau, 2006). HaCat cells harbor UV signature mutations of p53 gene, and mutated forms of p53 protein persistently reside in the nucleus (Chouinard et al., 2002; Lehman et al., 1993). This is in contrast to the wild-type form of p53, which can be transported from the cytoplasm to the nucleus. Despite their mutational status, p53 proteins have been shown to be partially functional in promoting UVB-induced apoptosis in HaCat cells (Henseleit et al., 1997). In our study, although depletion of either clock gene did not affect UVB-induced early activation of ATR-CHK1-p53 mediated DNA damage checkpoints in HaCat cells, it was able to block the expression of p53 target genes such as p21 and PUMA at later time points post UVB irradiation (Figure 4c). It remains to be elucidated whether BMAL1 and CLOCK can control UVB-induced apoptosis and DNA damage responses by regulating the activity of mutant p53 in HaCat cells.
The roles of p21 in mediating DNA damage responses have been evaluated in p53-deficient DLD-1 colon cancer cells (Therrien, Loignon, Drouin, & Drobetsky, 2001). Ablation of the endogenous p21 protein has been shown to give rise to higher efficacy of DNA damage repair, enhanced clonogenic survival, and reduced apoptosis in response to UVB irradiation in p53−/− DLD-1 cells (Therrien et al., 2001). In the current study, the UVB-induced expression of p21 was significantly decreased by BMAL1 or CLOCK depletion in HaCat cells (Figure 4c). Moreover, the outcomes of clock disruption on UVB responses in HaCat cells (Figures 1d, 2a and 3) are comparable to those described in p53−/−p21−/− DLD1 cells. Therefore, we speculate that BMAL1 and CLOCK may control UVB-induced apoptosis and DNA damage responses, at least in part via its action on the p21 expression in p53-defective HaCat keratinocytes.
Studies with other cell types indicate that BMAL1 could modulate the p21 expression via p53-dependent or p53-independent mechanisms (Grechez-Cassiau, Rayet, Guillaumond, Teboul, & Delaunay, 2008; Mullenders, Fabius, Madiredjo, Bernards, & Beijersbergen, 2009). A large-scale shRNA barcode screening has identified BMAL1 as a potential regulator of p53 transcription activity (Mullenders et al., 2009). In p53+/+ HCT116 colon cancer cells, knockdown of BMAL1 strongly suppresses γ-irradiation induced p21 expression, albeit it has no effect on the accumulation of p53 proteins (Mullenders et al., 2009). On the other hand, BMAL1 has also been shown in hepatocytes to regulate the p21 expression in a p53-independent manner through controlling the expression of RORs and REV-ERBs, which in turn control the transcription of p21 gene by binding to the ROR response element within its gene promoter (Grechez-Cassiau et al., 2008). Studies are underway to clarify which mechanism is involved in the clock control of UVB-induced p21 expression in HaCat cells.
In contrast to its effect on HaCat cells, depletion of CLOCK strongly suppressed UVB-induced p53 phosphorylation and p53 protein increase in HKCs (Figure 6b). The regulatory effects of CLOCK in UVB responses are likely attributed to its impact on the p53 expression or activity. It has been indicated that proliferating cells are more sensitive to UVB-induced DNA damage and apoptosis (Gaddameedhi et al., 2015; Pantazis, 1980). On the other hand, terminal differentiation protects keratinocytes against UVB-induced apoptosis (Mandinova et al., 2008). Therefore, the reduced susceptibility of HKCs to UVB-triggered apoptosis could also be attributed to the altered life cycle in BMAL1 or CLOCK depleted HKCs (Figure 6c).
In summary, this study uncovers important functions of BMAL1 and CLOCK in controlling apoptosis and DNA damage responses to UVB irradiation in immortalized HaCat cells and HKCs. HaCat cells harbor UV-type p53 mutations (Lehman et al., 1993) that are often found in clones of keratinocytes within the sun-exposed human skin (Jonason et al., 1996) and squamous cell carcinoma (Brash et al., 1991). Therefore, our results from HaCat cells can lead to better understanding of how clock genes regulate cell-fate determination and DNA damage responses in noncancerous or precancerous keratinocytes that carry p53 mutations in human skins. Studies to further elucidate and understand the underlining mechanism of circadian controls on UVB responses in normal and mutant keratinocytes, as well as other cell types of human skin, are currently underway. Given the implication of UVB on photoaging and photocarcinogenesis, these efforts will be significant and beneficial for prevention and treatment of skin cancers and other skin-related diseases.
ACKNOWLEDGMENTS
Authors are grateful for generous start-up funding from the School of Pharmaceutical Science and Technologies at Tianjin University, as well as from the School of Pharmacy and Graduate School at University of Wisconsin–Madison. J.D. also acknowledges the generous support from The National Institutes of Health (K01AR062132). The authors thank Professor Richard P. Hsung of School of Pharmacy at the University of Wisconsin–Madison for invaluable discussion and the preparation of this manuscript.
CONFLICTS OF INTEREST
The authors state no conflicts of interest.