Muscle regeneration potential and satellite cell activation profile during recovery following hindlimb immobilization in mice
Abstract
Reduced muscle activity leads to muscle atrophy and function loss in patients and animal models. Satellite cells (SCs) are postnatal muscle stem cells that play a pivotal role in skeletal muscle regeneration following injury. The regenerative potential, satellite cell numbers, and markers during recovery following immobilization of the hindlimb for 7 days were explored. In mice exposed to 7 days of hindlimb immobilization, in those exposed to recovery (7 days, splint removal), and in contralateral control muscles, muscle precursor cells were isolated from all hindlimb muscles (fluorescence-activated cell sorting, FACS) and SCs, and muscle regeneration were identified using immunofluorescence (gastrocnemius and soleus) and electron microscopy (EM, gastrocnemius). Expression of ki67, pax7, myoD, and myogenin was quantified (RT-PCR) from SC FACS yields. Body and grip strength were determined. Following 7 day hindlimb immobilization, a decline in SCs (FACS, immunofluorescence) was observed together with an upregulation of SC activation markers and signs of muscle regeneration including fusion to existing myofibers (EM). Recovery following hindlimb immobilization was characterized by a program of muscle regeneration events. Hindlimb immobilization induced a decline in SCs together with an upregulation of markers of SC activation, suggesting that fusion to existing myofibers takes place during unloading. Muscle recovery induced a significant rise in muscle precursor cells and regeneration events along with reduced SC activation expression markers and a concomitant rise in terminal muscle differentiation expression. These are novel findings of potential applicability for the treatment of disuse muscle atrophy, which is commonly associated with severe chronic and acute conditions.
1 INTRODUCTION
Skeletal muscle dysfunction and mass loss are characteristic features in patients with chronic diseases including cancer of several types and acute conditions such as critical illness. Impaired muscle function and atrophy limit the patients’ quality of life as exercise tolerance and daily life activities can be seriously hampered especially following prolonged intensive care unit (ICU) stay. Furthermore, skeletal muscle dysfunction and atrophy have also been shown to predict morbidity and mortality in patients regardless of the underlying condition (Barreiro, Bustamante, et al., 2015; Barreiro, Sznajder, Nader, & Budinger, 2015; Fearon et al., 2011; Maltais et al., 2014; Marquis et al., 2002; Patel et al., 2014; Seymour et al., 2010; Swallow et al., 2007; Vogelmeier et al., 2017). Several factors and biological mechanisms have been demonstrated to be involved in the pathophysiology of skeletal muscle mass loss (Barreiro, 2017; Barreiro, Bustamante, et al., 2015; Barreiro & Gea, 2015, 2016; Barreiro & Sznajder, 2013; Maltais et al., 2014). Deconditioning and reduced muscle activity are counted among the most relevant factors leading to muscle atrophy and loss of function in patients (Barreiro, Bustamante, et al., 2015; Barreiro & Gea, 2015; Fearon et al., 2011; Maltais et al., 2014; Marquis et al., 2002; Seymour et al., 2010) and animal models (Chacon-Cabrera, Gea, & Barreiro, 2017; Chacon-Cabrera, Lund-Palau, Gea, & Barreiro, 2016).
Biological mechanisms such as increased oxidative stress, mitochondrial alterations, enhanced protein breakdown, epigenetic events, and signaling pathways mediate muscle mass loss in experimental models of cancer-induced cachexia, chronic heart failure, and disuse muscle atrophy and in cachectic patients with chronic respiratory conditions (Barreiro & Gea, 2015, 2016; Barreiro & Sznajder, 2013; Barreiro et al., 2016; Chacon-Cabrera et al., 2014, 2016; Chacon-Cabrera, Fermoselle, Salmela, Yelamos, & Barreiro, 2015; Chacon-Cabrera, Gea, et al., 2017; Chacon-Cabrera, Mateu-Jimenez, et al., 2017; Fermoselle et al., 2012; Puig-Vilanova, Ausin, Martinez-Llorens, Gea, & Barreiro, 2014; Puig-Vilanova et al., 2015; Puig-Vilanova, Lloreta, et al., 2014). Recently, we have demonstrated that a specific program of molecular and cellular events characterized by increased muscle proteolysis and injury was observed in the gastrocnemius of mice following hindlimb unloading for several time-points, while reloading of the muscle improved all those alterations (Chacon-Cabrera et al., 2016). Additionally, in the same mouse model, muscle-enriched microRNAs, especially miR-486, through paired box (pax) seven regulation was shown to delay muscle cell differentiation, probably via acetylation of fork-head box (FoxO) 1 and 3, in the unloaded gastrocnemius (Chacon-Cabrera, Gea, et al., 2017). Interestingly, reloading of the muscle after splint removal induced a rise of the histone deacetylase sirtuin-1 along with a decrease in acetylation levels of the transcription factors FoxO 1 and 3, thus enabling the muscle fibers to regenerate in the mice (Chacon-Cabrera, Gea, et al., 2017). We concluded that the kinetics of the biological events leading to skeletal muscle mass loss and recovery were tightly regulated in the gastrocnemius of mice exposed to different time-points of muscle unloading and reloading (Chacon-Cabrera et al., 2016; Chacon-Cabrera, Gea, et al., 2017). Whether the potential of muscle regeneration was altered in response to disuse remained unexplored in the former investigations.
Satellite cells are postnatal muscle stem cells that play a pivotal role in the regeneration of skeletal muscle following injury. Satellite cell populations are heterogeneous and may differ in features such as their myogenic differentiation predisposition, gene expression profile, lineage potential of nonmyogenic fates, and stemness. Interestingly, different studies have also demonstrated modifications in satellite cell numbers in response to different stimuli and models of disuse muscle atrophy (Arentson-Lantz, English, Paddon-Jones, & Fry, 2016; Snijders et al., 2014; Suetta et al., 2013). On this basis, we focused the current investigation on the assessment of the regenerative potential and satellite cell numbers during recovery following immobilization of the hindlimb for 7 days. This experimental approach enabled us to mimic a physiological condition (immobilization) compared to other models in which myotoxins have been used to trigger muscle regeneration following injury in mice. The 7-day time-point was chosen on the basis of previous studies (Chacon-Cabrera et al., 2016; Chacon-Cabrera, Gea, et al., 2017), in which early and late biological regeneration events could be identified. Furthermore, we also hypothesized that a differential pattern of myogenic precursor cell markers may be expressed in the satellite cells of the hindlimb muscles exposed to unloading and reloading, respectively. Accordingly, we sought to specifically investigate in the hindlimb muscles of mice exposed to 7-day periods of unloading and reloading: 1) muscle progenitor cell counts isolated from all the hindlimb muscles using fluorescent-activated cell sorting (FACS); 2) levels of satellite cells and regeneration events using histological (immunofluorescence, gastrocnemius, and soleus) and electron microscopy analyses (gastrocnemius); and 3) to analyze the specific pattern of myogenic precursor cell markers in the satellite cell yields obtained from the FACS analyses. The model of disuse-induced muscle atrophy and recovery employed in the current investigation has been previously well-validated (Chacon-Cabrera et al., 2016; Chacon-Cabrera, Gea, et al., 2017).
2 MATERIALS AND METHODS
2.1 Animal experiments and ethics approval
Female C57BL/6J mice (10 weeks old, weight ∼20 g) were obtained from Harlan Interfauna Ibérica SL (Barcelona, Spain). Mice were kept under pathogen-free conditions in the animal house facility at Barcelona Biomedical Research Park (PRBB), with a 12:12 hr light: dark cycle.
Mice were exposed to unilateral hindlimb immobilization as previously described to reproduce a model of disuse muscle atrophy (Chacon-Cabrera et al., 2016; Chacon-Cabrera, Gea, et al., 2017). Briefly, the left hindlimb was shaved with clippers and was enveloped using surgical tape. The hindlimb was introduced in a 1.5 ml microcentrifuge tube with cover and bottom lids removed, while maintaining the foot in a plantar-flexed position to induce the maximal atrophy of the target limb muscles: gastrocnemius, soleus, tibialis anterior (TA), extensor digitorum longus (EDL), and quadriceps femoris (QF). The entire procedure was carried out using mouse restrainers, without anesthesia. As the weight of the tube was approximately 0.6 g, it did not interfere with the usual mobility of the mice. In this study, for satellite cell isolation experiments a total number of 40 mice were employed. Animals were further subdivided into the two following groups: 1) 7-day immobilization group (7-day I, N = 20/group); and 2) 7-day recovery group (7-day R, N = 20/group), in which the splint was removed following the 7-day immobilization period to let the animals move freely in their cages in order to evaluate muscle recovery. The target samples were obtained by pooling together the gastrocnemius, soleus, TA, EDL, and QF muscles from two different mice belonging to the same study group. The gastrocnemius, soleus, TA, EDL, and QF from two different mice belonging to the contralateral hindlimb from the 7-day I or 7-day R groups of mice served as the control muscles. For the immunofluorescence and electron microscopy experiments a total number of 16 additional mice were employed (see below specific details for this type of experiments). The animals were also divided into two groups (7-day I and 7-day R mice, n = 8/group). The gastrocnemius muscle was cut into two fragments that were used for immunofluorescence and electron microscopy analyses, respectively. Given the very small size of the soleus, only histological (immunofluorescence) analyses could be conducted on this muscle.
All animal experiments were conducted in the animal facilities at Parc de Recerca Biomèdica de Barcelona (PRBB). This controlled study was designed in accordance with the European Community Directive 2010/63/EU and Spanish Legislation (Real Decreto 53/2013, BOE 34/11370-11421, 2013) for the use and care of animals followed in our institution (PRBB animal facilities) and the Helsinki convention for the use and care of animals. Ethical approval was obtained by the Animal Research Committee (Animal welfare department in Catalonia, Spain, EBP-13-1485).
2.2 In vivo measurements in the mice
In all the study animals, food intake was measured daily, and food and water were supplied ad libitum for the entire duration of the immobilization or recovery periods. In all mice, body weight and limb strength were determined on day 0, and right at the end of the immobilization or recovery time-points (day 7, as described above) using a scale and a grip strength meter (Bioseb, Vitrolles, France), respectively, as previously reported (Barreiro & Gea, 2016; Chacon-Cabrera et al., 2015, 2014, 2016; Chacon-Cabrera, Gea, et al., 2017; Chacon-Cabrera, Mateu-Jimenez, et al., 2017). In the two groups of animals, total body weight and limb strength gain variables were calculated as the percentage of the measurements performed at the end of the study period (7-day I or 7-day R) with respect to the same measurements obtained at baseline (day 0 or on day 7 following the immobilization protocol) in the 7-day I and 7-day R study groups, respectively.
2.3 Sacrifice and sample collection
Mice from all experimental groups were sacrificed after the corresponding immobilization or recovery time-cohort. Each mouse was previously inoculated intraperitoneally with 0.1 ml sodium pentobarbital (60 mg/Kg). In all cases, the pedal and blink reflexes were evaluated in order to verify total anesthetic depth. Animal sacrifice took place following diaphragm extraction. For isolation of muscle cell progenitor experiments the following muscles were obtained from all the animals at the time of sacrifice: gastrocnemius, soleus, TA, EDL, and QF (entire muscles in all cases). As above mentioned, all study muscles from the hindlimb were pooled together by groups of two for each experimental condition (7-day I and 7-day R), as well as from the contralateral control hindlimb muscles, thus a total of ten samples were analyzed in each group (contralateral control muscles and both 7-day I and 7-day R mice). For immunofluorescence and electron microscopy experiments, gastrocnemius, and soleus muscles were obtained at the time of sacrifice in each experimental cohort. As soleus muscle was very small, only immunofluorescence analyses, but not electron microscopy, were conducted (see below specific details). For stem cell progenitor isolation experiments, the muscle samples were preserved in cold Dubelcco's Modified Eagle's Medium (DMEM) to be immediately processed as described below. For identification of several muscle regeneration markers, RNA was extracted from the isolated satellite cell yields (see below).
2.4 Satellite cell isolation using FACS
A schematic representation of these methodologies is shown in Figure 1. Immediately after dissection, muscles were processed following a modified version of a previously described protocol (Pasut, Oleynik, & Rudnicki, 2012) (Figure 1). Pools of gastrocnemius, soleus, TA, EDL, and QF muscles were first minced with scissors and secondly with a razor blade (Electron microscopy science, Hatfield, PA). The minced muscles were collected in a 50 ml tube containing 40 ml of cold DMEM to be subsequently washed to remove fat tissue. Enzymatic digestion with a DMEM media containing 2.5 U/ml collagenase (Serva Electrophoresis, Heidelberg, Germany) and 2.5 U/ml dispase (Sigma–Aldrich, Sant Louis, MO) was immediately performed at 37°C in an agitation bath for 10 min.
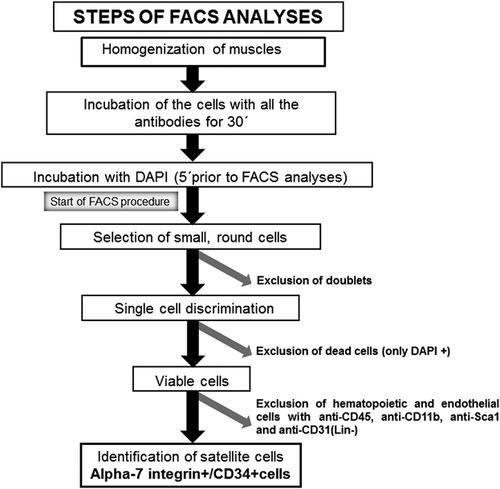
The digestion procedure was repeated four times and the digested muscle solution was then filtered through a 100 μm mesh filter (Corning, New York, NY). Immediately afterwards, the digestion was stopped by adding two volumes of 10% fetal bovine serum (FBS) in phosphate buffered saline (PBS), and the muscle solution was filtered through a 70 μm mesh filter (Corning). The filtered solution was centrifuged at 300g for 5 min. The pellet was kept and the supernatant was centrifuged again in order to recover the maximum amount of cells. Finally, the two cell pellets were combined in a fresh tube to be re-suspended in FACS buffer (1 ml PBS solution containing 2.5% goat serum [Sigma–Aldrich]). The number of cells was counted using a Neubauer chamber.
Prior to incubations with antibodies, the cells were washed in 20 ml cold DMEM and centrifuged at 1,700 rpm for 10 min to recover the cell pellet. Cells were resuspended in 1 × 106 cell/100 μl FACS buffer. All these cells were incubated with antibodies used to specifically identify the satellite cells: Phycoerythrin (PE)-conjugated anti-alpha-7 integrin (Ablab, Vancouver, Canada), a heterodimeric integral membrane protein critical for the modulation of cell-matrix interactions, and allophycocyanin (APC)-conjugated anti-CD34 (BD Pharmigen, San Jose, CA), as a cell surface sialomucin (a mucopolysaccharide molecule containing sialic acid) with reported anti-adhesive, motile, and pre-proliferative properties for 30 min. Additionally, the cells from both study groups of mice were incubated at the same time (30 min) with specific antibodies to exclude other cell type populations that might have also been present in the muscle extracts, namely endothelial cells, leukocytes, and hematopoietic cells: phycoerythrin (PE)-cyanine7 (Cy7)-conjugated anti-CD31 (Biolegend, San Diego, CA), a marker of endothelial cells, both anti-CD11b (Biolegend), and anti-CD45 (Biolegend) as markers of leukocytes, and anti-Sca-1 (Biolegend), a marker of hematopoietic stem cells. The excluded cell populations were named negative-lineage (Lin [−]).
Subsequently afterwards, all the study cells were incubated with 4′,6-diamino-2-fenilindol (DAPI) in order to exclude the dead cells (cells DAPI+ exclusively) 5 min prior to the start of FACS analyses (Figure 1, FACS Aria II SORP, BD Biosciences, San Jose, CA). Once DAPI+ cells and other cell types (endothelial, leukocytes, and hematopoietic) were excluded, the cells identified using FACS were small and of round shape indicating a relatively low complexity. Cells stained for PE-conjugated anti-alpha-7 integrin and APC-conjugated anti-CD34 were sorted out and were named alpha-7 integrin+/CD34+ muscle progenitors (Figure 1). Muscle progenitor cells were expressed as the percentage of alpha-7 integrin+/CD34+ progenitors to the total numbers of isolated viable cells. These cells were specifically preserved following the sorting experiments to be further analyzed as described below.
2.5 Satellite cell RNA extraction
Total RNA was first isolated from the yield of sorted satellite cells using Trizol reagent following the manufacturer's protocol (Life technologies, Carlsbad, CA). Total RNA concentrations were determined spectrophotometrically using the NanoDrop 1000 (Thermo Scientific, Waltham, MA).
2.6 Procedures of mRNA reverse transcription (RT)
A single RT was performed from which all the target genes of the study were analyzed. First-stranded cDNA was generated from mRNA using oligo(dT)12–18 primers and the Super-Script III reverse transcriptase following the manufacturer's instructions (Life Technologies).
2.7 Quantitative real time-PCR amplification (qRT-PCR)
TaqMan based qPCR reactions were performed using the ABI PRISM 7900HT Sequence Detector System (Life Technologies) together with commercially available gene expression assays. The probes corresponding to the following genes involved in muscle regeneration were tested: pax7 (Mn01354484_m1, Life Technologies), myogenic differentiation 1 (myoD) (Mm00440387_m1, Life Technologies), marker of cell proliferation ki-67 (ki67) (Mm01278617_m1, Life Technologies), and myogenin (Mm00446194_m1, Life Technologies). The housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (gapdh) (Mm99999915_g1, Life Technologies) served as the endogenous control for mRNA gene expression. Reactions were run in duplicates, and mRNA data were collected and subsequently analyzed using the sequence detection system relative quantification software version 2.4 (Applied BioSystems, Foster City, CA), in which the comparative CT method (2−△△CT) for relative quantification was employed (Livak & Schmittgen, 2001).
2.8 Satellite cell identification using immunofluorescence microscopy
The protocol used in these experiments was similar to that published in previous studies (Arentson-Lantz et al., 2016; Snijders et al., 2014; Suetta et al., 2013). Gastrocnemius and soleus muscles were fixed in 4% paraformaldehyde solution, pH 6.9 (EMD Millipore corporation, Billerica, MA) and were embedded progressively with increasing concentrations of sucrose. They were subsequently embedded in tissue-tek optimal cutting temperature (OCT) compound (Sakura Finetek, Torrance, CA) to be snap-frozen in 2-methyl-butane immersed in liquid nitrogen as previously described (Barthel & Raymond, 1990). Ten micrometer frozen sections were cut using a cryostat-microtome (Leica CM3050S, Leica Biosystems, Wetzlar, Germany) at −20°C and were mounted on glass slides. Immunofluorescence staining was used to detect satellite cells in both quiescent and proliferative states using specific antibodies (see below). Briefly, muscle cross-sections were air-dried for 30 min and were rinsed with PBS for another 15 min. PBS was used to rinse the sections among the different incubation steps. After rinsing, the sections were put in cold methanol for six more minutes. Then, the sections were boiled using a hot bath in 0.1 M citrate buffer (pH 6.0) for 12 min, and were then blocked with 10% goat serum in PBS for 2 hr. Subsequently, sections were incubated with Mouse IgG blocking reagent (MOM) (Vector Laboratories, Burlingame, CA) for 30 min. Afterwards, they were incubated overnight with a mixture of two antibodies: mouse monoclonal anti-pax7 antibody (1:20; sc-81648, Santa Cruz Biotechnology Inc., Dallas, TX) and rabbit polyclonal anti-ki-67 antibody (1:100; ab15580, Abcam, Cambridge, UK), prepared in an antibody solution (1% goat serum dissolved in PBS, at 4°C). Anti-pax7 antibody was used to detect satellite cells, while anti-ki-67 antibody was used to detect proliferating cells (namely blood cells and proliferating myoblasts) (Arentson-Lantz et al., 2016; Snijders et al., 2014; Suetta et al., 2013). The double staining of cells with both anti-pax7 and anti-ki-67 simultaneously, detected proliferating satellite cells. Following incubation with the primary antibodies and after rinsing with PBS, the sections were incubated at room temperature with the corresponding secondary antibodies for 1 hr: anti-mouse IgG1 Alexa Fluor 488 antibody and anti-rabbit IgG (H + L) Alexa Fluor Plus 555 (1:400 both, Invitrogen, Thermo Fisher Scientific), also prepared in an antibody solution. Finally, the sections were mounted using the fluorescent mounting medium DAPI G-Fluoromount medium (Southern Biotech, Birmingham, AL), which specifically stained DNA (allowing identification of all nuclei) in the muscle sections. A fluorescence microscope (×40 objective, Nikon Eclipse Ni, Nikon, Tokyo, Japan) coupled with a digitizing camera was used to identify and count the number of the satellite cells (ten fields) in each study sample. In each muscle section from gastrocnemius and soleus of the two study groups of mice, results were expressed as the percentage of either pax7-positive, ki-67-positive, or both pax7- and ki-67 double-stained nuclei to the total number of counted myonuclei in the 10 fields.
2.9 Identification of satellite cells and regeneration events using electron microscopy
For these specific experiments only the gastrocnemius (larger size) could be used. Gastrocnemius muscles were gently excised into very small fragments. These pieces were then further cut into two 1 × 0.5 mm-fragments on a smooth surface using tweezers and a surgical blade. The muscle fragments were immediately immersed into a fixative solution containing 2% glutaraldehyde in cacodylate buffer (0.2M, pH 7.4) for 24 hr and were then post-fixed in 1% osmium tetroxide solution in the cacodylate buffer for one more hour. The samples were subsequently dehydrated in a graded series of ethanol from 70% to 100%, were then transitioned into the intermediate solvent propylene oxide, to be finally embedded with increasing concentrations of an Epon resin (until 100% of epon resin-embedding was reached). Subsequently, samples were embedded longitudinally in a mold containing fresh resin, and were polymerized at 60°C for 24 hr. Semithin sections of 0.4 μm each were then obtained using a glass knife, and were immediately stained with toluidine blue in order to identify the specific region to be thin-sectioned at 50–80 nm using a diamond knife (Diatome, Nidau, Switzerland) and an ultramicrotome (Ultracut E, Reichert-Jung, Austria). The thin sections were placed onto 250-mesh copper/nickel grids and were stained with uranyl acetate and lead citrate. Satellite cells were identified and counted using a transmission electron microscope (FEI CM100 TEM; Philips, Eindhoven, The Netherlands) with an attached Veleta digital camera (iTEM, Olympus, Tokyo, Japan) and iTEM software was used to capture the images (Davies, 1999). Satellite cells were identified as small round-shaped cells located between the basal lamina and the sarcolemma of the myofibers. Fifty fields were counted in each study sample.
The following parameters were assessed in the ultrastructural muscle preparations from the study muscle samples: 1) number of satellite cells, located between the basal lamina and the sarcolemma, in the measured area (mm2); 2) early regeneration events characterized by the presence of internal myonuclei located in groups in the measured area (mm2); and 3) late regeneration events identified as the presence of isolated internal myonuclei in the measured area (mm2). Numbers of all these three ultrastructural events were counted in 50 fields of all the study samples and are illustrated in Table 2.
2.10 Statistical analysis
Results are presented as mean values (standard deviation). The normality of the study variables was explored using the Shaphiro–Wilk test. Food intake and percentage of change of both total body weight and limb strength variables are represented in Table 1, whereas the biological variables are represented in Table 2 and Figures (mean and standard deviation). The following statistical approaches were used in the study for different purposes. First, the variables food intake and percentage of change of total body weight and limb strength variables in mice from the 7-day I cohort were compared with mice from the 7-day R cohort using the unpaired Student's t-test. Secondly, one-way analysis of variance (ANOVA) was used to assess potential differences in all the study biological variables among the three study groups of muscles samples: 7-day I, 7-day R and the control muscles from the contralateral hindlimb. Tukey post hoc analysis was used to adjust for multiple comparisons. A level of significance of p < 0.05 was established in the study. Comparisons of biological variables of the muscles obtained from the contralateral hindlimb showed no significant differences between the 7-day I and the 7-day R groups of mice. Thus, for the sake of clarity and conciseness, this group has been named contralateral control muscles in the results below.
| 7-day I mice, n = 20 | 7-day R mice, n = 20 | |
|---|---|---|
| Food intake (grams/24 hr) | +4.7 (0.6) | +4.8 (0.4) n.s. |
| Total body weight, % gain | −3.7 (4.5) | +3.0 (4.7)*** |
| Limb strength gain, % gain | −12.9 (7.6) | +16.2 (13.5)** |
- Data are presented as mean values (standard deviation). Total body weight and limb strength gain variables were calculated in each animal group as described in methods section. Comparisons were made between the two groups of mice for each study variable (see statistical analyses in methods section).
- I; immobilization, R, recovery.
- Statistical significance is represented as follows: n.s., non-significant.
- **, p < 0.01, and ***, p < 0.001 between 7-day I and 7-day R mice.
3 RESULTS
3.1 Physiological characteristics of the study animals
As shown in Table 1, food intake did not differ between the 7-day I and 7-day R mice. The variables percentage of body weight and limb strength gain were significantly increased in the 7-day R compared to 7-day I mice (Table 1).
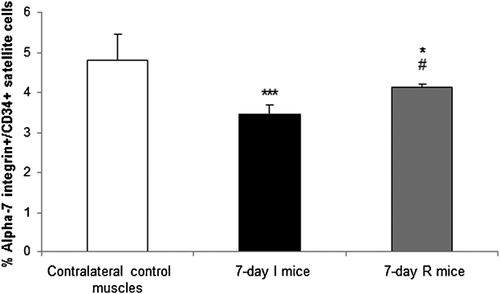
3.2 Disuse reduces satellite cell numbers in muscles
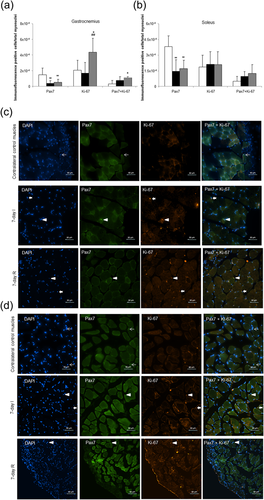
The number of alpha-7 integrin+/CD34+ satellite cells of the 7-day I group was significantly reduced compared to the contralateral control muscles (Figure 2). Alpha-7 integrin+/CD34+ satellite cell numbers were significantly higher in the 7-day R mice compared to the 7-day I group, although they did not reach levels detected in the contralateral control muscles (Figure 2). Immunofluorescence pax7-positive cells were also significantly reduced in gastrocnemius and soleus muscles of the 7-day I animals compared to the contralateral control muscles (Figure 3a–d, respectively). Levels of immunofluorescence pax7-positive cells were also lower in 7-day R muscles compared to contralateral control muscles in both gastrocnemius and soleus muscles (Figure 3a–d, respectively). In the gastrocnemius, but not the soleus, numbers of proliferating nuclei (ki-67 positively stained) were significantly higher in the 7-day R mice compared to both 7-day I and contralateral control muscles (Figure 3a–d). Additionally, in the gastrocnemius, but not the soleus, specific proliferating satellite cells (both pax7 and ki-67 double-stained) were significantly greater in the 7-day R group compared to contralateral control muscles (Figure 3a–d).
3.3 Ultrastructural analyses of satellite cells and muscle regeneration
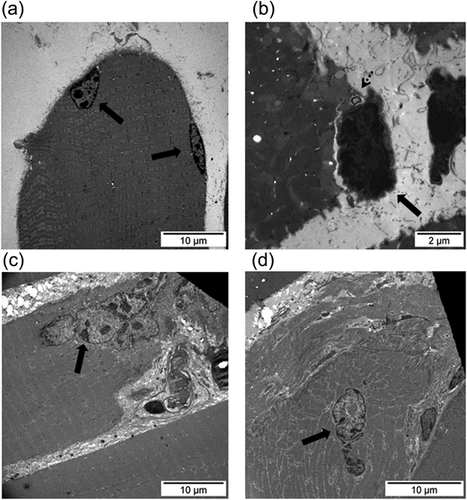
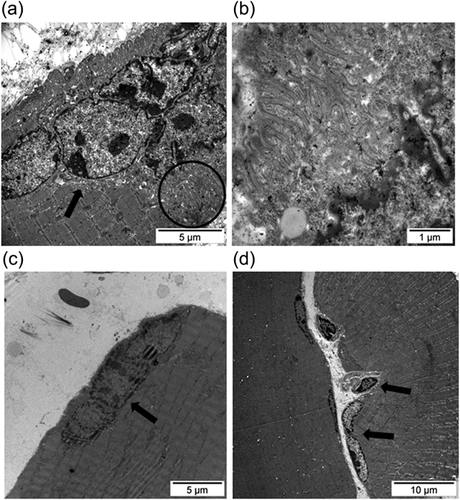
Examples of the normal ultrastructure of muscle sections and of a satellite cell are shown in Figures 4a and 4b, respectively. Compared to contralateral control muscles, ultrastructural satellite cell counts were significantly greater in the gastrocnemius of the 7-day R mice, whereas no significant differences were seen in the muscles of the 7-day I animals (Table 2). Numbers of grouped internal nuclei (early regeneration phase) were significantly greater in the 7-day I and 7-day R mice compared to the contralateral control muscles (Table 2 and Figure 4c). Compared to contralateral control muscles, in the gastrocnemius, counts of isolated nuclei (marker of late regeneration phase) were significantly lower in the 7-day I mice, while they were significantly greater in the 7-day R animals (Table 2 and Figure 4d). Satellite cell fusion to existing myofibers was demonstrated in the gastrocnemius ultrastructural sections of the 7-day I mice (Figure 5a–c) and the 7-day R animals (Figure 5d). Sarcomeric membrane folding took place following satellite cell internalization (Figure 5b).
| Contralateral control muscles (n = 8) | 7-day I mice (n = 8) | 7-day R mice (n = 8) | |
|---|---|---|---|
| SC counts (SC/mm2) | 13.1 (6.3) | 18.2 (6.9) | 22.4 (6.4)** |
| Muscle in early regeneration phase (grouped internal myonuclei/mm2) | 3.6 (2.9) | 7.7 (3.3)* | 7.6 (5.4)* |
| Muscle in late regeneration phase (isolated internal myonuclei/mm2) | 10.7 (3.2) | 5.9 (2.7)* | 16.7 (4.4)**,### |
- Data are presented as mean values (standard deviation).
- I, immobilization; R, recovery; SC, satellite cells.
- Statistical significance is represented as follows: n.s., non-significant.
- *, p < 0.05 and ** p < 0.01 between either 7-day I or 7-day R mice, respectively, and the contralateral control muscles; ###, p < 0.001 between 7-day I and 7-day R animals. See methods section for detailed information on the quantification procedure of the study parameters.
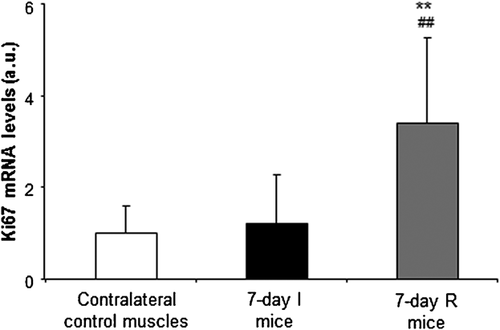
3.4 Disuse induces a specific pattern of regenerative markers in muscles
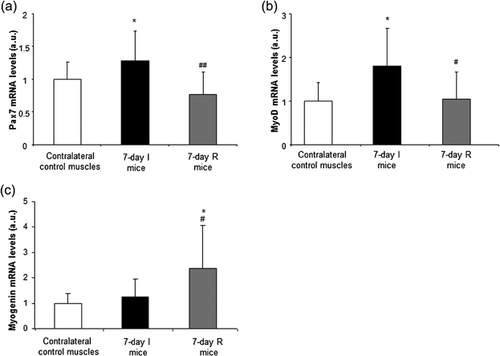
Levels of ki67 gene expression were significantly higher in the 7-day R group compared to both 7-day I mice and the contralateral control muscles (Figure 6). Pax7 and MyoD gene expression levels were significantly higher in the 7-day I mice compared to the contralateral control muscles, while the expression of these markers was significantly lower in hindlimb muscles from 7-day R animals compared to 7-day I mice (Figures 7a and 7b, respectively). Myogenin expression did not significantly differ between the 7-day I and the contralateral control muscles, whereas a significant rise in this marker was observed in muscles of the 7-day R animals compared to both the 7-day I mice and the contralateral control muscles (Figure 7c).
4 DISCUSSION
In the study, a large-scale approach, in which satellite cells were isolated from all the limb muscles using FACS, was followed. The rationale to use such methodologies was to identify a pure population of muscle progenitors as demonstrated in previous studies (Yin, Rice, & Rudnicki, 2013). In fact, one of the main goals in the study was to characterize the gene expression profile of the isolated progenitor cells during recovery following a period of hindlimb muscle disuse for 7 days, which was defined as a key time-point to analyze early and late events as previously shown in this experimental model (Chacon-Cabrera et al., 2016; Chacon-Cabrera, Gea, et al., 2017). Furthermore, the use of methodologies such as histological procedures enabled us to define the localization and numbers of active and proliferating satellite cells in the study muscles. Besides, electron microscopy analyses also revealed that differential events of early and late regeneration phases took place in the muscles of the two study groups of mice.
In the current investigation, the study hypothesis has been confirmed to a great extent. A specific program of muscle regeneration was induced during recovery following hindlimb immobilization. The number of progenitor muscle cells was reduced in limb muscles isolated from mice exposed to a 7-day period of hindlimb disuse. Histological analyses also revealed that pax7-positive satellite cells were reduced in both slow- and fast-twitch muscle types of the 7-day I mice. Additionally, ultrastructural events of late muscle regeneration also decreased in the gastrocnemius of the latter animals. In fact, these findings are consistent with what has been found in other models of disuse, in which a significant decline in muscle satellite counts was demonstrated along with severe atrophy in young healthy subjects at rest (Arentson-Lantz et al., 2016; Mackey et al., 2009) and in experimental models of disuse muscle atrophy (Darr and Schultz, 1989; Mitchell & Pavlath, 2004; Wang et al., 2007). In elderly subjects, a decline in muscle satellite cells was also observed following immobilization for two weeks using a whole-leg casting (Suetta et al., 2013). Another study, however, revealed no significant changes in muscle satellite cell counts in the quadriceps muscle of healthy young individuals exposed to several weeks of one-legged knee immobilization using a cast (Snijders et al., 2014). Discrepancies among studies could be attributed to differences in the experimental models, age of the study subjects, previous activity of the target individuals, and/or methodologies used to identify the satellite cell numbers. A major strength and novelty in the current study was the use of three different approaches to identify satellite cells and muscle regeneration features in the mice exposed to unloading and reloading.
Importantly, removal of the splint (recovery period) induced a rise in the number of progenitor cells in the limb muscles of the mice during recovery. Although in these mice the content of satellite cells did not reach the baseline levels observed in the contralateral control muscles, it was significantly greater than in the 7-day I animals. These results imply that proliferation of muscle progenitors is a key step in the initiation of the muscle regeneration process during the recovery period. In line with this, numbers of proliferating satellite cells and ultrastructural cell counts were greater in the gastrocnemius of the 7-day R mice compared to control muscles as well as ultrastructural events of late muscle regeneration (isolated internal nuclei). In the soleus muscle, however, no differences were seen among the study groups probably due to the different fiber type composition of this muscle. These findings suggest that the fast-twitch gastrocnemius may have contributed to a great extent to the increased numbers of progenitor cells detected in the 7-day R muscles during the FACS analyses. Importantly, in the animals, reestablishment of normal muscle activity following a resting period may mimic what happens in response to exercise training in humans. As such, strength training was also shown to induce a significant increase in satellite cell numbers along with the expression of myogenic regulatory and growth factors in the quadriceps muscle of healthy young subjects (Hanssen et al., 2013).
Levels of proliferating cells in general as measured by the ki-67 marker were only significantly increased in muscle progenitor cells in the animals exposed to 7-day recovery compared to levels detected in the control muscles and the 7-day immobilized mice. As mentioned above, numbers of proliferating satellite cells identified as those positively stained for both pax7 and ki-67 antigens were also greater in the gastrocnemius of 7-day R mice. These findings are in line with those previously reported in which counts of active satellite cell as identified exclusively by ki-67 expression levels also increased following muscle activity in healthy humans (Mackey et al., 2009). Interestingly, the rise in ki-67 expression also corresponded to that seen in the muscle progenitor cells during recovery. It should be mentioned that in the current study, proliferating satellite cells were detected using two antibodies since other cell types (e.g., hematopoietic cells) might have also proliferated during the recovery phase in the study muscles. We considered this to be a more accurate approach than that exclusively based on ki-67 staining.
Characterization of the muscle progenitors from a molecular standpoint has revealed that the expression of markers such as a pax7 and myoD was upregulated in the muscle progenitors of mice during unloading, while it reached baseline levels during reloading. These findings suggest that despite the decrease in muscle progenitors observed during unloading, satellite cells were not in G0 phase as myoD is expressed in myogenic precursor cells but not in quiescent satellite cells (Yin et al., 2013). The current observations are in line with those reported in models of disuse muscle atrophy, in which the expression of markers of satellite cell activation was upregulated, while satellite cell counts were also reduced (Arentson-Lantz et al., 2016; Snijders et al., 2014; Suetta et al., 2013).
The process of satellite cell activation during skeletal muscle injury including unloading is rather complex. For instance, periods of satellite cell proliferation and fusion to the existing fiber syncytium were demonstrated in the gastrocnemius of mice exposed to hindlimb suspension (Ferreira et al., 2006). In the present investigation, fusion of satellite cells was also observed in the gastrocnemius of muscles exposed to immobilization for 7 day (Figure 5). On this basis, it would be possible to conclude that activation of satellite cells should have taken place early during unloading, thus, leading to the fusion of those cells to the differentiated myofibers immediately afterwards. These findings most likely account for the decrease in muscle progenitors seen in the 7-day I mice as revealed by both the FACS (all hindlimb muscles) and histological analyses (gastrocnemius). Furthermore, ultrastructural events of early regeneration (grouped internal nuclei as a marker of myofiber fusion) were, indeed, identified and increased in the mice exposed to 7-day I compared to control muscles. Collectively, these observations are also consistent with the decrease in pax7 and myoD mRNA expression levels detected in the hindlimb muscles of animals during recovery in the present study. Moreover, mRNA expression levels of myogenin were upregulated in the latter muscles, which implied that during reloading, terminal differentiation was underway following the required decline in the expression of satellite cell activation markers (Yablonka-Reuveni & Rivera, 1994).
5 CONCLUSIONS
Recovery following hindlimb immobilization was characterized by a specific program of muscle regeneration (cellular and molecular) events. Hindlimb immobilization induced a decline in muscle progenitor cells together with an upregulation of markers of satellite cell activation, suggesting that fusion to existing myofibers takes place during unloading as demonstrated by the ultrastructural analyses. Muscle recovery as a result of splint removal induced a significant rise in muscle precursor cells and regeneration events along with a reduction in the expression of satellite cell activation markers and a concomitant rise in terminal muscle differentiation expression. These are novel, relevant findings of potential applicability for the treatment of disuse muscle atrophy, which is commonly associated with severe chronic and acute conditions.
ACKNOWLEDGMENTS
The authors are very grateful to the Cell Biology Group (Professor Pura Muñoz-Cànoves) at Universitat Pompeu Fabra and the CRG/UPF FACS Unit both from Barcelona Biomedical Research Park (PRBB), Barcelona (Spain) for their help with satellite cell isolation procedures. The authors are also very thankful to Maria Mercedes Simón-Grimaldos for her help with electron microscopy sampling and processing and to Mukaddes Izci for her help with part of the immunofluorescence microscopy experiments. Additionally, the authors are also thankful to Mr. Xavier Duran-Jordà for his statistical advice and data analyses.
EDITORIAL SUPPORT
None to declare.
CONFLICTS OF INTEREST
The authors declare no conflict of interest in relation to this study.




