Mammalian target of rapamycin as a therapeutic target in osteoporosis
Abstract
The mechanistic target of rapamycin (mTOR) plays a key role in sensing and integrating large amounts of environmental cues to regulate organismal growth, homeostasis, and many major cellular processes. Recently, mounting evidences highlight its roles in regulating bone homeostasis, which sheds light on the pathogenesis of osteoporosis. The activation/inhibition of mTOR signaling is reported to positively/negatively regulate bone marrow mesenchymal stem cells (BMSCs)/osteoblasts-mediated bone formation, adipogenic differentiation, osteocytes homeostasis, and osteoclasts-mediated bone resorption, which result in the changes of bone homeostasis, thereby resulting in or protect against osteoporosis. Given the likely importance of mTOR signaling in the pathogenesis of osteoporosis, here we discuss the detailed mechanisms in mTOR machinery and its association with osteoporosis therapy.
1 INTRODUCTION
Osteoporosis is known as a representative age-associated human musculoskeletal disease that presents increased morbidity and mortality and major socioeconomic burden (Sattui & Saag, 2014; Ström et al., 2011; Willson, Nelson, Newbold, Nelson, & LaFleur, 2015). To our knowledge, imbalance of skeletal homeostasis, an active coupling process that occurs via bone formation and bone resorption, results in osteoporosis (Hendrickx, Boudin, & Van Hul, 2015). To date, multifarious signaling pathways and critical molecules have been digged out to explore the mechanism of osteoporosis, like canonical Wnt/β-catenin pathway (Baron & Kneissel, 2013; Canalis, 2013; Lerner & Ohlsson, 2015) and RANKL/RANK pathway (Crockett, Mellis, Scott, & Helfrich, 2011; Lorenzo, 2017; Luo, Ren, Li, Lian, & Lin, 2016). However, the detailed pathogenesis of osteoporosis remains elusive. Currently, bisphosphonates and the anabolic agent teriparatide, which can suppress bone resorption and stimulate bone formation, respectively, are recommended as first-line drugs for osteoporosis (Compston et al., 2017; Cosman et al., 2014; Ip et al., 2013; Papaioannou et al., 2010; Qaseem, Forciea, McLean, Denberg, & Clinical Guidelines Committee of the American College of Physicians, 2017; Watts et al., 2010) (Table 1). However, providing effective therapeutic strategy remains a challenge, due to the intricate molecular mechanisms, the ambiguous therapeutic targets, inherent inadequacies and severe adverse events, such as rash, a decrease in weight, sciatica, and asthma after bisphosphonates therapy (Kennel & Drake, 2009), and nausea, diarrhea, insomnia, pharyngitis, hypercalcemia, and viral infection after teriparatide therapy (Saag et al., 2007). Thus, new therapeutic strategies for osteoporosis treatment are still needed.
| Organizations | Osteoporosis patients | Pharmacologic approaches | References |
|---|---|---|---|
| Clinical Guidelines Committee of the American College of Physicians (ACP) | Women:
(1) pharmacologic therapy for 5 years; (2) 65 years of age or older who are at a high risk for fracture based on a discussion of patient preferences, fracture risk profile, and benefits, harms, and costs of medications. |
(1) Alendronate, risedronate, zoledronic acid, or denosumab to reduce the risk for hip and vertebral fractures;
(2) Menopausal estrogen therapy or menopausal estrogen plus progestogen therapy or raloxifene in osteoporotic women. |
Qaseem et al. (2017) |
| Men who have clinically recognized osteoporosis. | Bisphosphonates to reduce the risk for vertebral fracture. | ||
| National Osteoporosis Guideline Group (NOGG). | (1) Postmenopausal women age 50 years or over;
(2) Continuation of bisphosphonate treatment beyond 3–5 years in individuals age ≥75 years, those with a history of hip or vertebral fracture, those who sustain a fracture while on treatment, and those taking oral glucocorticoids. |
(1) First-line therapy: Alendronate or risedronate;
(2) The most appropriate alternatives: intravenous bisphosphonates or denosumab if patients are intolerant of oral bisphosphonates or in whom they are contraindicated; (3) Additional options: raloxifene or hormone replacement therapy; (4) Teriparatide for those at very high risk, particularly for vertebral fractures. |
Compston et al. (2017) |
| Men age 50 years or over. | (1) First-line therapy: Alendronate or risedronate;
(2) The most appropriate alternatives: zoledronic acid or denosumab if first-line drugs are contraindicated or not tolerated; (3) Additional options: teriparatide. |
||
| National Osteoporosis Foundation (NOF) | (1) Patients with hip or vertebral (clinical or asymptomatic) fractures;
(2) Patients with T-scores ≤−2.5 at the femoral neck, total hip, or lumbar spine by DXA; (3) Postmenopausal women and men age 50 and older with low bone mass (T-score between −1.0 and −2.5, osteopenia) at the femoral neck, total hip, or lumbar spine by DXA and a 10-year hip fracture probability ≥3% or a 10-year major osteoporosis-related fracture probability ≥20% based on FRAX model. |
Bisphosphonates (alendronate, ibandronate, risedronate, and zoledronic acid), calcitonin, estrogen agonist/antagonist (raloxifene), estrogens and/or hormone therapy, tissue-selective estrogen complex (conjugated estrogens/bazedoxifene), parathyroid hormone 1–34 (teriparatide), and receptor activator of nuclear factor kappa-B (RANK) ligand inhibitor (denosumab). | Cosman et al. (2014) |
| The Osteoporosis Society of Hong Kong (OSHK) | Young postmenopausal women below 65 years of age and without a history of hip fracture. | (1) The preferred treatment: selective estrogen receptor modulator.
(2) Hormone replacement therapy may also be considered in postmenopausal women in the age range of 50–59 years or less than 10 years post-menopause. |
Ip et al. (2013) |
| Postmenopausal women aged 65 years or older. | (1) First-line therapy: bisphosphonates (alendronate, risedronate or zoledronic acid) or denosumab;
(2) Oral alternative for patients with a history of upper gastrointestinal disorders that may be a contra-indication for oral bisphosphonate therapy: Strontium ranelate. |
||
| Postmenopausal women with a more severe type of osteoporosis. | Teriparatide. | ||
| Scientific Advisory Council of Osteoporosis Canada | (1) Previous hip or spine fractures or multiple fractures;
(2) Treatment if 10-year risk of major osteoporotic fractures is>20%. (Risk assessment using the 2010 tool of the Canadian Association of Radiologists and Osteoporosis Canada). |
Women:
(1) Alendronate, risedronate, zoledronic acid and denosumab can be used as first-line therapies for prevention of hip, nonvertebral and vertebral fractures; (2) Raloxifene can be used as a first-line therapy for prevention of vertebral fractures; (3) Menopausal osteoporotic women with treatment for vasomotor symptoms, hormone therapy can be used as first-line therapy for prevention of hip, nonvertebral and vertebral fractures. (4) For menopausal women intolerant of first-line therapies, calcitonin or etidronate can be considered for prevention of vertebral fractures. |
Papaioannou et al. (2010) |
| Men:
(1) Alendronate, risedronate and zoledronic acid can be used as first-line therapies for prevention of fractures; (2) Testosterone is not recommended. |
|||
| American Association of Clinical Endocrinologists (AACE) Osteoporosis Task Force | (1) Those patients with a history of a fracture of the hip or spine;
(2) Patients without a history of fractures but with a T-score of −2.5 or lower; (3) Patients with a T-score between −1.0 and −2.5 if FRAX (see section 4.5) major osteoporotic fracture probability is ≥20% or hip fracture probability is ≥3%. |
(1) First line of therapy: alendronate, risedronate, zoledronic acid, and denosumab;
(2) Second-line agent: ibandronate; (3) Second- or third-line agent: raloxifene; (4) The last line of therapy: calcitonin; (5) Teriparatide for patients with very high fracture risk or patients in whom bisphosphonate therapy has failed; (6) Advise against the use of combination therapy. |
Watts et al. (2010) |
The mechanistic target of rapamycin (mTOR) is identified as a cellular key signaling used to respond to diverse environmental stimulus, control numerous processes that generate or consume a mass of energy and nutrients, regulate most major cellular functions, and assist most organisms in efficiently transformation between anabolic and catabolic states (Mathieu et al., 2012; Shimobayashi & Hall, 2014). For example, it plays an important role in regulating basic cell behaviors like growth and proliferation (Fingar & Blenis, 2004). Considering the complicated role of mTOR signaling in the context of cellular homeostasis, it is not surprising that its deregulation has been implicated in the pathogenesis of several human diseases, such as cancer (Chiarini, Evangelisti, McCubrey, & Martelli, 2015), obesity (Yang et al., 2012), type 2 diabetes (Suhara, Baba, Shimada, Higa, & Matsui 2017), and neurodegeneration (Zheng et al., 2016). Importantly, recently, inhibition of mTOR signaling is found to extend lifespan in model organisms and confer protection against mounting age-related diseases. Thus, drugs that targeting the mTOR signaling are expected to become widely used to reduce age-related pathologies in humans in the future (Johnson, Rabinovitch, & Kaeberlein, 2013).
Although mounting studies focus on the effect of mTOR signaling in bone homeostasis, the clinical applications of the activators/inhibitors of mTOR signaling on osteoporosis treatment have not been fully elucidated yet. Based on recent advances, the regulation of mTOR signaling may be the potential molecular mechanism of osteoporosis and may represent a novel therapeutic target. Therefore, based on prior knowledge, here we describe the biology of the mTOR signaling and further summarize its role in regulating bone homeostasis to identify potential mTOR-related therapeutic target for the pharmacological control of osteoporosis.
2 THE BIOLOGY OF mTOR SIGNALING
mTOR acts as the target of a molecule named rapamycin, also known as sirolimus, which is a macrolide produced by Streptomyces Hygroscopius bacteria. Till date, rapamycin and several derivative compounds (including everolimus, temsirolimus, ridaforolimus, umirolimus, and zotarolimus) have been applied for a variety of researches (Johnson, Rabinovitch, & Kaeberlein, 2013). mTOR is an atypical serine/threonine protein kinase that belongs to the phosphoinositide-3-OH kinase (PI3 K)-related kinase family, which principally regulating cellular growth and metabolism in response to the fluctuation of nutrition and hormone (Stanfel, Shamieh, Kaeberlein, & Kennedy, 2009). It interacts with several proteins to form two distinct complexes named mTOR complex 1 (mTORC1) and 2 (mTORC2).
These two complexes show different sensitivities to rapamycin as well as upstream inputs and downstream outputs (Benjamin, Colombi, Moroni, & Hall, 2011). mTORC1 comprises deptor, PRAS40, raptor, mLST8, mTOR, and TTI1-TEL2, and mTORC2 is comprised of deptor, mLST8, protor, rictor, mSIN1, mTOR, and TTI1-TEL2. Rapamycin binds to the FK506-binding protein FKBP12 and directly inhibits mTORC1 by disrupting the interaction between mTOR and raptor. Indirectly, chronic exposure of rapamycin sequesters mTOR from mTORC2 and inhibits mTORC2 assembly, which may be associated with metabolic complications, including glucose intolerance, and abnormal lipid profiles (Lamming et al., 2012).
However, previous studies about the upstream regulation and downstream outputs of mTORC1 are more than that of mTORC2. mTORC1 is activated by insulin, environmental nutrients (like amino acids), and other growth factors through PI3 K and AKT kinase signaling (Mathieu et al., 2012), and is inhibited by AMP-activated protein kinase (AMPK), a key sensor of cellular energy status. It can regulate lipid synthesis mainly through sterol-regulatory-element-binding protein transcription factors, while this mechanism is not completely understood. In addition, mTORC1 promotes protein synthesis through activation of the translation initiation promoter ribosomal protein S6 kinases (S6Ks) and through inhibition of the inhibitory mRNA cap binding 4E-binding protein 1 (4E-BP1). mTORC1 also regulates glycolysis and mitochondrial function through the hypoxic response transcription factor HIF-1α (Shimobayashi & Hall, 2014). Additionally, mTORC2 can inhibit FOXO3a through S6K1 and AKT. The complex also regulates actin cytoskeleton assembly through protein kinase C α (PKCα), Rho GTPases, and Ras proteins (Mathieu et al., 2012; Johnson et al., 2013) (Figure 1).

Furthermore, mTORC1 is known as the upstream suppressor of macro-autophagy (hereafter referred to simply as autophagy), which involves the isolation of intracellular organelles/macromolecules and the subsequent degradation and reuse (Cecconi & Levine, 2008; Mizushima, 2007; Ravikumar et al., 2004). mTORC1 is activated by growth factors or excess of nutrition etc, and then inhibits autophagy by phosphorylating the Ulk1/2/FIP200/autophagy gene (Atg) 13 complex (Fullgrabe, Klionsky, & Joseph, 2014). The isolation process begins with the appearance of a phagophore (referred to as autophagosome), which can encapsulate the waste cytosolic components to be degraded into the vesicle surrounded by a double isolation membrane. The Atg 12-Atg 5-Atg 7 complex and the lipidation of microtubule-associated light chain protein 3 (LC3) act as vital roles during autophagosome formation. Finally, the autophagosome fuses with a lysosome for content degradation and recycling (Glick, Barth, & Macleod, 2010) (Figure 2).
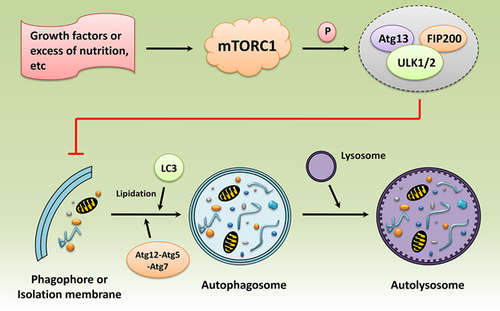
Therefore, mTOR signaling plays as a key regulatory nexus in modulating anabolic processes versus catabolic processes in response to nutrients, growth cues, and cellular energy status, and this process is important for cell growth, proliferation, survival, and homeostasis.
3 IMPLICATIONS OF mTOR SIGNALING IN OSTEOPOROSIS
On the basis of previous numerous types of clinical evidences (Bruyn et al., 2008; Lai et al., 2012; Su et al., 2009), mTOR signaling has been suggested as a new therapeutic target in various diseases, such as advanced stage renal cell carcinoma, breast cancer, tuberous sclerosis complex, several rare forms of cancer, systemic lupus erythematosus, rheumatoid arthritis, and systemic sclerosis (Garber, 2009; Mathieu et al., 2012; Vilar, Perez-Garcia, & Tabernero, 2011; Wander, Hennessy, & Slingerland, 2011). Recently, the effect of mTOR signaling on skeletal growth and development has also been widely explored. Several basic studies reveal that rapamycin can suppress the growth of body weight, alter the normal structure and dynamic equilibrium of growth plate, retard the growth of long bone, and influence fracture healing of femur (Álvarez-García et al., 2012, 2010, 2007; Holstein et al., 2008; Phornphutkul et al., 2009; Sanchez & He, 2009; Yang et al., 2015). Recently, anticancer synergy with the use of mTOR signaling inhibitors have been postulated in osteosarcoma treatment, suggesting that mTOR inhibition may also have a positive impact on bone (Moriceau et al., 2010). More importantly, preclinical studies have revealed that mTOR signaling is associated with bone loss (Spencer, Utting, Etheridge, Arnett, & Genever, 2006; Tchetina, Maslova, Krylov, & Myakotkin, 2015). However, more basic and clinical studies in elucidating the role of mTOR signaling in osteoporosis therapy are still required. This review will summarize recent studies on the role of mTOR signaling in bone homeostasis mediated by osteoblastic differentiation, adipogenic differentiation, osteocytes homeostasis, and osteoclast differentiation, and will highlight the potential therapeutic role of mTOR signaling in osteoporosis.
3.1 mTOR signaling in osteoblastic differentiation
Bone marrow mesenchymal stem cell (BMSC) acts as the precursor of osteoblast and adipocyte (Chen et al., 2016), and all of them are responsible for the forming, development, growth, and repair of bone. As reported, the impaired osteoblastogenesis along with the enhancement of adipogenesis may play a role in the imbalance of bone homeostasis, leading to decreased bone formation, bone mass loss, bone marrow fat accumulation, and ultimately osteoporosis (Burkhardt et al., 1987; Dong et al., 2014; Elbaz, Wu, Rivas, Gimble, & Duque, 2010; Griffith et al., 2006; Greco, Lenzi, & Migliaccio, 2015; Nuttall & Gimble, 2000; Yamaguchi, Komori, & Suda, 2000). Meanwhile, there is a reciprocal association and balance between the osteoblastic and adipogenic differentiation (Beresford, Bennett, Devlin, Leboy, & Owen, 1992). Currently, clinical anabolic drugs that enhance bone formation are limited to full-length parathyroid hormone (PTH 1–84) or its N-terminal fragment, teriparatide (PTH 1–34) (Canalis, 2010; Daddona, Matriano, Mandema, & Maa, 2011). However, both drugs have severe side effects as mentioned above (Kennel & Drake, 2009; Saag et al., 2007). Thus, it is necessary to hunt for more new therapeutic targets, which are associated with promoting osteoblastic differentiation.
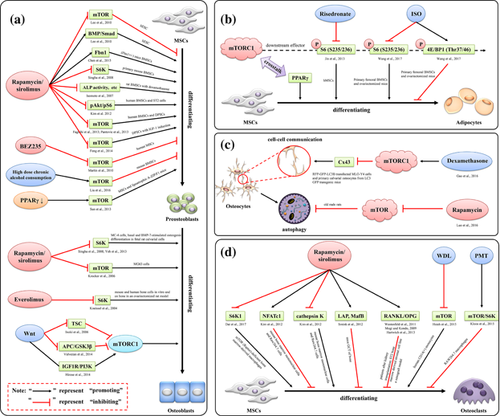
Though it is widely believed that the inhibition of mTOR signaling can promote osteoblastic differentiation, this still is a controversial issue up to now. A high-throughput small-molecule screening assay shows that rapamycin can promote osteoblast differentiation (Darcy et al., 2012). Simultaneously, rapamycin functions as a potent stimulator of osteoblastic differentiation of human embryonic stem cell (hESC), and it does so by inhibiting rapamycin-sensitive mTOR signaling and promoting BMP/Smad signaling (Lee et al., 2010). Likewise, blockage of mTOR signaling by osteoblastic-specific knockout or rapamycin treatment can rescue osteopenia phenotype in Fibrillin-1 (Fbn1)-deficient (Fbn1+/−) mice by improving osteoblastic differentiation and suppressing adipogenic differentiation of BMSCs (Chen et al., 2015). BEZ235, a newly developed dual PI3 K and mTOR inhibitor, has been reported to strongly promote osteoblastic differentiation in human MSCs by inhibiting PI3 K/mTOR signaling. In addition, BEZ235 can enhance de novo bone formation in calvarialorganotypic cultures (Martin et al., 2010).
In contrast, inhibition of mTOR signaling can also act as a negative role in regulating osteoblast differentiation. Rapamycin is reported to reduce the differentiation of MC3T3-E1 subclone4 (MC-4) cells and primary mouse BMSCs by down-regulating the expression of S6 K (Singha et al., 2008), rat BMSCs with dexamethasone intervention by decreasing alkaline phosphatase (ALP) activity, calcium content, and osteocalcin content (Isomoto et al., 2007), human BMSCs and ST2 cells by reducing the expression of pAKT/pS6 (Kim, Choi, Lee, Song, & Ji, 2012a). Similarly, sirolimus can reduce osteogenic differentiation of human BMSCs and osteoblasts (MG63 cells) by inhibiting mTOR signaling (Faghihi, Baghaban, Nekookar, Najar, & Salekdeh, 2013; Krocker et al., 2006). Everolimus, an oral mTOR signaling inhibitor, is reported to suppress osteoblastic differentiation on mouse and human bone cells in vitro and on bone in an ovariectomized rat model by inhibiting the expression of S6 K (Kneissel et al., 2004). Bone morphogenetic proteins (BMPs) can also promote osteoblast differentiation in vitro and in vivo, and its target is P70 ribosomal S6 K (P70S6 K), a downstream effector of mTOR. Additionally, rapamycin can inhibit basal and BMP-7-stimulated osteogenic marker expression, as well as bone nodule mineralization in fetal rat calvarial cells (Yeh, Ma, Ford, Adamo, & Lee, 2013).
Interestingly, the activation of mTOR signaling has also been found to promote osteoblast differentiation. IGF-1, a multifunctional peptide, activates mTOR signaling during osteogenic differentiation of human dental pulp stem cells (DPSCs), and rapamycin can block this process induced by IGF-1 (Feng et al., 2014). Also, AMPK can control osteogenic differentiation of DPSCs through both early mTOR inhibition (rapamycin)-mediated autophagy and late activation of AKT/mTOR signaling axis (Pantovic et al., 2013). Conversely, PI3 K/AKT/mTOR signaling cascade can be activated by high dose chronic alcohol consumption. And meanwhile, the reduced osteogenic differentiation, enhanced adipogenic differentiation of BMSCs, and osteopenia in a mouse model are observed (Liu et al., 2016).
As well known, the canonical Wnt/β-catenin signaling pathway is associated with osteogenic differentiation. It is initially activated by binding the Wnt ligand to Frizzled, the cell surface receptor, and low-density lipoprotein receptor-related protein 5 or 6, the co-receptors. In turn, the activated receptors result in the release of glycogen synthase kinase 3β (GSK3β) from the intracellular adenomatous polyposis coli (APC)-Axin complex. The GSK3β-mediated phosphorylation of β-catenin is then inhibited. Thereby, the transcription co-activator β-catenin is stabilized, and is translocated into the nucleus to induce the genes expression involved in osteoblasts differentiation (Gengyang et al., 2016; Niehrs, 2012). Recently, Wnt signaling is reported to promote mTORC1 activity by inhibiting the induction of the TSC complex (Inoki et al., 2006). In the absence of Wnt signalling, GSK3β can phosphorylate TSC2 at Ser1379 and Ser1383 in part through the AMPK-mediated phosphorylation of TSC2 at Ser1387. These phosphorylation events activate the TSC complex and thereby inhibit mTORC1. Additionally, studies in zebrafish and mice also reveal that Wnt and Frizzled can activate mTORC1 signaling through the loss of APC and the inhibition of GSK3β (Valvezan, Huang, Lengner, Pack, & Klein, 2014). Importantly, mTORC1 signaling can be activated by WNT7B, which has been implicated in embryonic and postnatal bone formation. And the inhibition of mTORC1 signaling in the osteoblast lineage alleviates the high-bone-mass phenotype induced by WNT7B, suggesting that WNT7B can promote bone formation in part through the activation of mTORC1 signaling (Karner, Esen, Okunade, Patterson, & Long, 2015). Similarly, mTORC1 signaling has been reported to promote osteoblast differentiation during regenerative outgrowth stage downstream of insulin-like growth factor 1receptor (IGF1R)/phosphatidylinositol-3 kinase (PI3 K) and Wnt signaling pathways in zebrafish caudal fin (Hirose, Shiomi, Hozumi, & Kikuchi, 2014). However, the implication of the crosstalk between mTOR signaling and Wnt/β-catenin signaling in osteoporosis remains indistinct, and more in vivo and in vitro studies are still necessary in the future (Figure 3).
Apart from regulating osteoblasts differentiation, mTOR signaling also acts as an excellent regulator in the proliferation, apoptosis, autophagy, energy metabolism, pleiotropic functions, and the maintenance of osteoblasts or osteoblast-like cells (Fan et al., 2015; Mori et al., 2008; Tassinary et al., 2015; Tseng, Yang, Lai, & Tang, 2010; Wang, Feng, Li, Chen, & Tang, 2016; Yang et al., 2013; Zeng, Jing, Zhang, Duan, & Xue, 2015). Therefore, mTOR signaling can be a positive or negative player in regulating osteogenic differentiation and bone formation, and this regulating process may be the potential pathophysiological mechanism of osteoporosis, suggesting that mTOR signaling activators/inhibitors may be the potential therapeutic drugs by targeting osteoblast differentiation.
3.2 mTOR signaling in adipogenic differentiation
As discussed above, adipogenic differentiation is also important for bone homeostasis. Previous studies have indicated that an excessive amount of bone marrow adipocytes may be a significant negative risk factor for skeletal health (Greco et al., 2015), suggesting that osteoporosis may be associated with increased adipose tissue in bone marrow (Burkhardt et al., 1987; Griffith et al., 2006; Nuttall & Gimble, 2000). Therefore, inhibition of adipogenic differentiation in bone marrow while simultaneously promoting osteogenesis may be a therapeutic approach for osteoporosis.
As reported, the inhibition of mTOR signaling can reduce adipogenic differentiation of BMSCs. Risedronate, a nitrogen-containing bisphosphonate (Bone et al., 2004; Harris et al., 1999), is reported to inhibit the phosphorylation of S6 on S235/236, a direct downstream effector of mTORC1, in adipogenic differentiated human MSCs (hMSCs) (Jin et al., 2013). Similarly, the phosphorylation of S6 (S235/236) can be inhibited and the phosphorylation of 4E/BP1 (Thr37/46) can be promoted by isopsoralen (ISO), the main active ingredient extracted from the seeds of Psoralea corylifolia, suggesting that ISO prevents BMSCs adipogenesis by inhibiting mTORC1 signaling (Wang et al., 2017).
Nuclear receptor peroxisome proliferator-activated receptor-γ (PPARγ) is an important transcription factor, which can reduce osteoblastic differentiation in BMSCs indirectly via working in an adipogenesis dependent manner, due to the reciprocal relationship between osteoblastic and adipogenic differentiation. Interestingly, PPARγ can also regulate osteogenesis in mouse and human osteoblasts directly (Jackson & Demer, 2000; Maurin, Chavassieux, & Meunier, 2005; Mbalaviele et al., 2000). One recent study indicates that osteoblast-targeted over-expression of PPARγ significantly reduces bone mass in mice (Cho et al., 2011). Also, over-expression of PPARγ induces the trans-differentiation of osteoblasts to adipocytes (Kim, Her, Kim, & Shin, 2005). Importantly, mounting evidences have shown that mTORC1 is critical for PPARγ-mediated adipogenesis (Cybulski, Polak, Auwerx, Rüegg, & Hall, 2009; Kim & Chen, 2004; Peterson et al., 2011; Polak et al., 2008; Xiang et al., 2013). Suppression of PPARγ is found to significantly increase osteoblast differentiation in MSCs and in lipoatrophic A-ZIP/F1 mice by activating mTOR signaling directly (Sun et al., 2013). Accordingly, the inhibition of mTOR signaling may play a negative role in regulating BMSC adipogenesis and may be the potential therapeutic target for osteoporosis.
3.3 mTOR signaling in osteocytes homeostasis
Osteocytes are the most abundant type of cells in bone. They are terminally differentiated and long-lived cells that are buried in the bone matrix and connected to one another and to the bone surface through an intercellular connections network via osteocytic dendritic processes (Bonewald, 2006). Osteocytes contribute bone mineral homeostasis, the local control of bone remodeling of their lacunae, and systemic phosphate regulation (Bonewald, 2011; Xiong et al., 2011). And in order to implement these functions, osteocytes synthesize and secrete plentiful specific regulatory proteins, such as SOST, RANKL, dentin matrix acidic phosphoprotein, matrix extracellular phosphoglycoprotein (MEPE), and fibroblast growth factor 23 (FGF23), which conducing to the regulation of calcium and phosphorus metabolism (Belanger, 1969; Bonewald, 2007; Feng, Ye, & Schiavi, 2009; Fukumoto, 2009; Fukumoto & Martin, 2009).
So far, studies about the role of mTOR signaling in regulating osteocytes homeostasis are still limited. Luckily, recent data show that mTOR signaling may participate in the cell–cell communication between osteocytes. As reported, Cx43 is a major hemichannel protein, which maintains dendritic connection between neighboring osteocytes (Bejarano et al., 2012; Doty, 1981) and bone homeostasis in vivo and in vitro (Bivi et al., 2012; Doty, 1981; Ishihara et al., 2008; Loiselle, Jiang, & Donahue, 2013; Watkins et al., 2011; Zhang et al., 2011). And dexamethasone-induced AKT-mTORC1 signaling is responsible for the degradation of connexin 43 (Cx43) in vivo and in vitro (Gao et al., 2016). After the activation of mTORC1 signaling, the dendritic processes were shortened and the dendritic connections were reduced, inducing impairment of cell-cell communication between osteocytes. In addition, recently, rapamycin is found to reduce the severity of age-related bone changes in trabecular bone of old male rats at least partly by activating autophagy of osteocytes, suggesting that mTOR signaling may present negative effect on osteocyte autophagy and rapamycin may be a feasible therapeutic approach for senile osteoporosis (Luo, Yang, et al., 2016). Therefore, mTOR signaling may present negative role in osteocytes homeostasis, but more detailed studies about the relationship between mTOR signaling and osteocytes homeostasis are necessary in the future.
3.4 mTOR signaling in osteoclast differentiation and bone resorption
Bone resorption is carried out by terminally-differentiated multinucleated cells, osteoclasts, which are derived from hematopoietic progenitors and differentiated from monocytes (Boyle, Simonet, & Lacey, 2003). This process is controlled by modulating the expression of osteoclast inducing a variety of cytokines, growth factors, and hormones, such as macrophage colony-stimulating factor (M-CSF) and receptor activator of NF-κB ligand (RANKL) (Asagiri & Takayanagi,, 2007; Leibbrandt & Penninger, 2008; Negishi-Koga & Takayanagi, 2009). Osteoclasts resorb bone by attaching to its surface, sealing off the ruffled border (Ma, Li, Hock, & Yu, 2012), and pumping protons and degradative enzymes into the sealed basolateral membrane, thereby dissolving mineral and decalcifying the bone matrix in the acidic environment.
As reported, inhibition of mTOR signaling can reduce osteoclast differentiation. In regulatory-associated protein of mTOR (Raptor)-deficient bone marrow-derived macrophages, lower mTORC1 signaling and retarded osteoclast differentiation are observed, and the impaired osteoclast differentiation can be rescued by using enforced expression of constitutively active S6K1. Furthermore, rapamycin can inhibit this process and osteoclast-specific gene expression by targeting S6K1 (Dai et al., 2017). Similarly, rapamycin can inhibit Epo-dependent and -independent osteoclast differentiation in mouse bone marrow mononuclear cells and Raw264.7 cells. Also, Epo can increase NFATc1 expression and decrease cathepsin K expression in an mTOR-independent manner, resulting in an increase of osteoclast numbers and a decrease in resorption activity (Kim, Jung, et al., 2012b). Rapamycin also decreases osteoclast differentiation in mice giant cell tumor (GCT) of bone by translationally regulating the balance between the transcription factor C/EBPβ long (LAP) and short (LIP) protein isoforms (Smink, Tunn, & Leutz, 2012). In addition, Sirolimus reduces serum levels of bone resorption markers in patients after kidney transplantation and profoundly reduce osteoclast differentiation and subsequently diminish hydroxyapatite resorption in vitro by regulating RANKL/OPG pathway (Westenfeld et al., 2011). Vitamin D (3) is reported to modulate mTOR signaling, and the combination of mTOR inhibitor and vitamin D (3) effectively inhibit osteoclast differentiation and function in chronic inflammatory condition, such as rheumatoid arthritis (RA), by increasing NFATc1 expression and decreasing cathepsin K expression (Kim et al., 2012). Wedelolactone (WDL), a natural coumarin isolated from plants, is found to inhibit breast cancer-induced osteoclast differentiation, which is generated from human CD14(+) monocytes cultured with M-CSF/RANKL, by decreasing AKT/mTOR signaling (Hsieh et al., 2015).
As well known, RANKL/RANK pathway is critical for osteoclast differentiation (Gengyang et al., 2016). In this signaling pathway, RANKL derived from osteoblast acts as a determinant of osteoclast-mediated bone resorption by binding to RANK on osteoclast precursors, and then is suppressed by osteoprotegerin (OPG), a decoy receptor for RANK ligand that can inhibit osteoclast differentiation (Benslimane-Ahmim et al., 2011; Rachner et al., 2009). As reported, rapamycin can induce the upregulation of OPG in the ST2 bone marrow derived stromal cell line (Mogi & Kondo, 2009). Additionally, in a xenograft model, increased OPG expression is correlated with a delay to pathologic fracture by inhibiting mTOR signaling (Hartwich et al., 2013).
Conversely, activation of mTOR/S6 K signaling by Pasteurella multocida toxin (PMT) has been reported to inhibit the differentiation of RAW264.7 macrophages into osteoclasts (Kloos et al., 2015). Also, rapamycin, alone or in synergy with transforming growth factor-beta (TGF-beta1), directly enhances osteoclast differentiation of RAW264.7 monocyte-macrophage cells in the presence of RANK-ligand (RANKL) (Shui, Riggs, & Khosla, 2002) (Figure 3).
mTOR signaling not only regulates osteoclasts differentiation, but also plays a critical role in mediating osteoclasts proliferation and survival (Álvarez-García et al., 2010; Cejka et al., 2010; Glantschnig, Fisher, Wesolowski, Rodan, & Reszka, 2003; Gnant et al., 2013; Hadji, Coleman, & Gnant 2013; Hussein, Tiedemann, Murshed, & Komarova, 2012; Ma et al., 2012; Sugatani & Hruska, 2005), which ulteriorly emphasizing the vital function of mTOR signaling in maintaining bone homeostasis. Overall, mTOR signaling serves as a double-edged sword in regulating osteoclast differentiation and bone resorption, suggesting that mTOR signaling may be potential molecular mechanism and therapeutic target of osteoporosis. However, the role of mTOR signaling in the interplay among osteoclasts with other cells (like BMSCs, osteoblasts, osteocytes, and adipocytes) and environmental conditions (like vasculogenesis) is still unclear.
Additionally, mTOR signaling can also regulate other cells which play role in skeletogenesis. Reportedly, rapamycin reduces the proliferation of chondrocytes, endotheliocytes, and periosteum cells, destroy mature and hypertrophic chondrocytes, and inhibits vasculogenesis of chondrocytes (Alvarez-Garcia et al., 2010; Álvarez-García et al., 2012, 2007; Holstein et al., 2008; Sanchez & He, 2009). Interestingly, bafilomycin A1 and chloroquine (both are inhibitors of autophagy) are found to activate mTORC1 signaling in autophagy-independent manner unexpectedly, which is observed exclusively in chondrocytes but not in osteoblasts, fibroblasts, or liver cells (Newton, Vuppalapati, Bouderlique, & Chagin, 2015).
4 IMPLICATIONS OF THE CROSSTALK BETWEEN mTOR SIGNALING AND OTHER SIGNALINGS IN OSTEOPOROSIS
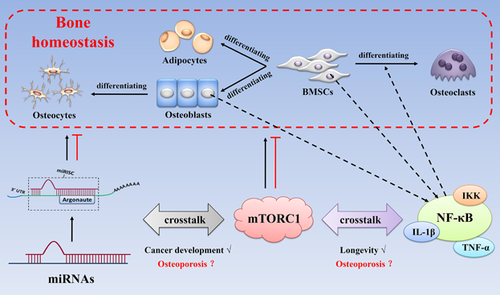
As discussed above, mTOR signaling participates in maintaining bone homeostasis by interacting with a series of molecular signalings, including canonical Wnt/β-catenin signaling pathway, PPARγ signaling, and RANKL/RANK pathway. Furthermore, recent studies reveal that the crosstalk between mTOR signaling and other novel signalings may provide new directions in exploring the potential mechanisms and therapeutic methods of osteoporosis.
MicroRNAs (miRNAs) are a class of short (19–25 nucleotides in length), endogenous, and noncoding RNAs that regulate the expression of a wide variety of genes at the posttranscriptional level by binding to 3′-untranslated regions (3′UTRs) of target messenger RNAs (mRNAs) (Kim, 2005; Kim, Han, & Siomi, 2009; Landgraf et al., 2007). They are also known as key regulators of a wide variety of pathophysiological processes, such as metabolism, cellular differentiation, proliferation, apoptosis, and autophagy (Alvarez-Garcia and Miska, 2005; Kloosterman & Plasterk, 2006). And the role of miRNAs in regulating bone formation and resorption has also been reviewed (Gengyang et al., 2016). Thus, it is clear that both miRNAs and mTOR signaling are crucial for maintaining bone homeostasis. Interestingly, recent studies reveal that there is interplay between miRNAs and the mTOR signaling during cancer development (Gibbings et al., 2012; Jing, Han, Sui, Xie, & Pan, 2015; Lan et al., 2014; Zhang, Huang, Wang, Chang, & Zheng, 2017), whereas a correlational report on osteoporosis is not yet available.
Inflammation participates in the development of several age-related disorders (Chung et al., 2009). And IKK/NF-κB signaling pathway is associated with the regulation of inflammation and innate and adaptive immune reactions (Hayden & Ghosh, 2011; Lawrence, 2009). Simultaneously, the fundamental roles of NF-κB signaling in osteoclast differentiation and activity have been well defined (Boyle et al., 2003; Lin et al., 2014; Xu et al., 2009), and the biological functions of NF-κB signaling activation in osteoblasts and MSCs have also been clarified (Chang et al., 2013, 2009; Cho et al., 2010; Lin et al., 2015), although the detailed regulation of their bone formation ability remains unclear. Moreover, by using transgenic animal models, the stimulation of proinflammatory cytokines (TNF-α, and IL-1β etc), or the TLR agonists, the roles of NF-κB signaling in bone homeostasis have been further illuminated (Hwa Cho, Bae, & Jung, 2006; Itoh et al., 2003; Lombardo et al., 2009; Mo et al., 2008; Takami, Kim, Rho, & Choi, 2002). Interestingly, recent studies show that the crosstalk between mTOR signaling and NF-κB signaling is associated with longevity and healthspan. As reported, IKKα and IKKγ interact with mTOR signaling directly, and the suppression of mTOR signaling and raptor by siRNA decrease the binding activity of NF-κB signaling (Dan et al., 2008). In addition, IKKα/β can activate mTOR signaling directly while suppressing TSC1 (Dan & Baldwin, 2008; Lee et al., 2007; Salminen & Kaarniranta, 2009). Although both the roles of mTOR signaling and NF-κB signaling in bone homeostasis have been revealed, studies about the effects of their crosstalk are still limited. Luckily, in a BOLERO-2 trial, breast cancer patients with bone involvement who received everolimus treatment have a delayed tumor progression in the skeleton as a result of direct osteoclasts suppression by interfering with the NF-κB signaling, in addition to the general suppressor effect on the cancer cells (Simone et al., 2015).
Therefore, the crosstalk of mTOR signaling with other novel signalings (like miRNAs and NF-κB, etc) may be the potential molecular mechanism of osteoporosis and may represent a novel therapeutic target for the pharmacological control of osteoporosis (Figure 4).
5 THERAPEUTIC POTENTIAL OF mTOR SIGNALING IN OSTEOPOROSIS
mTOR signaling is associated with aging and age-related diseases (Simon et al., 2013). And to date, some inhibitors of mTOR signaling have been applied in a series of clinical trials (Benjamin et al., 2011; Faivre, Kroemer, & Raymond, 2006). For example, sirolimus is initially approved by the FDA for preserving the function of renal allograft (Calne et al., 1989), and now there are more than 1,800 clinical trials have been registered (US National Library of Medicine, ) to test this drug in diverse disease conditions, like autoimmune, inflammatory, degenerative diseases, and cancer. Nevertheless, it is necessary to test them against a broad spectrum of age-related diseases. Interestingly, exploratory analyses in BOLERO-2 reveal that everolimus can suppress bone turnover levels, such as osteoclast metabolism (bone specific ALP), bone formation (amino-terminal propeptide of type I collagen), and bone resorption (C-terminal cross-linking telopeptide of type I collagen) markers, as well as reduce the incidence of breast cancer progression in bone, suggesting that everolimus may have a positive effect on bone health (Gnant et al., 2013). However, the role of mTOR signaling still represents a novel area of research in the osteoporosis field. Importantly, this paper indicates that mTOR signaling is deeply involved in the regulation of bone homeostasis. Given that the biological roles are crucial in bone tissues, activators/inhibitors of mTOR signaling may be the very attractive new therapeutics to prevent bone loss.
However, further studies are required if mTOR signaling-based therapeutics are to be used clinically, and many more questions are raised. For example, how to pinpoint the variation tendency (up/down regulation) of mTOR signaling in regulating bone homeostasis, as well as the optimal dosage and treatment duration of activators/inhibitors of mTOR signaling? What toxic adverse effects may occur with the employ of mTOR signaling activators/inhibitors (like mucositis, rash, increased risk of infections, hyperglycaemia, hyperlipidaemia, and hypophosphataemia, which have been observed in patients who have renal transplant with rapamycin or everolimus therapy (Brattström et al., 1998; Qi, Huang, Yao, Shen, & Min, 2013; Soefje, Karnad, & Brenner, 2011))? Then how to reduce the toxic side effects? How can mTOR signaling activators/inhibitors be effectively delivered in vivo? All of these questions will need to be answered in the future.
ACKNOWLEDGMENTS
This work was supported by National Natural Science Foundation of China [81503591, 81674000], Science and Technology Projects of Guangdong Province [2016A020226006, 2014A020221021], Natural Science Foundation of Guangdong Province [2016A030313645, 2014A030310082], Excellent Doctor Project of the First School of Clinic Medicine of Guangzhou University of Chinese Medicine [YB201602, YB201501], Excellent Young Scholars Project of the First Affiliated Hospital of Guangzhou University of Chinese Medicine [2015QN03], A grant from Guangzhou University of Chinese Medicine for Excellent Young Scholars Project [KAB110133K04] and Disciplinary Construction Fund of Education Department in Guangdong Province for Young Researcher Project [2013LYM-0012]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
DISCLOSURE
The authors have no conflict of interest to disclose.




