LncRNA-PVT1 promotes pancreatic cancer cells proliferation and migration through acting as a molecular sponge to regulate miR-448
Abstract
The identification and characterization of long non-coding RNAs (lncRNAs) in diverse biological process has currently developed rapidly. LncRNA-PVT1, located adjacent to the MYC locus on chromosomal region 8q24, has been reported to be associated with many biological processes. However, the function and mechanism of PVT1 in pancreatic carcinoma (PC) is poorly understood. In this present study, we first measured the level of PVT1 in the PC cell lines and tissues by quantitative real-time PCR (qRT-PCR), and then employed loss-of-function and gain-of-function approaches to explore the association between PVT1 expression levels and PC cell proliferation/migration ability. Furthermore, bioinformatics analysis was utilized to show that PVT1 contains binding site for miR-448 and an inverse correlation between PVT1 and miR-448 was obtained in PC specimens. Additionally, dual luciferase reporter assay, RNA-binding protein immunoprecipitation (RIP) and applied biotin-avidin pulldown system were applied to further confirm that PVT1 directly bind with microRNA binding site harboring in the PVT1 sequence. Then, SERBP1 was identified as a target of miR-448 according to the gene expression array analysis of PC clinical samples. Together, we revealed that PVT1 functions as an endogenous “sponge” by competing for miR-448 binding to regulate the miRNA target SERBP1 and, therefore, promotes the proliferation and migration of PC cells.
1 INTRODUCTION
Pancreatic carcinoma (PC) is the one of the most common and malignant cancer in the world. Due to its early aggressive nature and lacking of early diagnostic and prognostic markers, the overall prognosis remains unsatisfied, with 5-year survival rate to be ∼8% (Puleo et al., 2015; Siegel, Miller, & Jemal, 2016). Hence, it is urgent to explore the molecular mechanism underlying the molecular mechanisms of carcinogenesis and progression of PC and exploring targeted signaling pathways for cancer treatment.
Long non-coding RNAs (lncRNAs) are a type of RNA molecule larger than 200 nucleotides that do not encode proteins (Ponting, Oliver, & Reik, 2009). Recent reports highlighted the important role of lncRNAs in roles in gene regulation and various aspects of tumor cellular homeostasis, including tumor cell growth, development, differentiation, proliferation, apoptosis, and metastasis (Li et al., 2017; Niu et al., 2017; Xi & Song, 2016; Zuo et al., 2017). Long-noncoding RNA (lncRNA) plasmacytoma variant translocation 1 (PVT1), located at 8q24.21, has been reported aberrantly expressed in many human cancers. For instance, Zhou et al. (2016) showed that PVT1 could promote osteosarcoma development by acting as a molecular sponge to regulate miR-195; Huang et al. (2016) reported that PVT1 overexpression is a poor prognostic biomarker and regulates migration and invasion in small cell lung cancer (Huang et al., 2016); and in 2016, feedback loop of lncRNA-PVT1 and FOXM1 were confirmed to facilitate gastric cancer growth and invasion (Du et al., 2016). However, the expression level and biological function of PVT1 in PC still remains unknown.
The aim of current study is to investigate the role of PVT1 in the progression and migration of PC. Our findings show that PVT1 is significantly overexpressed in PC tissues and silencing of PVT1 impairs proliferation ability and migration capacity of PC cells. Moreover, for the first time, we identify an important role for VT1 in pancreatic cancer cells. Furthermore, our results suggested that PVT1 exhibited oncogenic activity through competitive sponging miR-448 and then regulating the expression of SERBP1, which might be novel signaling pathways for cancer treatment.
2 MATERIALS AND METHODS
2.1 Patients
Pancreatic samples were obtained from the Department of Surgery, The First Affiliated Hospital, Wenzhou Medical University. All patients did not receive chemotherapy or radiotherapy before the surgery. All samples were frozen in liquid nitrogen immediately after resection and stored at −80°C until use. This study was approved by the institutional review boards of all of the hospitals involved in the study, and the written informed consent was obtained from all patients.
2.2 Cell lines
Human PDAC cell lines PANC-1, AsPC-1, and HEK 293T cell were obtained from ATCC (the American Type Culture Collection, Manassas, VA). Patu 8988, BxPC-3, SW 1990 cells and normal pancreatic cells HPDE6-C7 were purchased from the Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences (Shanghai, China). All cell lines were cultured in 37°C in a 5% CO2 humidified in DMEM or RPMI 1640 (Gibco) with 10% fetal bovine serum (FBS, HyClone).
2.3 Cell cytoplasm/nucleus fraction isolation
NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific, Waltham, MA) was employed to prepare cytoplasmic and nuclear extracts from PDAC cells. RNAs extracted from each of the fractions were subjected to following RT-qPCR analysis to demonstrate the levels of nuclear control transcript (MALAT1), cytoplasmic control transcript (GAPDH), lncRNA PVT1, and miR-448.
2.4 Quantitative real time PCR analyses
The total RNA was extracted from the tissues or cultured cells using TRIzol reagent (Invitrogen, Carlsbad, CA), according to the manufacturer's instructions. Reverse transcription was performed with PrimeScript RT reagent Kit (Takara, Dalian, Liaoning, China). qRT-PCR was performed with SYBR Prime Script RT-PCR Kits (Takara, Japan) based on the manufacturer's instructions. The target gene expression level was calculated with the 2−ΔΔCt method, which was normalized to GAPDH mRNA. All assays were performed in triplicate. The expression levels were relative to the fold change of the corresponding controls, which were defined as 1.0.
2.5 Cell viability
Cell viability was assessed via 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyl-trtrazolium bromide (MTT) assay. 5 × 103 cells/well were seeded in a 96-well flat-bottomed plate for 24 hr, then transfected with corresponding sh-RNA or pc-DNA3.1 and cultured in normal medium. At 0, 24, 48, 72, and 96 hr after transfection, the MTT solution (5 mg/ml, 20 µl) was added to each well. Following incubation for 4 hr, the media was removed and 100 µl DMSO were added to each well. The relative number of surviving cells was assessed by measuring the optical density (O.D.) of cell lysates at 560 nm. All assays were performed in triplicate.
2.6 Colony formation assay
The indicated cells were plated into six-well plates (1 × 103 cells/well) and incubated in DMEM or RPMI 1640 with 10% FBS at 37°C. Two weeks later, cells were washed with PBS, fixed in methanol for 30 min and stained with 1% crystal violet dye, and the number of colonies was counted. All assays were performed in triplicate.
2.7 Cell migration and invasion assays
Cell migration were measured by transwell chamber (8 μm pore size, Corning). 48 hr after transfection, cells in serum-free media were placed into the upper chamber. Media containing 10% FBS was added into the lower chamber. Following 48 hr incubation, cells remaining in upper membrane were wiped off, while cells that migrated were fixed in methanol, stained with 0.1% crystal violet and counted under a microscope. Three independent experiments were carried out.
2.8 Wound healing assays
Cell migration capacity was calculated by wound healing assay. 2 × 105 cells with or without transfection were plated into 12-well plates and incubated in DMEM or RPMI 1640 with 10% FBS at 37°C. After reaching 100% confluence, cells were wounded by scraping with a 200 µl tip, following washed three times in serum-free medium and incubated in regular medium. Wounds were observed at 0 and 48 hr. The cell migration distance was calculated by subtracting the wound width at each time point from the wound width at the 0 hr time point. Three independent assays were assayed.
Cells were lysed in RIPA lysis buffer (P0013, Beyotime) and nuclear proteins were extracted using lysis buffer (P0028, Beyotime), all the procedures were following the manufacturer's protocol. Subsequently the cell lysates were boiled in 5× SDS–PAGE loading buffer for 10 min and then resolved by 8% SDS–PAGE and transferred to nitrocellulose membrane. The following antibodies were used in this study: SERBP1 was purchased from Cell signaling technology. GAPDH (Proteintech) was referred as internal reference. Bound antibodies were visualized with the ECL kit (P0018, Beyotime).
2.9 Dual luciferase reporter assay
Cells (293 T) were seeded at 3 × 104 cells/well in 24-well plates and allowed to settle overnight. The next day, cells were co-transfected with pmirGLO-PVT1-WT or -MUT reporter plasmids and miR-448 mimic. And also cells were co-transfected with pmirGLO-SERBP1-WT or -MUT reporter plasmids and miR-448 mimic. Twenty-four hours after transfection, the relative luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI) and normalized against Renilla luciferase activity.
2.10 RNA-binding protein immunoprecipitation
RIP experiments were performed using the Magna RIP RNA-binding protein immunoprecipitation kit (Millipore, Billerica, MA) and the Ago2 antibody (Abcam, Cambridge, MA) following the manufacturer's protocol. Co-precipitated RNAs were subjected to RT-qPCR analysis.
2.11 RNA-Pulldown assay
SW1990 cells were transfected with biotinylated miRNA, collected 48 hr after transfection. The cell lysates were incubated with M-280 streptaviden magnetic beads (Invitrogen). The bound RNAs were purified using TRIzol reagent (Invitrogen) for further RT-qPCR analysis.
2.12 Statistical analysis
Statistical analyses were performed using SPSS 17.0 software (SPSS, Chicago, IL). Differences between two groups were assessed using Student's t-test (two-tailed). Correlations between PVT1 and miR-448 or SERBP1 were analyzed by Spearman rank correlation. Each experiment was performed at least three times. Results of experiments are displayed as mean ± SD. A p-value <0.05 was considered to indicate statistical significance.
3 RESULTS
3.1 LncRNA-PVT1 is overexpressed in pancreatic cancer and was negatively correlates with clinical outcome of pancreatic cancer patients
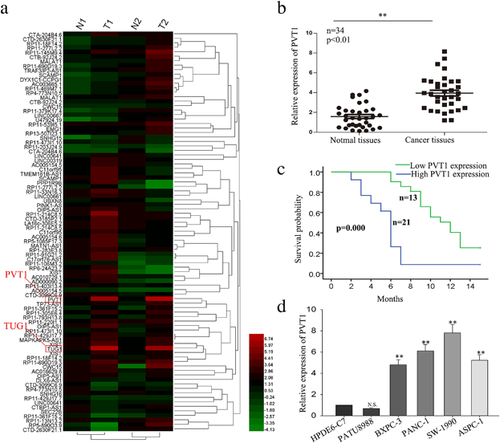
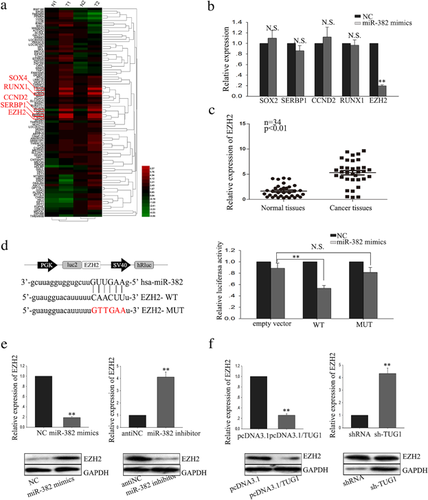
To explore the potential role of lncRNAs in regulating the progression of human PC, we previously conducted lncRNA expression array analysis using clinical samples (Figure 1a). The array results revealed that lncRNA-PVT1 and lncRNA-TUG1 were highly expressed in pancreatic tissues. The biology function of TUG1 has been discussed in our other study. Therefore, in the present study, we focus on the biology function of PVT1. To further confirm the expression level of PVT1, we assessed the PVT1 expression level in 34 paired PC tissues and corresponding adjacent pancreatic tissue samples by reverse transcription and quantitative real-time PCR (RT-qPCR). As present in Figure 1b, the expression level of PVT1 was significantly higher in tumor tissues. As shown in Table 1, the correlation between PVT 1 expression and clinical features revealed high level of PVT was correlated with large tumor size (p = 0.004), poor tumor differentiation (p = 0.001), TNM stage (p = 0.038), Vascular infiltration (p = 0.009), distant metastasis (p = 0.000), and negatively correlated with overall survival (OS) of patients with pancreatic cancer (Figure 1c), which indicated that upregulated PVT might contribute to the development of pancreatic cancer. Furthermore, we examined the expression level of PVT1 in five PC cell lines (PATU 8988, BxPC-3, PANC-1, SW 1990, AsPC-1) and a normal human pancreatic normal pancreatic epithelial cell line HPDE6-C7. Compared with HPDE6-C7 cells, PC cells exhibited significantly higher levels of PVT1 expression except for PATU 8988 cells (Figure 1d). Together, these results suggest that PVT1 is might be involved in the progression of PC.
| Lnc-TUG1 expression | |||
|---|---|---|---|
| Variable | Low | High | p-value |
| Age | |||
| <60 | 12 | 12 | 0.715 |
| ≥60 | 4 | 6 | |
| Gender | |||
| Male | 13 | 11 | 0.270 |
| Female | 3 | 7 | |
| Tumor size | |||
| <2 | 14 | 3 | 0.000 |
| ≥2 | 2 | 15 | |
| Histological grade | |||
| High/moderate | 13 | 1 | 0.000 |
| Low | 3 | 17 | |
| TNM stage | |||
| I–II | 11 | 2 | 0.001 |
| III–IV | 5 | 16 | |
| Lymphatic invasion | |||
| Positive | 13 | 13 | 0.693 |
| Negative | 5 | 5 | |
| Vascular infiltration | |||
| Positive | 4 | 17 | 0.000 |
| Negative | 12 | 1 | |
| Distant metastasis | |||
| Positive | 3 | 17 | 0.000 |
| Negative | 13 | 1 | |
- Low/high by the sample median. Pearson χ2 test. p < 0.05 was considered statistically significant.
3.2 PVT1 facilitates the proliferation and migration of PC cells
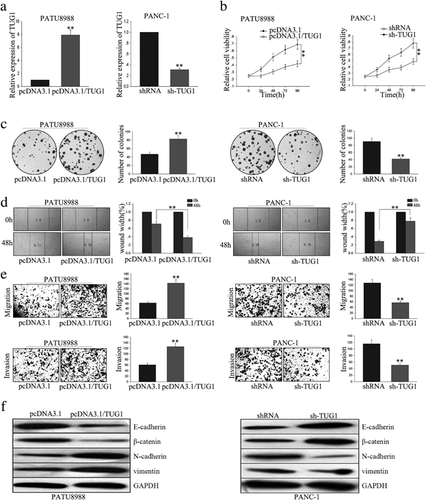
To begin identify the function of PVT1 on impacting cell proliferation and migration, we transfected PVT1 expression vector and shRNA/PVT1 into PATU 8988 and SW1990 cell lines, respectively. Satisfactory transfection efficiency was obtained after 48h as determined by RT-qPCR (Figure 2a). Results from MTT and colony formation assay showed that forced expression of PVT1 significantly enhanced the cells proliferation ability, on the contrary, PVT1 knockdown remarkably weakened the cells proliferation ability (Figure 2b,c). These data provide evidence of the growth-promoting role of PVT1 in vitro. Moreover, wound healing assay and transwell assay showed that overexpression of PVT1 facilitated cells migration, and vice versa in cells deletion of PVT1 (Figure 2d,e). Collectively, these results indicate that PVT1 involved in pancreatic cancer cells proliferation and migration.
3.3 PVT1 activity is partially dependent on its negative regulation of miR-448
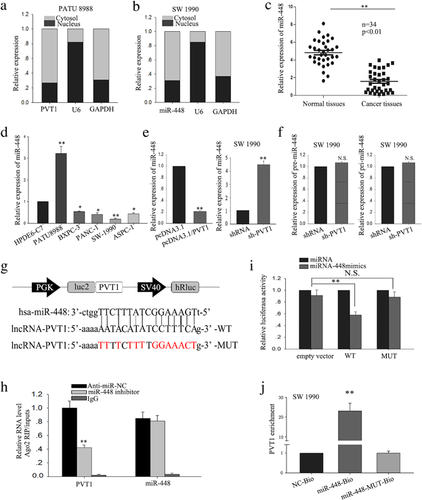
Besides characterizing the function of PVT1 on PC cells, we also sought to explore in further detail the mechanism by which PVT1 exerts its functions. Accumulating documents identified that many lncRNAs have been reported to function as competing endogenous (ce)RNAs by serving as sponges that bind and sequester away miRNAs (Wang et al., 2017; Wu et al., 2017; Xue, Ni, Jiang, & Li, 2017). To exam whether PVT1 exerts its function in PC cells through function as a ceRNA, we first measured the percentage of PVT1 in the cytoplasmic and nuclear fractions of PATU 8988 and SW1990 cells. As shown in Figure 3a, RT-qPCR of nuclear and cytoplasmic fractions of PATU 8988 and SW1990 cells present that PVT1 was located in the cytoplasm, providing prerequisite for function as a ceRNA. To dissect the underlying mechanism of PVT1, we applied starbase software (http://starbase.sysu.edu.cn/mirLncRNA.php) to predict the miRNAs bound to PVT1. We knockdown PVT1 by specifically sh-RNA in SW1990, and we found that down-regulated PVT1 had the most significant effect on miR-448 expression (Supplementary Table S1). Next, RT-qPCR of nuclear and cytoplasmic fractions of SW1990 cells validated that miR-448 was also located in the cytoplasm, indicating a potential reciprocal interaction between PVT1 and miR-328 (Figure 3b). Thus, we selected miR-448 as the next step research object. RT-qPCR showed that, in PC tissues, miR-448 was downregulated in the tumor specimens, which was inversely correlation with PVT1 (Figure 3c). Compared with HPDE6-C7 cells, PC cell lines displayed lower expression of miR-448, except for in PATU 8988 cells (Figure 3d). To further evaluate the potential regulating relationship between PVT1 and miR-448, PATU 8988, and SW1990 cells were transfected with PVT1 expression vector and shRNA/PVT1 to increase or decrease PVT1 expression, respectively. Forced expression of PVT1 resulted in a significant down-regulation of miR-448, while deletion of PVT1 significantly increased the level of miR-448 (Figure 3e). These results suggest that miR-448 was negatively regulated by PVT1. To confirm that PVT1 regulates miR-448 at a post-transcriptional level, the effect of PVT1 on pre-miR-448 and pri-miR-448 was measured (Figure 3f). And the results showed that PVT1 could not affect the level of pre-miR-448 and pri-miR-448. To explore whether PVT1-mediated miR-448 regulation through function as a ceRNA, we performed RNA-binding protein immunoprecipitation (RIP), dual luciferase reporter assay and applied biotin-avidin pulldown system. We sub-cloned full-length of PVT1 into the pmirGLO dual luciferase reporter vector and performed luciferase assays in SW1990 cell. As shown in Figure 3g, co-transfection of SW1990 cells with pmirGLO-PVT1-WT vector and miR-448 mimic significantly reduced luciferase reporter activity with respect to the negative control, while cells co-transfected with pmirGLO-PVT1-MUT vector and negative control showed a higher level of luciferase activity in comparison to the group of co-transfected with WT vector and negative control. And results from RIP showed that Ago2 protein was efficiently immunoprecipitated from cell extracts by Ago2 antibody (Figure 3h), which revealed that while PVT1 was detected in Ago2 immunoprecipitates from the control group, its levels were drastically reduced in Ago2 complexes purified from cells treated with miR-448 inhibitor, indicating that PVT1 is likely in the miR-448 RISC complex. Further, a biotin-avidin pulldown system was application to assay whether miR-448 could pull down PVT1. As shown in Figure 3j, PVT1 was pulled down by miR-448, but the bind site of PVT1 for miR-448 was mutated failure to pull down PVT1, indicating that PVT1 regulated miR-448 in a sequence-specific manner. Together, our findings revealed that PVT1 exerts inhibitory effects on miR-448 expression through function as a ceRNA and therefore directly sponging miR-448.
3.4 The function of PVT1 in PC cell could be abolished by miR-448
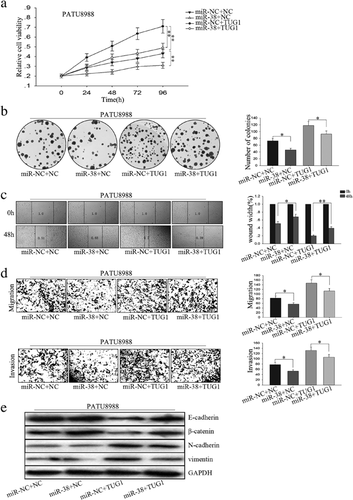
We transfected PATU 8988 with PVT1 expression vector and miR-448 mimic to study the effects of miR-448 on cell proliferation and migration mediated by PVT1. MTT and colony formation assay revealed that PVT1 promoted and miR-448 suppressed cell proliferation, while co-transfection of miR-448 mimic and PVT1 expression vector showed that PVT1 promoted cell proliferation was inhibited by miR-448 (Figure 4a,b). Moreover, results from wound healing assay and transwell assay demonstrated that PVT1 promoted and miR-448 suppressed PC cell migration, while co-transfection of miR-448 mimic and PVT1 expression vector showed that miR-448 abolished the migration-promoted function of PVT1 (Figure 4c,d). Those observations suggest that the effects of PVT1 overexpression on the promotion of PC cell proliferation and migration could be reversed by miR-448 mimics.
3.5 Knockdown of PVT1 inhibits SERBP1, a target of miR-448
Based on our previously conducted gene expression array analysis using clinical samples, we identified several genes (Figure 5a), which involved in PC progression and metastasis. To screen the appropriate target of miR-448, we first measured the identified genes expression in the SW1990 cells treated with miR-448 mimics. As present in Figure 5b, miR-448 showed significant effect on the level of SERBP1. RT-qPCR showed that, in PC tissues, SERBP1 was significantly up-regulated in the tumor specimens, which was positively correlation with PVT1 (Figure 5c). And bioinformatics analysis revealed that in the 3′UTR region of SERBP1 contains a bind site for miR-448 (Figure 5d, left). Moreover, results from dual luciferase reporter assay showed that co-transfection of SW1990 cells with pmirGLO-SERBP1-WT vector and miR-448 mimic significantly reduced luciferase reporter activity, on the contrary, cells co-transfected with pmirGLO-SERBP1-MUT vector and negative control showed a higher level of luciferase activity (Figure 5d, right). Furthermore, PATU 8988 and SW1990 cells were transfected with miR-448 inhibitor and miR-448 mimics to decrease or increase miR-448 expression, respectively. Overexpression of miR-448 led to a significant down-regulation of SERBP1, while knockdown of miR-448 significantly increased the level of SERBP1 both in mRNA and protein levels (Figure 5e). Additionally, results from RT-qPCR and western blot revealed that overexpression of PVT1 increased the level of SERBP1 while down-regulated of PVT1 led to reduction of SERBP1 both in miRNA and protein levels (Figure 5f). Collectively, these findings suggest that SERBP1 is a target of miR-448 and is positively regulated by PVT1.
3.6 Knockdown of SERBP1 suppresses the function of PVT1
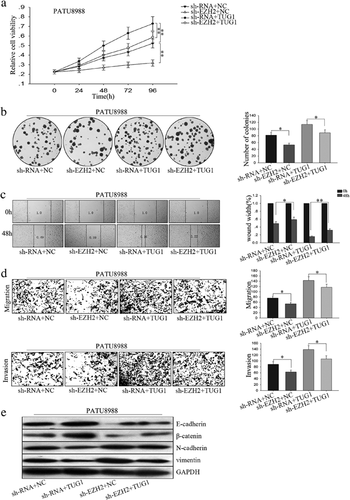
We transfected PATU8988 with PVT1 expression vector and sh-SERBP1 to study the effects of sh-SERBP1 on cell proliferation and migration mediated by PVT1. MTT and colony formation assay revealed that PVT1 promoted and sh-SERBP1 suppressed cell proliferation, while co-transfection of sh-SERBP1 and PVT1 expression vector showed that PVT1 promoted cell proliferation inhibited by sh-SERBP1 (Figure 6a,b). Moreover, results from wound healing and transwell assays demonstrated that PVT1 promoted and sh-SERBP1 suppressed PC cell EMT phenotype formation, while co-transfection of sh-SERBP1 and PVT1 expression vector showed that sh-SERBP1 reversed the EMT promoted by PVT1 to MET (Figure 6c,d). Those findings suggest that downexpression of SERBP1 inhibited PC cell proliferation and migration.
3.7 The expression level of PVT1 was negatively correlated with miR-448 and positively correlated with SERBP1 in PC tissues
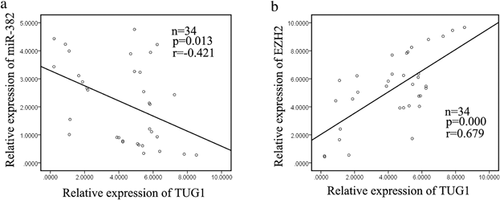
Finally, the relationship of the three molecules was analyzed. As shown in Figure 7a, miR-448 levels and PVT1 levels were negatively correlated (2-tailed Spearman's correlation, r = −0.560, p < 0.01). What's more, the level of SERBP1 showed a positively correlated with PVT1 levels (2-tailed Spearman's correlation, r = 0.64, p < 0.01; Figure 6b). Collectively, these data indicated that there is a regulatory signaling pathway in which PVT1 regulates SERBP1 by competitively sponging miR-448, inducing increased proliferation ability and migration capacity EMT phenotype formation PC cells (Table 1).
4 DISCUSSION
PC is one of the fetal and high-grade malignant cancers in the world (Siegel et al., 2016). During the past decade, despite tremendous efforts have been made to improve early detection and clinical outcomes of patients with PC, the overall prognosis remains very poor with ∼8% 5-year survival rate (Ansari, Gustafsson, & Andersson, 2015; Mohammed, Van Buren, & Fisher, 2014; Paulson, Tran Cao, Tempero, & Lowy, 2013; Siegel et al., 2016). Hence, exploring the underlying molecular mechanisms of carcinogenesis and cancer progression in PC is essential providing novel biomarkers for cancer detection and elucidating signaling pathways for cancer treatment. With the development of high-throughput DNA sequencing and array-based technologies, numerous new long non-coding RNAs (lncRNAs) were identified. Currently, mounting documents suggest that lncRNAs play critical roles in gene regulation and thus affect various aspects of tumor cellular homeostasis; for example, in 2017, Cui et al. (2017) reported that upregulated SNHG1 contributes to progression of non-small cell lung cancer through inhibition of miR-101-3p and activation of Wnt/β-catenin signaling pathway; and Sun et al. (2016) showed that long intergenic noncoding RNA 00511 acts as an oncogene in non-small-cell lung cancer by binding to EZH2 and suppressing p57. However, the function and molecular mechanism of lncRNAs in PC carcinogenesis are not well investigated. Investigating the underlying mechanism by which lncRNAs function in PC might facilitate the development of novel therapeutic targets. Therefore, investigating the mechanisms underlying the progression of PC might facilitate the development of novel treatments that improve patient prognosis.
PVT1, located adjacent to the MYC locus on chromosomal region 8q24, has been reported to be associated with many biological processes (Carramusa et al., 2007; Liu, Liu, Zhou, Mi, & Wang, 2015; Liu et al., 2016; Takahashi et al., 2014; Zhang, Bu, Liu, Zhang, & Li, 2015). Despite the implications of PVT1 in the regulation of a variety of transcription factors and physiological processes, the role of PVT1 in PC remains unexplored. In our study, according to the results of lncRNA expression array analysis of clinical PC samples and RT-qPCR of PC tissues, we demonstrated that the PVT1 is upregulated in PC. Furthermore, in comparison to the normal HPDE6-C cells, PVT1 expression was obviously increased in four PC cell lines except PATU 8988 cell lines. Then results of loss-of-function and gain-of-function approaches showed that PVT1plays a key role in PC cell proliferation and migration. Deletion of PVT1 significantly weakened PC cell growth ability and decreased cell migration capacity, while overexpression of PVT1 obtained opposite effects. Collectively, our findings suggested that PVT1 may function as an oncogene in PC, and its overexpression favors to PC development and progression. However, the detailed mechanism by which PVT1 functions in PC needed to be further investigated.
Recently, accumulating lncRNAs have been demonstrated as endogenous ceRNAs for specific miRNAs and regulate their function; for instance, in 2016, Lv et al. (2016) revealed that lncRNA Unigene56159 promotes epithelial–mesenchymal transition by acting as a ceRNA of miR-140-5p in hepatocellular carcinoma cells; and Wang et al. (2016) reported that lncRNA related to periodontitis interacts with miR-182 to upregulate osteogenic differentiation in periodontal mesenchymal stem cells of periodontitis patients. In our present study, we provide evidence that PVT1 may also function as ceRNA to competitively sponge miR-448 and affect its distribution on specific targets. Consistent to miR-448 mimic, deletion of PVT1 could suppress miR-448 target SERBP1, whereas ectopically forced expressed PVT1 inhibits miR-448 function, leading to suppression of its target gene SERBP1. Therefore, the influence of PVT1 on PC cells proliferation and migration could be attributed, at least partially, to function as a ceRNA competitively sponging miR-448. However, there are still flaws in our study. For example, the samples are too small and lacking in vivo assay. In our future study, we will enlarge our samples and further investigate the role of PVT1 in vivo.
Collectively, we show that the PVT1 is upregulated in PC tissues and cell lines. Its effects on cell proliferation and migration indicated its oncogenic property in PC tumorigenesis. In our study, PVT1 acts as a molecular sponge for miR-448 and regulates its target SERBP1. This reciprocal repression of PVT1 and miR-448 may highlight the important role of RNA-RNA interaction and provide novel insight into lncRNA-based mechanisms underlying various aspects of tumorigenesis.
ACKNOWLEDGMENTS
This study is supported by project co-sponsored by province and ministry (WKJ-ZJ-1706) and the technology innovation team of diagnosis and treatment of abdominal surgery of Wenzhou, Zhejiang Province, China (2016–354) and Zhejiang Provincial Natural Science Foundation of China (No. LY15H160056).
CONFLICTS OF INTEREST
No conflicts of interest to disclose.




