Effects of XIST/miR-137 axis on neuropathic pain by targeting TNFAIP1 in a rat model
Abstract
Non-coding RNAs have been reported to participate in the pathophysiology of neuropathic pain. The objective of our study was to investigate the biological role of XIST in neuropathic pain development. In our study, we identify and validate that lncRNA XIST was markedly increased and miR-137 was significantly decreased in chronic constriction injury (CCI) rats. XIST silencing alleviated pain behaviors including both mechanical and thermal hyperalgesia in the CCI rats. XIST was predicted to interact with miR-137 by bioinformatics technology and dual-luciferase reporter assays confirmed the correlation between XIST and miR-137. miR-137 was negatively modulated by XIST and upregulation of miR-137 greatly reduced neuropathic pain development in CCI rats. Moreover, we observed that tumor necrosis factor alpha-induced protein 1 (TNFAIP1) was enhanced in CCI rats and 3′-untranslated region (UTR) of TNFAIP1 was exhibited to be a target of miR-137 by bioinformatics prediction. TNFAIP1 can act as a crucial inflammation regulator by activating NF-kB activity. Overexpression of miR-137 significantly suppressed TNFAIP1 both in vitro and in vivo. Furthermore, upregulation of XIST reversed the inhibitory role of miR-137 in neuropathic pain development by inhibiting TNFAIP1. In conclusion, our current study indicates that XIST can positively regulate neuropathic pain in rats through regulating the expression of miR-137 and TNFAIP1. Our results imply that XIST/miR-137/TNFAIP1 axis may serve as a novel therapeutic target in neuropathic pain.
1 INTRODUCTION
Neuropathic pain is a kind of pain which is caused by damages in the somatosensory system or diseases (Cohen & Mao, 2014). A lot of factors can contribute to neuropathic pain, such as toxicity, surgery, radiation, and congenital disorders (Lema, Foley, & Hausheer, 2010). A population prevalence of neuropathic pain is likely to be estimated between 6.9% and 10% (van Hecke, Austin, & Khan, 2014). Currently, the molecular mechanism of neuropathic pain still remains unclear. It is crucial to find out the specific pathology of neuropathic pain and develop useful therapeutic treatment for neuropathic pain.
Long non-protein-coding RNAs (lncRNAs) are regarded as transcripts with more than 200 nucleotides length (Vance & Ponting, 2014). LncRNAs have served as important molecules which can regulate gene expression at transcriptional and post-transcriptional levels (Knauss & Sun, 2013). It has been reported that identifying abnormal lncRNAs in the spinal cord under neuropathic pain conditions helps to understand the genetic mechanisms of neuropathic pain (Jiang, Sun, & He, 2015). For example, a kind of lncRNA can contribute to neuropathic pain by inhibiting Kcna2 in primary afferent neurons (Zhao, Tang, & Zhang, 2013). LncRNA uc.48+ is reported to be responsible for diabetic neuropathic pain modulated by the P2X3 receptor in the dorsal root ganglia (Wang, Xu, & Zou, 2016). LncRNA XIST is correlated with polycomb repressive complex 2 and can regulate X-chromosome in female mammals (Cerase, Pintacuda, & Tattermusch, 2015). Besides this, XIST can serve as an oncogene in various cancers. XIST is reported to promote esophageal squamous cell carcinoma development through miR-101/EZH2 axis (Wu, Dinglin, & Wang, 2017). XIST exhibits oncogenic roles in human glioma by sponging miR-137 (Wang, Yuan, & Li, 2017). In addition, XIST can contribute to rat spinal cord injury by inhibiting miR-494 (Gu, Xie, & Liu, 2017). The biological roles of XIST in neuropathic pain development remain unclear.
microRNAs belong to a kind of small non-coding RNAs that can modulate gene expression through translational inhibition or mRNA degradation (Bartel, 2004). Recently, it has been reported that miRNAs can regulate nervous system genes and they can lead to neuronal network plasticity (Lopez-Gonzalez, Landry, & Favereaux, 2017). miR-150 can relieve neuropathic pain by targeting toll-like receptor 5 (Ji, Lu, & Huang, 2017). Through regulating neuropathic pain-associated genes, miR-183 cluster can scale mechanical pain sensitivity (Peng, Li, & Zhang, 2017). miR-449a is able to ameliorate neuropathic pain via inhibiting KCNMA1 and TRPA1, and upregulating TPTE (Lu, Ma, & Wang, 2017). miR-137 has been shown to participate in several kinds of nerve diseases. For instance, miR-137 can reduce Aβ-triggered neurotoxicity through inactivating NF-κB pathway and targeting TNFAIP1 in Neuro2a cells (He, Tan, & Zhang, 2017). miR-137 is associated with schizophrenia and can disrupt Nrg1a neurodevelopmental signal transduction (Thomas, Anderson, & Shah, 2017). The biological roles of miR-137 in neuropathic pain development remain to be explored.
TNF alpha-induced protein 1 (TNFAIP1) is an early response gene of endothelium (Liu, Sun, & Zhou, 2010). TNFAIP1 plays tumor suppressor roles in variety of diseases, such as anorexia nervosa, paclitaxel resistance, and cancers (Ando, Ishikawa, & Kawamura, 2001; Grinchuk, Motakis, & Kuznetsov, 2010; Zhu, Yao, & Wu, 2014). However, TNFAIP1 has been reported to contribute to some nerve diseases. It is indicated that TNFAIP1 can lead to Aβ25-35-attributed neurotoxicity by inactivating Akt signaling pathway (Liu, Yu, & Xun, 2016). In addition, TNFAIP1 has been greatly induced in both the transgenic C. elegans AD brains and post-mortem AD brains, which suggests that TNFAIP1 is involved in AD development (Link, Taft, & Kapulkin, 2003; Wu & Luo, 2005).
Currently, we aimed to investigate the role of XIST in neuropathic pain development. XIST was significantly increased in a rat model of neuropathic pain. Knockdown of XIST markedly attenuated the development of neuropathic pain by increasing miR-137. Overexpressing miR-137 relieved neuropathic pain by downregulating TNFAIP1. It was hypothesized that XIST exerted a role in promoting neuropathic pain by modulating miR-137/TNFAIP1.
2 MATERIALS AND METHODS
2.1 Ethical statement
The experimental procedures in present study were strictly performed according to the requirements in the guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Breeding and use of rats in this experiment severely followed the International Association for the study of pain guidelines for the care and protection of laboratory animals.
2.2 Animal studies
Sprague–Dawley rats (adult, female, weighed 180–200 g) were purchased from Shanghai Animal Laboratory Center. The rats were group-maintained every five rats per cage with a constant feeding environment at 20–25 °C. The rats had free access to both food and water. Seven days of feeding later, the rats were randomly assigned, including the normal control, sham operation, chronic constriction injury (CCI). One week after CCI surgery, the intrathecal administration was carried out. Rats were administered at 8:00 a.m. every morning, lasting for 10 days. After rats were scarified, the spinal cord tissues were collected for further investigations.
2.3 Cell culture
Rat microglial cells and HEK-293T cells were purchased from the American Type Culture Collection (Manassas, VA). All the cells were grown in Dulbecco's modified Eagle medium (DMEM, Lonza Inc. Walkersville, MD). 10% heat-inactivated fetal bovine serum (FBS; Invitrogen, Carlsbad, CA) and 1% penicillin/streptomycin (Sigma, St. Louis, MO) were added to the medium. The cells were cultured in a 5% CO2 incubator at 37 °C.
2.4 Neuropathic pain animal model
The animal model of neuropathic pain was set up by performing bilateral chronic constriction injury (CCI) method (Bennett & Xie, 1988). Intraperitoneal (i.p.) injection of sodium pentobarbital (40 mg/kg) was used to anesthetize rats. The sciatic nerves on both sides were exposed using a mid-thigh incision and separated from surrounding tissues. The rats subjected to sciatic nerve exposures and isolation without ligation served as sham control groups. After CCI surgery, the dorsal spinal cords of L4-L6 were harvested at day 0, 5, 10, and 15.
2.5 Intrathecal injection
Occipital muscles of the rats were isolated and the PE-10 polyethylene catheter was placed in the cisterna magna. Paralysis of bilateral hind limbs was carried out using injection of lidocaine (Sigma) and then the intrathecal implantation was performed. After the catheter was fixed, the incision was sealed. Two days later, the rats were subjected to CCI surgery. Recombinant lentivirus (Genepharma, Shanghai, China) were injected into the rats using a microinjection syringe through intrathecal catheter.
2.6 Real-time quantitative polymerase chain reaction
Total RNA and miRNA were extracted by using the RNAiso Plus. RNA reverse transcribed by the Prime Script TM RT Master Mix and qPCR was carried out by SYBR Premix Ex Taq II (TaKaRaBio Technology, Dalian, China). The primers used in qRT-PCR were demonstrated in Table 1. U6 RNA served as an internal microRNA control and GAPDH was used as an internal mRNA or lncRNA control. The Applied Biosystems 7900 RealTime PCR System (Applied Biosystems, Foster City, CA) was employed to perform Real-time PCR. The melting curve was used to observe the reliability of the PCR data. Threshold cycle (CT) values were taken and the relative expression of target genes was calculated using the equation 2−ΔΔCt.
| Genes | Forward (5′ ∼ 3′) | Reverse (5′ ∼ 3′) |
|---|---|---|
| GAPDH | CAAGGTCATCCATGACAACTTTG | GTCCACCACCCTGTTGCTGTAG |
| U6 | CTCGCTTCGGCAGCACA | AACGCTTCACGAATTTGCGT |
| XIST | AGCTCCTCGGACAGCTGTAA | CTCCAGATAGCTGGCAACC |
| miR-137 | GCGCTTATTGCTTAAGAATAC | CAGTGCAGGGTCCGAGGT |
| TNFAIP1 | CGAGCTCGTGCTGCCTGGGTCTCTGC | GCTCTAGAGCAGCTGCTCTGTCGGATGTTT |
2.7 Behavioral tests
paw withdrawal thermal latency (PWTL) and paw withdrawal mechanic threshold (PWMT) of rats' left hind paws were tested with 7370 Plantar Test (Ugo Basile, Italy) and 2390 Electronic von–Frey Anesthesiometer (IITC Life Science, Woodland Hills, CA). To evaluate PWTL, light source was pointed to rats' paws below the pelma. For each test, the positions were the same every time. When the rats responded with leg rising, the recorded time was considered PWTL for this test (accurate to 0.1 s). For another appraisal of PWMT, the light source was replaced by a probe touching the pelma of rats.
2.8 Transfection
According to the instructions of manufacturer, miR-137 mimics, inhibitors or their parental negative controls was transfected in the same way. LV-shXIST, LV-XIST, LV-miR-137, and LV-shTNFAIP1 were transfected into rat microglial cells or rats.
2.9 Luciferase activity assay
The wild-type (WT) or mutant (MUT) miR-137 binding site of XIST was designed and subcloned into pGL3 Basic vector (Promega, Madison,WI). The wild-type (WT) or mutant (MUT) miR-137 binding site in the 3′-UTR of TNFAIP1 was constructed and subcloned into pGL3 Basic vector (Promega). HEK-293T cells were seeded on 24-well plates for 24 hr. Mimics or inhibitors of miR-137 (RiboBio, Guangzhou, China) were cotransfected with 10 µg pLUC-WT-XIST or pLUC-MUT-XIST, pLUC-WT-TNFAIP1, or pLUC-MUT-TNFAIP1. Lipofectamine 2000 reagent (Invitrogen) was used to carry out the transfection. Luciferase activity was measured by the Dual-Luciferase Reporter Assay System (Promega).
2.10 Western blot analysis
Proteins were collected, loaded and then separated using 10% sodium dodecyl sulfate polyacrylamide gels. PVDF membrane (PolyVinylidene Fluoride) was used to transfer separated proteins on. After saturated in 5% defatted milk, membranes were incubated with primary antibodies. Next day, after membranes were washed with TBST, secondary antibodies conjugated with Horse Radish Peroxidase (HRP) were used to indicate the membranes at room temperature. The primary antibodies: anti-TNFAIP1 (1:1000, Abcam, Cambridge, Britain), anti-GAPDH (1:1000, Abcam) were employed.
2.11 Statistical analysis
All data were presented as the mean ± standard deviation (SD) of at least three experiments. Comparisons between quantitative variables were performed by the student's t-test and One-Way ANOVA analysis. Differences were considered significant when the p value was less than 0.05. (*p < 0.05, **p < 0.01). The SPSS 19.0 (SPSS Inc, Chicago, IL) were used to carry out statistical analysis.
3 RESULTS
3.1 XIST and TNFAIP1 were overexpressed while miR-137 was downregulated in CCI rats
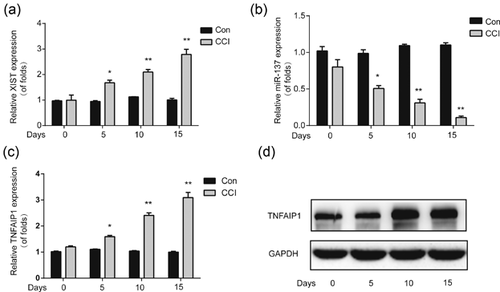
Firstly, to explore the roles of XIST, miR-137, and TNFAIP1 in neuropathic pain, the spinal cord of rats were collected from CCI models and subjected to qRT-PCR. Compared to the sham surgery group, XIST was significantly upregulated in CCI rats in a time-dependent manner (Figure 1a). Next, we observed that miR-137 expression was remarkably downregulated in CCI rats, which indicated a negative correlation between miR-137 and XIST in neuropathic pain development (Figure 1b). Moreover, TNFAIP1 expression was measured and it was demonstrated that TNFAIP1 were markedly increased in CCI rats (Figures 1c and 1d). These results exhibited that XIST/miR-137/TNFAIP1 axis was involved in neuropathic pain development in vivo.
3.2 Knockdown of XIST relieved neuropathic pain development in vivo
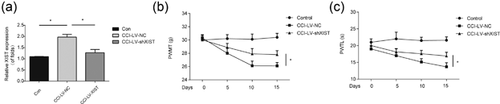
In addition, to test the biological fucntion of XIST in neuropathic pain progression, XIST was silenced in rats by carrying out intrathecal injection of lentivirus mediated infection of XIST shRNA (LV-shXIST). It was observed that XIST was significantly downregulated in CCI rats infected with LV-shXIST compared to the control group (Figure 2a). As it was shown in Figures 2b and 2c, both mechanical allodynia (Figure 2b) and thermal hyperalgesia (Figure 2c) were significantly alleviated by XIST knockdown. These results presented that downregulation of XIST was able to suppress neuropathic pain development in CCI rats.
3.3 miR-137 was a direct target microRNA of XIST
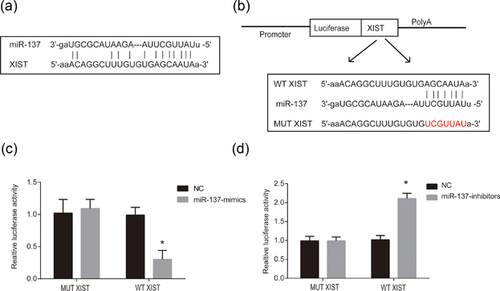
To figure out the direct target microRNA modulated by XIST in neuropathic pain, bioinformatics analysis was performed in our investigation. Interestingly, a conserved binding region between XIST and miR-137 was manifested (Figure 3a). To confirm the direct relationship between miR-137 and XIST, the wild-type binding site (WT-XIST) or mutant binding site (MUT-XIST) was constructed and transfected into the dual-luciferase vector (Figure 3b). In Figure 3c, cotransfection of miR-137 mimics and the luciferase vector containing WT-XIST led to a significant decrease of relative luciferase activity in HEK-293T cells (Figure 3c). Reversely, cotransfection of miR-137 inhibitors and the luciferase vector containing WT-XIST resulted in a great increase of relative luciferase activity in HEK-293T cells (Figure 3d). These in vitro assays suggested that miR-137 was a potential microRNA target of XIST. Overall, these results implied that XIST directly targeted miR-137 in vitro.
3.4 Upregulation of miR-137 inhibited neuropathic pain development
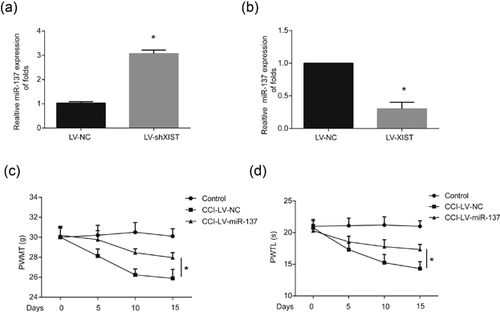
Next, to validate whether expression of miR-137 can be modulated by XIST in vitro, LV-shXIST or LV-XIST was transfected into rat microglial cells. It was shown that LV-shXIST upregulated miR-137 expression (Figure 4a) and LV-XIST downregulated miR-137 expression (Figure 4b). Besides these, to investigate the role of miR-137 playing neuropathic pain development, rats were infected with LV-miR-137. As it was exhibited in Figures 4c and 4d, both PWMT and PWTL were relieved by miR-137 overexpression. These data indicated that overexpression of miR-137 could inhibit neuropathic pain progression.
3.5 TNFAIP1 was a direct target of miR-137
Furthermore, to investigate the direct target of miR137, bioinformatics research was done in our study. A binding site between miR-137 and TNFAIP1 was exhibited in Figure 5a. To validate the direct association between miR-137 and TNFAIP1, the wild-type binding site (WT-TNFAIP1) and mutant binding site (MUT-TNFAIP1) was designed and transfected into the dual-luciferase vector (Figure 5b). Cotransfection of miR-137 mimics and the luciferase vector containing WT-TNFAIP1 decreased relative luciferase activity in HEK-293T cells (Figure 5c), while cotransfection of miR-137 inhibitors and the luciferase vector containing WT-TNFAIP1 increased relative luciferase activity in HEK-293T cells (Figure 5d). To sum up, these findings elaborated that TNFAIP1 was a potential target gene of miR-137.
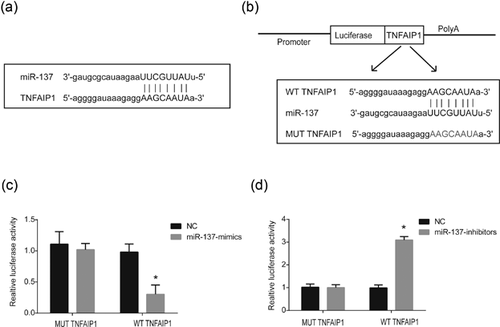
3.6 Downregulation of TNFAIP1 suppressed neuropathic pain progression in vitro and in vivo
Next, to find out whether TNFAIP1 can be influenced by miR-137 in vitro, LV-miR-137 or LV-NC was transfected into rat microglial cells. miR-137 was overexpressed by LV-miR-137 in rat microglial cells (Figure 6a). In addition, we observed that LV-miR-137 greatly upregulated TNFAIP1 mRNA expression and protein levels (Figures 6b and 6c). Besides these, to investigate the function of TNFAIP1 in neuropathic pain development, LV-shTNFAIP1 was infected with rats. It was exhibited in Figures 6d and 6e, neuropathic pain development was assessed by PWMT and PWTL. The pain was greatly reduced by TNFAIP1 downregulation. These data revealed that miR-137 could inhibit neuropathic pain progression by decreasing TNFAIP1.
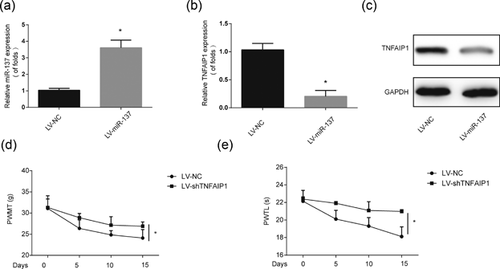
3.7 Overexpression of XIST enhanced neuropathic pain via upregulating TNFAIP1 in vitro and in vivo
To examine the correlation between XIST and TNFAIP1, rat microglial cells were infected with LV-XIST. Figure 7a showed that XIST was increased in rat microglial cells infected with LV-XIST. However, miR-137 was significantly downregulated by LV-XIST (Figure 7b). Consistently, in Figures 7c and 7d, TNFAIP1 expression was upregulated by ovexpressing XIST in vitro. Additionally, LV-miR-137 was able to reverse the expression of TNFAIP1 increased by LV-XIST in vitro (Figures 7c and 7d). Furthermore, miR-137 overexpression can reverse the effects of LV-XIST on mechanical allodynia (Figure 7e) and thermal hyperalgesia (Figure 7f) via downregulating TNFAIP1. In conclusion, these results suggested that overexpression of XIST can promote neuropathic pain development via sponging miR-137 and increasing TNFAIP1 in vitro and in vivo.
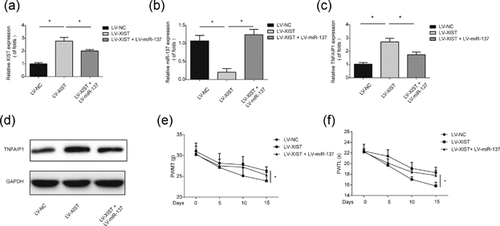
4 DISCUSSION
Recently, increasing roles of non-coding RNAs have been revealed in neuropathic pain (Zhou, Xiong, & Chen, 2017). In our present study, we observed that XIST/miR-137/TNFAIP1 axis was responsible for neuropathic pain development in CCI rats. XIST was upregulated in CCI rat models and it could promote neuropathic pain development by decreasing miR-137 and increasing TNFAIP1.
Many studies have reported the interactions between lncRNAs and microRNAs. It has been indicated that lncRNA can act as an inhibitory regulator of miRNA (Dykes & Emanueli, 2017). In cervical cancer, LncRNA TCONS_00026907 can promote progression and prognosis through targeting miR-143-5p (Jin, Chen, & Hu, 2017). By inhibiting miR-874, lncRNA XLOC_008466 can function as an oncogene in human non-small cell lung cancer (Yang, Li, & Zhang, 2017). LncRNA CRNDE can promote gastric cancer proliferation by sponging miR-145 (Hu, Du, & Zhang, 2017). XIST has been observed to be overexpressed and exerted crucial roles in several kinds of tumors, including osteosarcoma, breast cancer, and gastric cancer (Li, Wu, & Li, 2017; Ma, Zhou, & Luo, 2017; Schouten, Vollebergh, & Opdam, 2016). For instance, XIST is increased in osteosarcoma and is correlated with cell proliferation and poor prognosis (Li et al., 2017). High XIST expression can predict poor outcome in breast cancer patients (Schouten et al., 2016). Besides these, XIST can inhibit miR-497 and promote gastric cancer development via decreasing MACC1 (Ma et al., 2017). A recent research paper has reported that in rat spinal cord injury, XIST results in neuronal apoptosis via inhibiting AKT phosphorylation and is downregulated by miR-494 (Gu et al., 2017). This indicates that XIST is associated with nerve disease. However, there is no study focusing on the relevance between XIST and neuropathic pain. The underlying mechanisms of XIST remain largely poor understood in neuropathic pain progression. In our current study, we observed that XIST was upregulated in CCI rats and can promote neuropathic pain development. Bioinformatics analysis was used to predict the potential microRNA target of XIST in our study. We focused on the mechanism of XIST involved in neuropathic pain process.
microRNAs are short non-coding RNA which can degrade mRNA and repress translation through binding to the 3′-untranslational region (UTR) of their corresponding genes (Bartel, 2004). miR-137 has been identified to participate in some types of cancer. It is observed that miR-137 can inhibit glioblastoma multiforme cells proliferation and trigger brain tumor stem cells differentiation (Silber, Lim, & Petritsch, 2008). miR-137 can inhibit lung cancer tumor growth and enhance paclitaxel chemosensitivity in lung cancer (Shen, Wang, & Ge, 2016). In addition, noncoding variants in miR-137 gene locus can promote schizophrenia risk with genome-wide significance (Siegert, Seo, & Kwon, 2015). It is demonstrated that miR-137 plays an important role in the regulation of hippocampal neurodegeneration through CDC42 (Huang, Zhang, & Zheng, 2014). In brain and cultured primary neurons, overexpressing miR-137 is able to suppress dendritic morphogenesis, phenotypic maturation, and spine development (Smrt, Szulwach, & Pfeiffer, 2010). miR-137 can modulate serine palmitoyltransferase and amyloid beta, which is a novel target in sporadic Alzheimer's disease (Geekiyanage & Chan, 2011). These indicate that miR-137 plays important roles in inflammation-correlated and neural disease. By using bioinformatics, miR-137 was predicted as XIST microRNA target. It was found that miR-137 was markedly decreased in CCI rats. Overexpression of miR-137 greatly repressed neuropathic pain development. We proved that XIST can induce neuropathic pain in CCI rats by sponging miR-137. Dual-luciferase reporter assays confirmed the negative interaction between XIST and miR-137.
TNFAIP1can be activated by TNFα and IL-6 and it plays significant roles in many human diseases (Wolf, Marks, & Sarma, 1992). TNFAIP1 is abnormally expressed in various cancers (Zhou, Li, & Zhang, 2013; Zhu et al., 2014). Interestingly, in cerebral cortical neurons, Bacurd1/Kctd13 and Bacurd2/Tnfaip1 can interact with Rnd proteins and affect the long-term positioning and dendritic maturation (Gladwyn-Ng, Huang, & Ngo, 2016). Additionally, TNFAIP1 has been upregulated in Alzheimer's disease brains and is correlated with diabetic nephropathy (Link et al., 2003). TNFAIP1 is reported to promote the neurotoxicity induced by Aβ25-35 and it has been validated that miR-137 can reduce neurotoxicity by inhibiting TNFAIP1 in Neuro2a cells (Link et al., 2003; Liu et al., 2016). Hence, it is well known that TNFAIP1 can exert crucial rules in neural disease. Nevertheless, the association between miR-137 and TNFAIP1 in neuropathic pain is still not clear. In our investigation, we uncovered that TNFAIP1 was significantly induced in CCI rat. Moreover, it was observed that miR-137 can inhibit neuropathic pain through targeting TNFAIP1. When TNFAIP1 was suppressed by LV-sh TNFAIP1, neuropathic pain development was greatly inhibited. XIST can promote neuropathic pain via increasing TNFAIP1 expression. Further studies are needed to concentrate on the mechanism of TNFAIP1 in neuropathic pain development.
In conclusion, our study implied a critical role of XIST/miR-137/TNFAIP1 axis in neuropathic pain progression. We exhibited that inhibition of XIST alleviated neuropathic pain development of CCI rats via upregulating miR-137 and downregulating TNFAIP1. Both in vitro and in vivo assays were carried out to elucidate the mechanisms of XIST/miR-137/TNFAIP1 axis in regulating neuropathic pain development. Our findings indicate that XIST can be identified as a novel therapeutic target for neuropathic pain treatment.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.




