LncRNA DGCR5 promotes lung adenocarcinoma (LUAD) progression via inhibiting hsa-mir-22-3p
Abstract
Long non-coding RNAs (lncRNAs) serve critical roles in the pathogenesis of various cancers, including lung adenocarcinoma (LUAD). Herein, in this study, we aimed to investigate the biological and clinical significance of lncRNA DiGeorge syndrome critical region gene 5 (DGCR5) in LUAD. It was observed that DGCR5 was upregulated in LUAD tissues and LUAD cell lines. Inhibition of DGCR5 can prevent LUAD progression via playing anti-apoptosis roles. Both mRNA expression and protein levels of BCL-2 were increased by DGCR5 downregulation while reversely BAX was increased. Additionally, a novel microRNA target of DGCR5, hsa-mir-22-3p was identified through bioinformatics search and confirmed by dual-luciferase reporter system. Gain and loss-of-function studies were performed to verify whether DGCR5 exerts its biological functions through regulating hsa-mir-22-3p in vitro. Overexpression of DGCR5 was able to reverse the tumor inhibitory effect of hsa-mir-22-3p mimics. Furthermore, in vivo tests tumor xenografts were established to detect the function of DGCR5 in LUAD tumorigenesis. Downregulated DGCR5 expression was greatly associated with smaller tumor size, implying a favorable prognosis of LUAD patients. Taken these together, DGCR5 could be considered as a prognostic biomarker and therapeutic target in LUAD diagnosis and treatment.
1 INTRODUCTION
Lung cancer is still the most common cause of death in both men and women all around the world (Siegel, Naishadham, & Jemal, 2013). Non-small cell lung cancers (NSCLCs) accounts for almost 80 percent of lung cancers of which about 50% are LUAD. Despite improvements have been made in early diagnosis possibly by increasing technologies and newly developed therapies, the 5-year overall survival for LUAD patients remains low and the recurrence rate is still unsatisfactory (Martin & Leighl, 2017). It is of critical importance for us to elucidate the molecular mechanisms involved in LUAD development and identifying novel prognostic biomarkers and therapeutic targets for LUAD.
Recently, it has been reported that more than 90% of the transcripts from the human genome could not code for proteins (Wilusz, Sunwoo, & Spector, 2009). Long non-coding RNAs (lncRNAs) are a kind of non-protein coding transcripts with over 200 nucleotides in length (Wahlestedt, 2013). They are widely expressed in human cells and play crucial roles in various biological events, such as cell-cycle regulation, genomic expression, and cell differentiation (Ballantyne et al., 2016; Luo et al., 2015; Tang, Jiang, et al., 2015). Increasing evidence has revealed that aberrant expression of lncRNAs might be associated with the development and progression of various cancer types (Bolha, Ravnik-Glavač, & Glavač, 2017). For instance, lncRNA UCA1 can promote gall bladder cancer progression through epigenetically inhibiting p21 and E-cadherin expression (Cai et al., 2017). LincIN, a novel NF90-binding lncRNA, is upregulated in advanced breast tumors and involved in metastasis (Jiang et al., 2017). Additionally, many lncRNAs are biomarkers in lung cancer. MALAT-1, which can promote lung cancer metastasis to the brain via epithelial-mesenchymal transition was highly expressed and associated with poor prognosis in NSCLC (Shen et al., 2015). HOTAIR was overexpressed in advanced stage lung cancers and promote cancer cell migration and aggression, and was correlated with metastasis and poor prognosis (Liu et al., 2013; Nakagawa et al., 2013). LncRNA DiGeorge syndrome critical region gene 5 (DGCR5), also known as Linc00037, is down-regulated in Huntington's disease neurodegeneration (Johnson, 2012). Down-regulation of DGCR5 is associated with poor prognosis in hepatocellular carcinoma (Huang et al., 2016). However, few studies have been reported about the role of DGCR5 in LUAD. We observed that DGCR5 was upregulated in LUAD when consulting a cancer microarray database, Oncomine (p < 0.05, https://www.oncomine.org/resource/login.html).
MiRNAs, a class of short (18–24 nt), single stranded and noncoding RNAs, are involved in regulating gene transcription and expression via directly binding with the target mRNAs (Iorio & Croce, 2017). Recently, accumulating articles have revealed that lncRNAs exhibit potential function of interacting with microRNAs (miRNAs) as a sponge and regulate their expression and activity (Bayoumi et al., 2016; Yoon, Abdelmohsen, & Gorospe, 2014). In human breast cancer, lncRNA CRNDE activates Wnt/β-catenin signaling pathway through acting as a molecular sponge of microRNA-136 (Huan, Xing, Lin, Xui, & Qin, 2017). Key lncRNAs have been identified as competing endogenous RNAs for miRNA-mRNA in LUAD (Li, Ainiwaer, Sheyhiding, Zhang, & Zhang, 2016). In NSCLC, NEAT1 can upregulate EGCG-induced CTR1 and enhance cisplatin sensitivity (Jiang, Wu, Wang, Huang, & Feng, 2016). c-Myc-activated lncRNA H19 can downregulate miR-107 and promote cell cycle progression of non-small cell lung cancer (Cui et al., 2015). In pancreatic ductal adenocarcinoma, reciprocal regulation of DGCR5 and miR-320a can influence the cellular malignant phenotype and 5-FU response (Yong et al., 2017). By using some bioinformatics analysis, hsa-mir-22-3p was predicted to be a target gene of DGCR5. It is reported that hsa-mir-22-3p can regulate expression of the long non-coding RNA MEG3 in acute myeloid leukemia (Yao, Cao, et al., 2017). The endothelium is protected against ox-LDL-induced dysfunction by lncRNA MALAT1 via upregulation of hsa-mir-22-3p target genes CXCR2 and AKT (Tang, Jin, et al., 2015). H19 activates Wnt signaling and promotes osteoblast differentiation as a miRNA sponge for hsa-mir-22-3p, which is a negative regulator of osteogenesis and Wnt/β-catenin pathway (Liang et al., 2016). However, it is still unclear about the correlation between DGCR5 and hsa-mir-22-3p in LUAD.
The aim of this study is to investigate whether DGCR5 is associated with LUAD and identify the role of DGCR5 in the diagnosis or prognosis in LUAD. Our current study was designed to explore the underlying mechanisms of DGCR5 function in LUAD. We speculated that lncRNA-DGCR5 can serve as a competing endogenous RNA (ceRNA), which was involved in the progression and prognosis of LUAD through the inhibition of hsa-mir-22-3p both in vivo and in vitro. Therefore, DGCR5 can be a potential biomarker in the diagnosis of LUAD.
2 MATERIALS AND METHODS
2.1 Cell culture
Human lung carcinoma A549 cells, H460 cells, H1299 cells human normal lung epithelial cells BES-2B and HEK-293T cells were obtained from American Type Culture Collection (ATCC). All the cells were cultured in RPMI 1640 medium with 10% heat-inactivated fetal bovine serum (FBS, GIBCO, Carlsbad, CA), 100 U/ml penicillin and 100 mg/ml streptomycin supplemented. Cells were grown at 37°C in an appropriate incubator containing 5% CO2.
2.2 Lentiviral vector construction, production, and transfection
Human DGCR5 full- length cDNA was amplified by PCR from the mRNA of LUAD cells. Then, the shDGCR5 sequences were designed with shLuc as the negative control (NC). The objective products were cloned into pcDNA3.1 (Invitrogen, Carlsbad, CA). The constructed vectors and the lentivirus packaging vectors (pMD2.G, pMDL- G/P-RRE) were cotransfected into A549 and H1299 cells for 48 h, respectively. Lentiviruses were produced, harvested, and purified with ultracentrifugation. A549 and H1299 cells seeded in 24-well plates were transfected with lentivirus, using 8 µg/ml polybrene (Sigma, St. Louis, MO). Stable expression cells were screened in a medium containing 800 µg/ml G418 (Sigma). LV-shDGCR5, LV-DGCR5, LV-hsa-mir-22-3p or their corresponding negative control was transduced into LUAD cells. Hsa-mir-22-3p mimics, inhibitors or their parental negative controls were transfected into the LUAD cells or HEK-293T cells using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA).
2.3 CCK8 assay
The lentivirus infected A549 and H1299 cells (50,000 cells/well) were seeded in a 96-well plate and were incubated at 37°C. At 24, 48, 72, 96, and 120 hr, respectively, 100 µl of CCK8 solution (Dojindo Molecular Technologies, Japan) was added to each well. After 4 hr, a microplate reader (Bio Tek Instruments, Bio-Tek, Winooski, VT) was used to test the absorbance at 450 nm.
2.4 Flow cytometric analysis of the cell apoptosis
The treated A549 and H1299 cells were harvested and stained with FITC-Annexin V and Propidium iodide (PI). The images of cell apoptosis were obtained using the FACS Calibur (BD Biosciences, San Jose, CA) and analyzed using the Flowjo software (Tree Star Corp).
2.5 Quantitative real-time reverse transcription PCR (qRT-PCR)
Total RNA was extracted using the RNAiso Plus (TaKaRaBio Technology, Dalian, China). RNA reverse transcribed by the Prime Script TM RT Master Mix (TaKaRa Bio Technology), and qPCR was performed by SYBR Premix Ex Taq II (TaKaRaBio Technology, Dalian, China). The primers used for qRT-PCR are described in Table 1. Specific primers for hsa-mir-22-3p were obtained from RiboBio (Guangzhou, China). Human U6 RNA served as an internal microRNA control and GAPDH was used as an internal mRNA control. The Applied Biosystems 7300 RealTime PCR System (Applied Biosystems, Foster City, CA) was employed to do Real-time PCR. The fold change was calculated by the equation 2−△△Ct.
| Genes | Forward(5′–3′) | Reverse(5′–3′) |
|---|---|---|
| GAPDH | CAAGGTCATCCATGACAACTTTG | GTCCACCACCCTGTTGCTGTAG |
| U6 | CTCGCTTCGGCAGCACA | AACGCTTCACGAATTTGCGT |
| DGCR5 | CACGAGTGTAGTGCCCAGTT | GGTCAGGGACCTTTGTCGTT |
| BCL-2 | AGCGTCAACGGGAGATGTC | GTGATGCAAGCTCCCACCAG |
| BAX | CAGCTCTGAGCAGATCATGAAGACA | GCCCATCTTCTTCCAGATGGTGAGC |
2.6 Bioinformatics analysis
ChipBase (http://www.lncipedia.org), LncRNAdb (http://www.lncrnadb.org/), and StarBase (http://starbase.sysu.edu.cn/) were used to identify specific microRNAs regulated by DGCR5.
2.7 Luciferase activity assay
The wild-type (WT) or mutant (MUT) DGCR5 binding hsa-mir-22-3p was synthesized and subcloned into pGL3 Basic vector (Promega, Madison,WI). A total of HEK-293T cells were seeded on 24-well plates for 48h. Mimics or inhibitors of hsa-mir-22-3p (RiboBio, Guangzhou, China) were cotransfected with 10 µg pLUC-WT-DGCR5 or pLUC-MUT-DGCR5 with Lipofectamine 2000 reagent (Invitrogen). Luciferase activity was measured by the Dual-Luciferase Reporter Assay System (Promega, Madison,WI).
2.8 Protein extraction and Western blot
A lysis buffer, including a protease inhibitor cocktail (Sigma–Aldrich) was used to do protein extraction. Equal proteins were loaded and separated on 10% sodium dodecyl sulfate polyacrylamide gels. The separated proteins were transferred onto PVDF membrane (Millipore, Billerica, MA). The primary antibodies with the target proteins were used to incubate the PVDF membranes at 4°C overnight. The HRP-conjugated secondary antibodies were employed to incubate the treated PVDF membranes for 1 hr at room temperature. The primary antibodies used in our study were as followed: anti-Cleaved-Caspase-3, anti-BCL-2 (1:500, Abcam, Cambridge, Britain), anti-BAX (1:500, Abcam), and anti-GAPDH (1:500, Abcam). Protein bands were quantified using Eagle Eye II software.
2.9 Nude mouse xenograft studies
Thirteen mice (BALB/c, nude, female, aged 6 weeks, weighed 16–18 g, purchased from Shanghai Animal Laboratory Center) were housed in the Experimental Animal Center. The lights were turned on at 7:00 am and turned off at 5:00 pm in the center, with a 22 ± 1°C temperature and 55 ± 5% humidity. Six mice were injected subcutaneously in the front dorsum with parental A549 cells (5 × 106 each) and the other seven were injected with A549 infected with LV-shDGCR5. Two weeks later, tumor sizes were recorded every three days. Tumor lengths and widths were measured using calipers. Tumor volumes were calculated using: volume (mm3) = length × width × width/2. After 6 weeks, all mice were euthanized by cervical dislocation and tumors were collected. This study was performed in strict accordance with the requirements in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
2.10 Statistical analysis
All data are recorded as the mean ± SD. Comparison between groups was analyzed by a Student's t test or one-way analysis of variance (ANOVA). Differences were considered significant when the p value was less than 0.05. (*p < 0.05, ** p < 0.01). The SPSS 18.0 (SPSS, Inc, Chicago, IL) and GraphPad Prism v6.0 (Graphpad Software, Inc) were used to perform statistical analysis.
3 RESULTS
3.1 LncRNA-DGCR5 is significantly overexpressed in LUAD tissues and LUAD cells
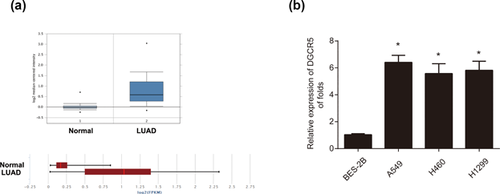
Firstly, to study the expression of lncRNA-DGCR5 in human LUAD tissues, we analyzed DGCR5 expression in LUAD tumor tissues and normal tissues provided by consulting a cancer microarray database, Oncomine (https://www.oncomine.org/resource/login.html), we observed that DGCR5 was greatly upregulated in LUAD tumors compared to the normal lung tissues derived from Selamat Lung (Figure 1a). Next, DGCR5 expression in LUAD cell lines including A549, H460, and H1299 cells was significantly overexpressed in comparison to human normal lung epithelial cell line BES-2B cells (Figure 1b). These data indicate that DGCR5 may play an oncogenic role in LUAD progression.
3.2 DGCR5 promotes the progression of LUAD cells
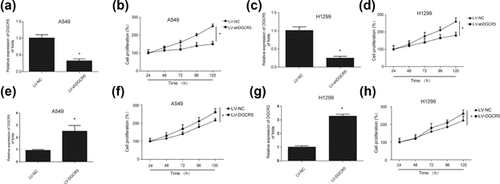
In order to investigate whether DGCR5 can influence the proliferation of LUAD cells, CCK8 assay was performed. CCK8 assay is often used to determine cell proliferation. LV-shDGCR5 was employed to knockdown the expression of DGCR5 in A549 cells and H1299 cell. A549 cells and H1299 cells were infected with LV-NC or LV-shDGCR5, qRT-PCR analysis exhibited the silencing effect (Figures 2a and 2c). We observed that the cell proliferation was significantly restrained after DGCR5 was inhibited in LUAD cells (Figures 2b and 2d). Reversely, when the cells were overexpressed with DGCR5 using LV-DGCR5 (Figures 2e and 2g), the cell surviving fraction was greatly promoted (Figures 2F and 2h). These results manifest that downregulation of DGCR5 can inhibit LUAD progression while upregulation of DGCR5 promotes LUAD progression respectively.
3.3 Decreased DGCR5 induced LUAD cell apoptosis in vitro
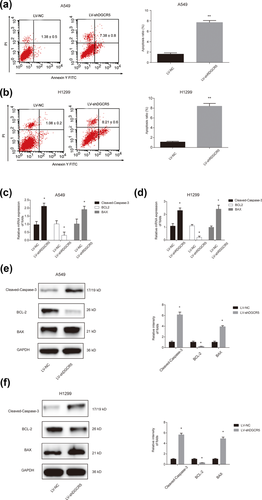
To explore the potential mechanism of LUAD progression regulated by DGCR5, flow cytometry was used. Our study indicated that apoptosis capacity of A549 cells and H1299 cells transfected LV-shDGCR5 was significantly increased when compared with the negative control group (Figures 3a and 3b). The ratios of apoptosis were greatly significant. In addition, Cleaved-Caspase-3, apoptosis-suppressing gene BCL-2 and apoptosis-promoting gene BAX were detected by qRT-PCR. In Figures 3c and 3d, it was exhibited that mRNA expression of BCL-2 was inhibited and BAX, Cleaved-Caspase-3 mRNA expression were increased in LUAD cells after DGCR5 was silenced. The result of Cleaved-Caspase-3, BCL-2, and BAX protein levels was consistent with Cleaved-Caspase-3, BCL-2, and BAX mRNA expression results in Figure 3e and 3f. These results reveal that decreased DGCR5 inhibits LUAD via suppressing cell apoptosis in vitro.
3.4 Hsa-mir-22-3p is a direct target of DGCR5
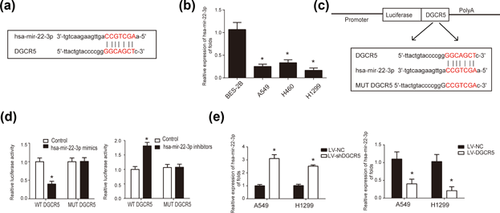
More and more reports have reported the interaction between lncRNA and microRNA. Therefore, to investigate the microRNAs regulated by DGCR5, bioinformatics analysis, including ChipBase, LncRNAdb and StarBase were carried out. According to these databases, we identified hsa-mir-22-3p as a target gene of DGCR5. The complementary binding sites between hsa-mir-22-3p and DGCR5 were shown in Figure 4a. Next, hsa-mir-22-3p expression in LUAD cell lines including A549, H460, H1299 cells was significantly downregulated compared to human normal lung epithelial cell line BES-2B cells (Figure 4b). This indicated a negative correlation between DGCR5 and hsa-mir-22-3p. To confirm that hsa-mir-22-3p is a direct target of DGCR5, we constructed luciferase reporter plasmids incorporating cDNA sequences of wild type DGCR5 and mutant hsa-mir-22-3p binding site (Figure 4c). Cotransfection of the luciferase reporter plasmid containing the wild type with hsa-mir-22-3p mimics in HEK-293T cells resulted in decreased reporter activity compared to the control. However, an increased reporter activity was observed when cotransfection of the luciferase reporter plasmid containing the wild-type with hsa-mir-22-3p inhibitors was performed (Figure 4c). Besides these, in Figure 4d to detect the altered expression of hsa-mir-22-3p regulated by DGCR5 in LUAD cells, DGCR5 knock down and DCGR overexpresion was carried out. From these results, we can speculate that hsa-mir-22-3p serves as a direct target of DGCR5.
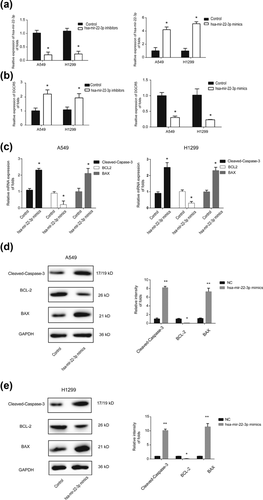
3.5 DGCR5 inhibits the apoptosis of LUAD cells via regulating hsa-mir-22-3p
To evaluate whether DGCR regulates the apoptosis of LUAD cells through hsa-mir-22-3p, hsa-mir-22-3p mimics, inhibitors, or their corresponding negative controls were transfected into LUAD cells. In Figure 5a, qRT-PCR was used to test the expression of hsa-mir-22-3p. Hsa-mir-22-3p mimics decreased DGCR expression and while hsa-mir-22-3p inhibitors can increase DGCR expression, respectively (Figure 5b). Next, we detected the expression of Cleaved-Caspase-3, BCL-2, and BAX. It was observed that hsa-mir-22-3p mimics inhibited BCL-2 mRNA expression and promoted BAX, Cleaved-Caspase-3 mRNA expression (Figure 5c). Moreover, protein level of BCL-2 was downregulated by hsa-mir-22-3p mimics and reversely BAX, Cleaved-Caspase-3, were upregulated by hsa-mir-22-3p overexpression in LUAD cells. This implied that hsa-mir-22-3p played a tumor suppressive role in LUAD progression. These results indicate that DGCR5 is able to inhibit the apoptosis of LUAD cells via regulating hsa-mir-22-3p.
3.6 DGCR5 reverses the inhibitory effects of hsa-mir-22-3p in LUAD progress in vitro
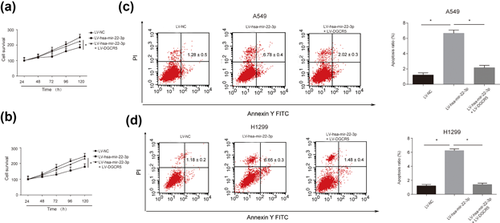
Furthermore, to test whether the inhibitory effects of hsa-mir-22-3p could be reversed by DGCR5, cotransfection of LV-hsa-mir-22-3p and LV-DGCR5 was carried out in LUAD cells. CCK8 assay in Figures 6a and 6b demonstrated that overexpressing DGCR5 was able to inhibit the suppressive role of hsa-mir-22-3p in LUAD progression. Besides this, flow cytometry showed that LV-hsa-mir-22-3p induced LUAD cell apoptosis and DGCR5 could reverse this phenomenon in both A549 cells and H1299 cells (Figures 6c and 6d). The apoptosis ratios exhibited a significant difference. These results suggest that DGCR5 can reverse the inhibitory effects of hsa-mir-22-3p in LUAD progress in vitro.
3.7 Downregulation of DGCR5 inhibits LUAD progress in vivo
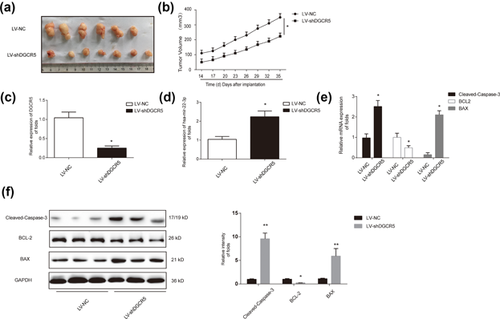
An A549 cell nude mouse xenograft model was established to determine whether or not downregulation of DGCR5 can inhibit LUAD progress in vivo. Two groups were set up in nude mice. Six Mice were injected subcutaneously in the front dorsum with parental A549 cells (5 × 106 each) and the other seven mice were injected with A549 infected with LV-shDGCR5. Tumors were peeled from nude mice subcutis (Figure 7a). In agreement with in vitro tests, DGCR5 inhibition group exhibited a better inhibitory effect on repressing tumor growth in a time-dependent manner (Figure 7b). Through upregulating hsa-mir-22-3p, LV-shDGCR5 group showed an anti-apoptosis role in vivo (Figures 7c and 7d). Meanwhile, DGCR5 silencing decreased BCL-2 and increased BAX, Cleaved-Caspase-3in mRNA levels and protein levels (Figures 7e and 7f). These data suggest that downregulation of DGCR5 could inhibit LUAD progress in vivo.
4 DISCUSSION
A growing number of studies have indicated that lncRNAs can determine some cellular processes in cancer, including proliferation, metastasis, and differentiation (Eades et al., 2014; Jiang et al., 2011). Many earlier studies have presented that lncRNAs are associated with carcinogenesis, metastasis, prognosis, and diagnosis, and function as oncogenes or cancer suppressor genes (Chen et al., 2017; Jen et al., 2017; Nie et al., 2017). Herein, more studies are required to elucidate the biological and molecular mechanisms of lncRNAs on cancer. In our current study, we observed that DGCR5 expression was higher in LUAD tissues than in normal tissues. DGCR5 was also increased in LUAD cells compared to the normal lung epithelial cells. Besides this, it was also found that DGCR5 accelerated the progression of the LUAD and can inhibit LUAD cell apoptosis. Therefore, we hypothesized that DGCR5 had an oncogene-effect in the development and progression of LUAD.
Studies have shown that BCL-2 prevented apoptosis in various cancer types (Yao, Sun, et al., 2017; Han et al., 2015). For another, BAX has been proved to induce process of apoptosis in some kind of cancers (Kang et al., 2017; Liu et al., 2014). It was demonstrated in our study that DGCR5 upregulated the expression of BCL-2 and downregulated the expression of BAX, Cleaved-Caspase-3 both in vitro and in vivo. This further confirmed that DGCR5 accelerated the progression of LUAD by inhibiting apoptosis. To our knowledge, this is the first report to show that DGCR5 can be regulated by hsa-mir-22-3p in LUAD.
Recently, a number of studies have shown that miRNAs can play significant roles in the biological process, such as cell proliferation, metastasis, and inflammation by targeting corresponding mRNAs in numerous cancers (Djuranovic, Nahvi, & Green, 2011; Ma et al., 2017; Xu et al., 2017). For instance, miR-23a can promote TGF-β1-induced EMT and tumor metastasis in breast cancer cells through targeting CDH1 and activating Wnt/β-catenin signaling (Ma et al., 2017). MicroRNA-1296 can inhibit metastasis and epithelial-mesenchymal transition of hepatocellular carcinoma by inhibiting SRPK1-mediated PI3K/AKT pathway (Xu et al., 2017). In lung cancer cells, microRNA-664 is able to promote the tumor proliferation, migration, and invasion (Zhu et al., 2017). In our study, a novel microRNA target of DGCR5, hsa-mir-22-3p was identified through bioinformatics search and confirmed by dual-luciferase reporter system. Currently, it was found in our investigation that hsa-mir-22-3p can modulate expression of DGCR5. Hsa-mir-22-3p mimics can inhibit DGCR5 expression while hsa-mir-22-3p inhibitors reversed it, which indicated a negative correlation between hsa-mir-22-3p and DGCR5. Further research on mechanism of hsa-mir-22-3p regulating DGCR5 is warranted. The expression rate of hsa-mir-22-3p in cancerous tissues was significantly decreased in gastric cancer (Jafarzadeh-Samani et al., 2017). Hsa-mir-22-3p can promote apoptosis of osteosarcoma cells by inducing cell cycle arrest (Gai et al., 2017). Via targeting CD147, hsa-mir-22-3p has shown an inhibitory role in hepatocellular carcinoma cell migration and invasion (Luo et al., 2017). However, the molecular mechanism of hsa-mir-22-3p in LUAD remains unknown. In our study, we showed that hsa-mir-22-3p downregulated the expression Bcl-2 and upregulated BAX in vitro. Furthermore, it was also confirmed that hsa-mir-22-3p inhibited the proliferation of LUAD and induced LUAD cell apoptosis. MicroRNA bioinformatics are needed to find out the target mRNA gene of hsa-mir-22-3p.
Recent studies have demonstrated that interactions between lncRNAs and miRNAs are involved in a wide range of human carcinomas (Ergun & Oztuzcu, 2015; Liz & Esteller, 2016; Tay, Rinn, & Pandolfi, 2014). For example, the lncRNA GAS5 suppresses cell growth and epithelial-mesenchymal transition in osteosarcoma through repressing the miR-221/ARHI Pathway (Ye et al., 2017). LncRNA UCA1 can promote renal cell carcinoma proliferation via epigenetically repressing p21 expression and negatively regulating miR-495 (Lu et al., 2017). For another, microRNA-26a inhibits proliferation and metastasis of human hepatocellular carcinoma by regulating DNMT3B-MEG3 axis (Li et al., 2017). In lung cancer, by regulating lncRNA nuclear enriched abundant transcript 1 (NEAT1), microRNA-449a inhibits lung cancer cell growth (You et al., 2014). As reported previously, in pancreatic ductal adenocarcinoma, reversed interaction between DGCR5 and miR-320a can affect the cellular malignant phenotype and 5-FU response (Yong et al., 2017). From these, we can observe that there is a complex regulation network between lncRNAs and miRNAs involved in a variety of cancers. In our study, we observed a binding region between DGCR5 and hsa-mir-22-3p. Our study showed that DGCR5 could function as a competing endogenous lncRNA in LUAD for sponging hsa-mir-22-3p. Hsa-mir-22-3p and DGCR5 appear to negatively regulate each other. Further studies are warranted to elucidate the DGCR5/hsa-mir-22-3p regulation network and determine whether DGCR5 mediates hsa-mir-22-3p directly. Besides this, more studies can focus on whether DGCR5 can interact with other target microRNA genes.
Our findings in LUAD cell lines and xenografts support the use of DGCR5 as an oncogene to promote LUAD development. This is the first reporting of a possible mechanism for a crosstalk between DGCR and hsa-mir-22-3p crosstalk in LUAD. We showed that when DGCR5 was silenced, the expression level of hsa-mir-22-3p was significantly upregulated in vitro and in vivo, indicating that DGCR5 negatively regulated hsa-mir-22-3p transcription through targeted binding. These results suggest DGCR5 as a potential target for LUAD treatment.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.




