The combination of plasma fibrinogen and neutrophil lymphocyte ratio (F-NLR) is a predictive factor in patients with resectable non small cell lung cancer
Abstract
The prognostic value of inflammation indexes in non small cell lung cancer (NSCLC) was not established. Therefore, we assessed the clinical applicability of the F-NLR score, which is based on fibrinogen (F) and the neutrophil-lymphocyte ratio (NLR), and the glasgow prognostic score (GPS) to predict the prognoses of NSCLC patients. We retrospectively identified 515 patients with stage I/II/IIIA who underwent surgery at our institution, and evaluated their preoperative serum levels of CRP, albumin, fibrinogen, neutrophil count, and the lymphocyte count. The cut-off values of the fibrinogen level and NLR were determined with receiver operating characteristic (ROC) curve. GPS was classified into three groups as previously described. The disease free survival (DFS) and overall survival (OS) were calculated by the Kaplan–Meier method. Categorical variables were compared using the χ2 test. Survival curves were estimated using the Kaplan–Meier method, and the Cox proportional hazard model was used to assess the prognostic factors. The F-NLR was significantly associated with sex (p = 0.000), smoking history (p = 0.014), lesion type (p = 0.000), histologic type (p = 0.000), T stage (p = 0.000), venous invasion (p = 0.000), lymphatic invasion (p = 0.000), and TNM stage (p = 0.000). The 5-year DFS rates in F-NLR groups 0, 1, and 2 were 46.7%, 36.4%, 30.1%, respectively (p = 0.000), and the 5-year overall survival (OS) rates in the above three groups were 52.0%, 39.8%, 32.1%, respectively (p = 0.000). Multivariate analysis showed that venous invasion (p = 0.036), lymph node metastasis (p = 0.000), and F-NLR (p = 0.034) were independent prognostic factors for DFS. Age (p = 0.015), venous invasion (p = 0.024), lymph node metastasis (p = 0.000), and F-NLR (p = 0.019) were independent prognostic factors for OS. Thus, F-NLR was the independent prognostic factor for both the DFS and OS. And patients with a high-risk preoperative F-NLR group may benefit from adjuvant therapy by subgroup analysis. Our results demonstrated that F-NLR, a novel inflammation-based grading system, as well as the GPS, appeared to have value as a promising clinical predictor of the prognosis for the resectable non small cell lung cancer patients.
1 INTRODUCTION
Lung cancer is the major cause of morbidity and mortality worldwide. Non-Small Cell Lung Cancer (NSCLC) comprises approximately 85% of all lung cancers and has become a leading cause of cancer-related mortality (Molina, Yang, Cassivi, Schild, & Adjei, 2008). Despite the progress in radical resection and adjuvant therapy (radiotherapy and chemotherapy) after surgery, NSCLC still shows a poor 5-year survival rate. Although there were several certain biomarkers had been identified as survival-related with NSCLC (Brundage, Davies, & Mackillop, 2002; Matsuoka, Sumitomo, & Misaki, 2007; Okada, Nishio, & Sakamoto, 2004; Tomita, Shimizu, Ayabe, Yonei, & Onitsuka, 2010), their clinical use was limited due to various reasons. Therefore, identifying biomarkers which are effective and easy-obtained for the early detection and postoperative follow-up of NSCLC is of clinical significance.
Systemic inflammatory response has been improved to play an important role in the progression of cancers in the last decades (Balkwill & Mantovani, 2001; Mantovani, Allavena, Sica, & Balkwill, 2008). The inflammation-based prognostic systems have been confirmed in numerous studies. The Glasgow Prognostic Score (GPS), which combines serum CRP with albuminemia and has been demonstrated to be a predictive factor in various cancers, is regarded as a prognostic milestone among all the systems (Forrest, McMillan, & McArdle, 2003; McMillan, 2013). Other prognostic systems such as neutrophil to lymphocyte ratio (NLR) (Cho et al., 2014; McNamara et al., 2014; Tajiri et al., 2015), platelet to lymphocyte ratio (PLR) (Kwon, Kim, & Oh, 2012; Li et al., 2015), and fibrinogen (He et al., 2017; Luo, Kim, Kim, Lee, & Song, 2017; Seebacher et al., 2017), can help predict the progression, metastasis, and prognosis of cancer patients.
Recent studies have emphasized that hyperfibrinogenemia is involved in the malignant behaviors of various types of cancer (Kijima et al., 2017; Lu et al., 2011; Qiu et al., 2012). And the elevated preoperative plasma fibrinogen levels are associated with worse outcome in various malignancies. The NLR reflects the status of the systemic inflammatory response in host immune surveillance, and an imbalance of the host immune surveillance system facilitates tumor proliferation and metastasis (Vesely & Schreiber, 2013). However, the fibrinogen and NLR have not been simultaneously assessed as markers of tumor progression and prognosis in patients with non small cell lung cancer. Then we combined the serum fibrinogen and NLR together first time to predict its clinical value in the prognosis of NSCLC patients.
2 MATERIALS AND METHODS
2.1 Patients
We retrospectively reviewed data from 515 patients (with 334 men and 181 women, median age 60 years old) with resectable non small cell lung cancer who received surgery at Cangzhou Central Hospital between June 2008 and June 2012. The criteria for inclusion in this study included: complete pulmonary resection and systematic node dissection of the hilar and mediastinal lymph nodes, histologically confirmed NSCLC and complete clinical, laboratory, imaging, and follow-up data. And the exclusion criteria included: preoperative chemotherapy/radiotherapy or died perioperative period, positive surgical margins, advanced disease (e.g., malignant pleural effusion/involvement or distant metastasis), clinical evidence of infection or other bone marrow, hematological, or autoimmune disease, a history of lung cancer, or a second primary cancer diagnosed within 5 years of the lung cancer index. Clinicopathological information of the patients were obtained from the medical records, including sex, age, smoking history, tumor location, lesion type, resection type, histologic type, tumor size, lymph node metastasis, and TNM stage. The TNM stage was assessed according to American Joint Committee on Cancer (AJCC) staging manual (seventh edition) (Edge & Compton, 2010). Ethical approval was obtained from the Ethical Committees of Cangzhou Central Hospital.
2.2 F-NLR and GPS evaluation
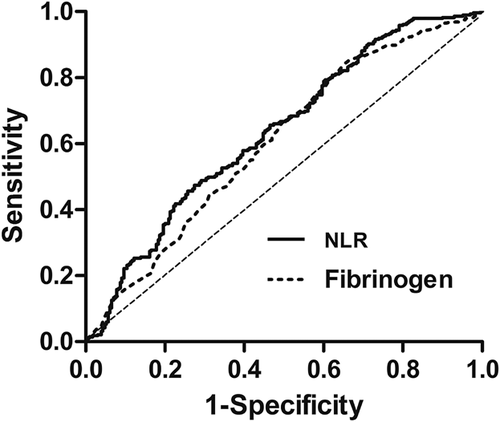
Routine laboratory measurements including the serum levels of CRP, albumin, and fibrinogen were extracted in a retrospective fashion from the medical records. The NLR was calculated by dividing the neutrophil count by the lymphocyte count. The cutoff value was found as 2.21 for NLR and 3.38 g/L for fibrinogen level according to the Youden index by ROC curve (Figure 1). For these values, sensitivity was determined as 49.8% and 65.7%, specificity as 72.6% and 54.7%, and area under the concentration-time curve (AUC) as 0.635 and 0.604, respectively. The F-NLR score was classified into three groups based on each cut-off value of plasma fibrinogen and NLR as follows: F-NLR score of 2: both hyperfibrinogenemia (>3.38) and high NLR (>2.21), F-NLR score of 1: one of these hematological abnormalities, and F-NLR score of 0: neither hyperfibrinogenemia nor high NLR. GPS was classified into three groups, as previously described: GPS of 2: both an elevated CRP (>10 mg/L) and hypoalbuminemia (<35g/L), GPS of 1: one of these hematological abnormalities, and GPS of 0: neither an elevated CRP nor hypoalbuminemia.
2.3 Statistical analysis
Categorical variables were compared using the χ2 test. Receiver operating characteristic (ROC) curves and the area under the curve (AUC) were used to assess the predictive value of fibrinogen concentrations and NLR. Kaplan–Meier survival curves were generated, and the differences in survival rate were determined using the log-rank test. Prognostic factors were assessed by univariate and multivariate analyses (Cox's proportional hazards regression model). All statistical analyses were performed using SPSS 17.0 statistical software (SPSS, Inc., Chicago, IL). P-values <0.05 were considered statistically significant.
3 RESULTS
3.1 The clinical baseline characteristics of NSCLC patients
The overall demographic and clinical characteristics of all the patients were summarized in Table 1. A total of 515 NSCLC patients (64.9% male and 35.1% female) who met the inclusion criteria were enrolled in this study. There were 256 (49.7%) patients who were younger than 60-year old in this study, 330 (64.1%) patients had smoking history, 442 (85.8%) patients underwent lobectomy, and 73 (14.2%) underwent pneumonectomy. Patients with clinical stages I, II, and IIIA accounted for 42.3%, 21.2%, and 36.5%, respectively. Of all the patients, 361 (70.1%) tumors were located in peripheral and the left ones were located in the central. Patients with the histologic type of squamous cell carcinoma, adenocarcinoma, and other types accounted for 47.4%, 40.2%, and 12.4%, respectively. The median follow-up for survivors was 54.0 months (range 3.0–108.0 months).
| Variable | Number (%) or mean ± SD |
|---|---|
| Sex | |
| Male/Female | 334 (64.9%)/181 (35.1%) |
| Age (years) | 60.4 ± 9.3 |
| ≤60/>60 | 256 (49.7%)/259 (50.3%) |
| Smoking history | |
| None/Yes | 185 (35.9%)/330 (64.1%) |
| Tumor location | |
| Left/right | 209 (40.6%)/306 (59.4%) |
| Lesion type | |
| Peripheral/Central | 361 (70.1%)/154 (29.9%) |
| Resection type | |
| lobectomy/pneumonectomy | 442 (85.8%)/73 (14.2%) |
| Histologic type | |
| SqCC/Adenocarcinoma/Others | 244 (47.4%)/207 (40.2%)/64 (12.4%) |
| Tumor size, mm | 44.6 ± 21.3 |
| T stage | |
| T1/T2/T3 | 179(34.8%)/284 (55.1%)/52 (10.1%) |
| Venous invasion | |
| Negative/Positive | 396 (76.9%)/119 (23.1%) |
| Lymphatic invasion | |
| Negative/Positive | 183 (35.5%)/332 (64.5%) |
| Lymph node metastasis | |
| Negative/Positive | 283 (55.0%)/232 (45.0%) |
| TNM stage | |
| I/II/IIIA | 218 (42.3%)/109 (21.2%)/188 (36.5%) |
| F-NLR | |
| 0/1/2 | 175 (34.0%)/194 (37.7%)/146 (28.3%) |
| GPS | |
| 0/1/2 | 380 (73.8%)/94 (18.3%)/41 (8.0%) |
- GPS, glasgow prognostic score; SqCC, squamous cell carcinoma.
3.2 Correlation analysis between F-NLR/GPS and clinicopathological factors in NSCLC patients
In this study, we investigated the correlations between the F-NLR/GPS and the clinicopathological parameters for the NSCLC patients. The results were presented in Table 2. For F-NLR, several factors had no significant differences among three groups except for sex (p = 0.000), smoking history (p = 0.014), lesion type (p = 0.000), histologic type (p = 0.000), T stage (p = 0.000), venous invasion (p = 0.000), lymphatic invasion (p = 0.000), and TNM stage (p = 0.000). And GPS showed obvious correlations with the clinicopathological features investigated including sex (p = 0.001), age (p = 0.045), histologic type (p = 0.000), T stage (p = 0.004), venous invasion (p = 0.001), lymphatic invasion (p = 0.002), and TNM stage (p = 0.014).
| F-NLR | GPS | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | 0 | 1 | 2 | p | 0 | 1 | 2 | p |
| Sex | 0.000 | 0.001 | ||||||
| Male | 98 (56.0%) | 123 (63.4%) | 113 (77.4%) | 228 (60.0%) | 74 (78.7%) | 32 (78.0%) | ||
| Female | 77 (44.0%) | 71 (36.6%) | 33 (22.6%) | 152 (40.0%) | 20 (21.3%) | 9 (22.0%) | ||
| Age (years) | 0.086 | 0.045 | ||||||
| ≤60 | 98 (56.0%) | 94 (48.5%) | 64 (43.8%) | 200 (52.6%) | 42 (44.7%) | 14 (34.1%) | ||
| >60 | 77 (44.0%) | 100 (51.5%) | 82 (56.2%) | 180 (47.4%) | 52 (55.3%) | 27 (65.9%) | ||
| Smoking history | 0.014 | 0.207 | ||||||
| None | 101 (57.7%) | 122 (62.9%) | 107 (73.3%) | 235 (61.8%) | 66 (70.2%) | 29 (70.7%) | ||
| Yes | 74 (42.3%) | 72 (37.1%) | 39 (26.7%) | 145 (38.2%) | 28 (29.8%) | 12 (29.3%) | ||
| Tumor location | 0.461 | 0.242 | ||||||
| Left | 74 (42.3%) | 82 (42.3%) | 53 (36.3%) | 155 (40.8%) | 42 (44.7%) | 12 (29.3%) | ||
| Right | 101 (57.7%) | 112 (57.7%) | 93 (63.7%) | 225 (59.2%) | 52 (55.3%) | 29 (70.7%) | ||
| Lesion type | 0.000 | 0.241 | ||||||
| Peripheral | 147 (84.0%) | 137 (70.6%) | 77 (52.7%) | 270 (71.7%) | 67 (71.3%) | 24 (58.5%) | ||
| Central | 28 (16.0%) | 57 (29.4%) | 69 (47.3%) | 110 (28.9%) | 27 (28.7%) | 17 (41.5%) | ||
| Histologic type | 0.000 | 0.000 | ||||||
| SqCC | 54 (30.9%) | 94 (48.5%) | 96 (65.8%) | 159 (41.8%) | 59 (62.8%) | 26 (63.4%) | ||
| Adenocarcinoma | 102 (58.3%) | 74 (38.1%) | 31 (21.2%) | 174 (45.8%) | 25 (26.6%) | 8 (19.5%) | ||
| Others | 19 (10.9%) | 26 (13.4%) | 19 (13.0%) | 47 (12.4%) | 10 (10.6%) | 7 (17.1%) | ||
| T stage | 0.000 | 0.004 | ||||||
| T1 | 90 (51.4%) | 65 (33.5%) | 24 (16.4%) | 147 (38.7%) | 22 (23.4%) | 10 (24.4%) | ||
| T2 | 79 (45.1%) | 111 (57.2%) | 94 (64.4%) | 200 (52.6%) | 62 (66.0%) | 22 (53.7%) | ||
| T3 | 6 (3.4%) | 18 (9.3%) | 28 (19.2%) | 33 (8.7%) | 10 (10.6%) | 9 (22.0%) | ||
| Venous invasion | 0.000 | 0.001 | ||||||
| Negative | 148 (84.6%) | 153 (78.9%) | 95 (65.1%) | 307 (80.8%) | 64 (68.1%) | 25 (61.0%) | ||
| Positive | 27 (15.4%) | 41 (21.1%) | 51 (34.9%) | 73 (19.2%) | 30 (31.9%) | 16 (39.0%) | ||
| Lymphatic invasion | 0.000 | 0.002 | ||||||
| Negative | 142 (81.1%) | 126 (64.9%) | 64 (43.8%) | 259 (68.2%) | 56 (59.6%) | 17 (41.5%) | ||
| Positive | 33 (18.9%) | 68 (35.1%) | 82 (56.2%) | 121 (31.8%) | 38 (40.4%) | 24 (58.5%) | ||
| Lymph node metastasis | 0.340 | 0.057 | ||||||
| Negative | 103 (58.9%) | 106 (54.6%) | 74 (50.7%) | 220 (57.9%) | 46 (48.9%) | 17 (41.5%) | ||
| Positive | 72 (41.1%) | 88 (45.4%) | 72 (49.3%) | 160 (42.1%) | 48 (51.1%) | 24 (58.5%) | ||
| TNM stage | 0.000 | 0.014 | ||||||
| I | 98 (56.0%) | 80 (41.2%) | 40 (27.4%) | 177 (46.6%) | 32 (34.0%) | 9 (22.0%) | ||
| II | 18 (10.3%) | 46 (23.7%) | 45 (30.8%) | 75 (19.7%) | 22 (23.4%) | 12 (29.3%) | ||
| IIIA | 59 (33.7%) | 68 (35.1%) | 61 (41.8%) | 128 (33.7%) | 40 (42.6%) | 20 (48.8%) | ||
3.3 Univariate and multivariate analyses for DFS
The age (p = 0.044), lesion type (p = 0.014), resection type (p = 0.034), T stage (p = 0.002), venous invasion (p = 0.000), lymphatic invasion (p = 0.038), lymph node metastasis (p = 0.000), adjuvant radiotherapy (p = 0.033), F-NLR (p = 0.000), and GPS (p = 0.000) were significantly associated with DFS in the univariate analysis (Table 3). Multivariate analysis demonstrated that the venous invasion (p = 0.036), lymph node metastasis (p = 0.000), and F-NLR (p = 0.034) were independent prognostic factors for DFS (Table 3).
| Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Number of patients | HR | 95%CI | p-value | HR | 95%CI | p-value |
| Sex (male/female) | 334/181 | 0.820 | 0.654–1.029 | 0.087 | |||
| Age (≤60/>60) years | 256/259 | 1.246 | 1.006 –1.545 | 0.044 | 1.202 | 0.966–1.497 | 0.099 |
| Smoking history (none/yes) | 185/330 | 0.884 | 0.706–1.107 | 0.282 | |||
| Tumor location (left/right) | 209/306 | 1.094 | 0.880–1.361 | 0.418 | |||
| Lesion type (peripheral/central) | 361/154 | 1.332 | 1.059–1.676 | 0.014 | 1.052 | 0.812–1.361 | 0.703 |
| Resection type (lobectomy/pneumonectomy) | 442/73 | 1.377 | 1.024–1.851 | 0.034 | 1.066 | 0.771–1.475 | 0.699 |
| Histologic type (SqCC/adenocarcinoma/others) | 244/207/64 | 1.138 | 0.976–1.327 | 0.100 | |||
| T stage (T1/T2/T3) | 179/284/52 | 1.301 | 1.097–1.543 | 0.002 | 1.105 | 0.918–1.330 | 0.292 |
| Venous invasion (negative/positive) | 396/119 | 0.649 | 0.510–0.825 | 0.000 | 0.761 | 0.590–0.983 | 0.036 |
| Lymphatic invasion (negative/positive) | 183/332 | 1.263 | 1.013–1.576 | 0.038 | 1.092 | 0.854–1.395 | 0.483 |
| Lymph node metastasis (negative/positive) | 283/232 | 2.192 | 1.765–2.722 | 0.000 | 2.089 | 1.676–2.605 | 0.000 |
| Adjuvant chemotherapy (none/yes) | 245/270 | 0.893 | 0.721–1.106 | 0.300 | |||
| Adjuvant radiotherapy (none/yes) | 416/99 | 0.743 | 0.566–0.977 | 0.033 | 0.803 | 0.609–1.059 | 0.121 |
| F-NLR (0/1/2) | 175/194/146 | 1.358 | 1.184–1.558 | 0.000 | 1.190 | 1.013–1.398 | 0.034 |
| GPS (0/1/2) | 380/94/41 | 1.375 | 1.170–1.616 | 0.000 | 1.154 | 0.976–1.365 | 0.095 |
- CI, confidence interval; HR, hazard ratio.
3.4 Univariate and multivariate analyses for OS
The sex (p = 0.025), age (p = 0.024), smoking history (p = 0.041), lesion type (p = 0.003), resection type (p = 0.006), T stage (p = 0.001), venous invasion (p = 0.001), lymphatic invasion (p = 0.012), lymph node metastasis (p = 0.000), adjuvant radiotherapy (p = 0.016), F-NLR (p = 0.000), and GPS (p = 0.000) were significantly associated with OS in the univariate analysis (Table 4). Multivariate analysis demonstrated that the age (p = 0.015), venous invasion (p = 0.024), lymph node metastasis (p = 0.000), and F-NLR (p = 0.019) were independent prognostic factors for OS (Table 4).
| Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Number of patients | HR | 95%CI | p-value | HR | 95%CI | p-value |
| Sex (male/female) | 334/181 | 0.767 | 0.608–0.967 | 0.025 | 0.920 | 0.705–1.200 | 0.539 |
| Age (≤60/>60) years | 209/306 | 1.286 | 1.034–1.599 | 0.024 | 1.321 | 1.056–1.653 | 0.015 |
| Smoking history (none/yes) | 185/330 | 0.786 | 0.624–0.991 | 0.041 | 0.936 | 0.717–1.221 | 0.625 |
| Tumor location (left/right) | 256/259 | 1.125 | 0.901–1.404 | 0.300 | |||
| Lesion type (peripheral/central) | 361/154 | 1.421 | 1.128–1.792 | 0.003 | 1.057 | 0.812–1.374 | 0.681 |
| Resection type (lobectomy/pneumonectomy) | 442/73 | 1.508 | 1.123–2.025 | 0.006 | 1.141 | 0.825–1.578 | 0.425 |
| Histologic type (SqCC/adenocarcinoma/others) | 244/207/64 | 1.087 | 0.928–1.273 | 0.301 | |||
| T stage (T1/T2/T3) | 179/284/52 | 1.344 | 1.129–1.599 | 0.001 | 1.098 | 0.908–1.328 | 0.333 |
| Venous invasion (negative/positive) | 396/119 | 0.657 | 0.514–0.841 | 0.001 | 0.741 | 0.571–0.962 | 0.024 |
| Lymphatic invasion (negative/positive) | 183/332 | 1.333 | 1.065–1.669 | 0.012 | 1.129 | 0.880–1.448 | 0.341 |
| Lymph node metastasis (negative/positive) | 283/232 | 2.254 | 1.808–2.810 | 0.000 | 2.180 | 1.743–2.727 | 0.000 |
| Adjuvant chemotherapy (none/yes) | 245/270 | 0.839 | 0.675–1.043 | 0.114 | |||
| Adjuvant radiotherapy (none/yes) | 416/99 | 0.708 | 0.537–0.933 | 0.016 | 0.749 | 0.672–1.004 | 0.063 |
| F-NLR (0/1/2) | 175/194/146 | 1.433 | 1.245–1.649 | 0.000 | 1.216 | 1.033–1.432 | 0.019 |
| GPS (0/1/2) | 380/94/41 | 1.394 | 1.181–1.646 | 0.000 | 1.136 | 0.955–1.350 | 0.149 |
3.5 F-NLR as predictive factor for the selection of treatment pattern in NSCLC patients
By subgroup analysis, the 5-year DFS rates in the surgery and surgery + adjuvant therapy (radiotherapy and chemotherapy) groups differed significantly for the F-NLR 2 patients and were 22.5%, 34.8%, respectively (p = 0.028). The 5-year OS rates in above two groups were 24.3%, 37.0%, respectively (p = 0.020). And there was no significant difference for both the DFS and OS in the F-NLR 0/1 patients. Results were presented in Figure 5.
3.6 F-NLR and GPS as prognostic factors
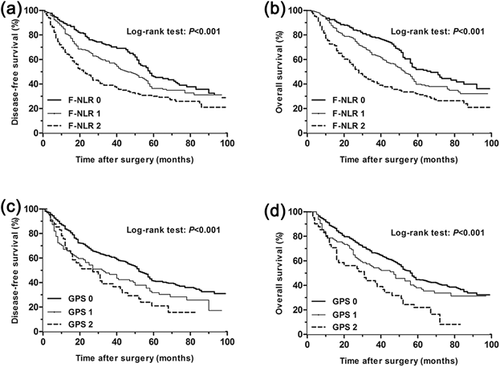
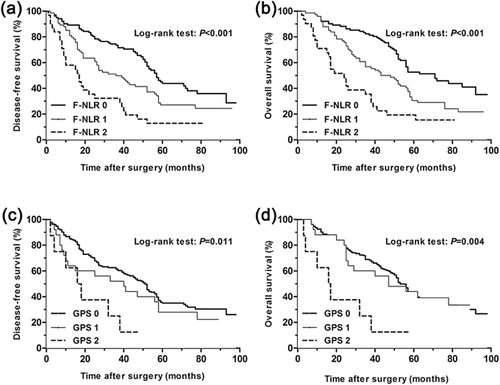
For all the patients, the 5-year disease free survival (DFS) rates in F-NLR groups 0, 1, and 2 differed significantly and were 46.7%, 36.4%, 30.1%, respectively (p = 0.000, Figure 2). The 5-year overall survival (OS) rates in F-NLR groups 0, 1, and 2 were 52.0%, 39.8%, 32.1%, respectively (p = 0.000, Figure 2). The 5-year disease free survival rates in GPS groups 0, 1, and 2 were 41.6, 31.8, and 21.0%, respectively (p = 0.000, Figure 2), and the 5-year overall survival rates for the three groups were 44.7%, 38.1%, 22.0%, respectively (p = 0.000, Figure 2). Furthermore, subgroup analysis also indicated that the GPS and F-NLR scores were both significantly related to DFS and OS in the histologic type of squamous cell carcinoma and adenocarcinoma. Results were presented in Figures 3 and 4. A higher GPS or F-NLR score was associated with significantly poorer prognosis. Univariate analysis showed that F-NLR and GPS scores were both significantly related to DFS and OS (Tables 3 and 4), and multivariate analysis further proved the F-NLR score as an independent prognostic factor for both the DFS and OS (p = 0.034 and p = 0.019, respectively, Tables 3 and 4).

4 DISCUSSION
Inflammatory and immune cells are the basic constituents of the tumor microenvironment. A systemic inflammatory response of the tumor cells is important in the process of tumor growth, progression and metastasis by creating an advantageous microenvironment and suppressing antitumor immunity (Fridman et al., 2011). The systemic inflammatory response breaks balance of circulating white blood cell constituents (Satomi et al., 1995). Thus it influences the number of neutrophils and lymphocytes in the white blood cell during cancer progression. The neutrophil-lymphocyte ratio (NLR) has been previously demonstrated as a representative prognostic marker in various malignancies as a systemic inflammatory response (Li et al., 2014; Mano et al., 2013; Sharaiha et al., 2011). And many studies have confirmed the close relationship between hyperfibrinogenemia and tumor progression (Son et al., 2013; Takeuchi et al., 2007; Wang et al., 2014). Then the clinical value of combined serum fibrinogen and NLR (F-NLR) was further demonstrated in various studies (Arigami, Uenosono, Ishigami et al., 2016; Arigami, Uenosono, Matsushita et al., 2016). Therefore, we combined the serum fibrinogen and NLR together as F-NLR to assess its association with clinicopathological characteristics and prognosis. To the best of our knowledge, this is the first study to investigate its clinical value in NSCLC patients.
Several recent studies have analyzed GPS as a potential predictor of the responses to chemotherapy, radiotherapy, and prognosis in various malignancies (Crumley, McMillan, McKernan, McDonald, & Stuart, 2006; Leitch, Chakrabarti, & Crozier, 2007; McMillan, Crozier, & Canna, 2007). It was first studied by Forrest et al. as a new prognostic factor in patients with advanced NSCLC in 2003 based on the serum CRP and albumin levels (Forrest et al., 2003). In addition, Yotsukura et al. (2016) has shown that GPS is associated with survival of resectable stages I and II NSCLC patients.
Based on these previous studies, we retrospectively collected the preoperative data from 515 patients with resectable non small cell lung cancer. The present study identified a significant association between F-NLR and clinicopathological characteristics, including the sex, smoking history, lesion type, histologic type, T stage, venous invasion, lymphatic invasion, and TNM stage. And we also found that GPS was significantly associated with the clinicopathological characteristics which were similar to F-NLR. This may indicate that the ability of F-NLR to predict tumor growth, progression and metastasis is similar to GPS.
In order to evaluate whether high-risk patients based on F-NLR 2 would benefit from adjuvant therapy compared with surgery alone, we performed a Kaplan–Meier analysis and log-rank test. The results of the high-risk subgroup demonstrated a significance difference in both DFS and OS (p = 0.028 and p = 0.020, respectively) (Figure 5). And this may indicate that patients with a high-risk preoperative F-NLR group would benefit from adjuvant therapy.

In the further univariate and multivariate analyses, we demonstrated that F-NLR was an independent predictive factor for both DFS and OS in patients with NSCLC, and the patients with a high F-NLR score seemed to have a poor prognosis compared with the patients with low F-NLR score. The GPS was both significantly related to DFS and OS in the univariate analysis. However, the GPS was not an independent predictive factor for both DFS and OS in the further multivariate analyses, which was different from the results of Yotsukura et al. (2016). This may due to our smaller sample data or the addition of stage IIIA patients. The subgroup analysis indicated that the GPS and F-NLR scores were both significantly related to DFS and OS in the histologic type of squamous cell carcinoma and adenocarcinoma, and further studies will be needed to elucidate the detailed biomechanism.
Although we could not draw a positive conclusion for GPS in the multivariate analyses, our results suggested that F-NLR and GPS have value as clinical predictors for the clinicopathological characteristics and prognosis upon resectable NSCLC patients. The fact that these markers can be analyzed using conventional equipment makes them practical and inexpensive. However, the potential limitations of the current study included the use of a retrospective analysis and a small sample size. In addition, we included patients with the stages I, II, and IIIA, which may cause a selection bias. Thus, larger prospective studies will be needed to confirm these preliminary results.
In conclusion, a grading system based on the F-NLR score, as well as the GPS, has clinical value as a predictive factor of the prognosis upon resectable non small cell lung cancer. We hope that this economical and practical index will serve as a useful biomarker for planning therapeutic strategies for patients with resectable non small cell lung cancer.
CONFLICTS OF INTEREST
The authors declare that there is no conflicts of interest regarding the publication of this article.




