An Optimized CRISPR/Cas12a Assay to Facilitate the BRAF V600E Mutation Detection
Funding: This work was supported by the Tehran University of Medical Sciences (1401-3-366-57150).
ABSTRACT
Background
Accurate detection of the BRAF V600E (1799T > A) mutation status can significantly contribute to selecting an optimal therapeutic strategy for diverse cancer types. CRISPR-based diagnostic platforms exhibit simple programming, cost-effectiveness, high sensitivity, and high specificity in detecting target sequences. The goal of this study is to develop a simple BRAF V600E mutation detection method.
Methods
We combined the CRISPR/Cas12a system with recombinase polymerase amplification (RPA). Subsequently, several parameters related to CRISPR/Cas12a reaction efficiency were evaluated. Then, we conducted a comparative analysis of three distinct approaches toward identifying BRAF V600E mutations in the clinical samples.
Results
Our data suggest that CRISPR/Cas detection is considerably responsive to variations in buffer conditions. Magnesium acetate (MgOAc) demonstrated superior performance compared to all other examined additive salts. It was observed using 150 nM guide RNA (gRNA) in an optimized reaction buffer containing 14 mM MgOAc, coupled with a reduction in the volumes of PCR and RPA products to 1 μL and 3 μL, respectively, resulted in an enhanced sensitivity. Detection time was decreased to 75 min with a 2% limit of detection (LOD), as evidenced by the results obtained from the blue light illuminator. The CRISPR/Cas12a assay confirmed the real-time PCR results in 31 of 32 clinical samples to identify the BRAF V600E mutation status, while Sanger sequencing detected BRAF V600E mutations with lower sensitivity.
Conclusion
We propose a potential diagnostic approach that is facile, fast, and affordable with high fidelity. This method can detect BRAF V600E mutation with a 2% LOD without the need for a thermocycler.
1 Introduction
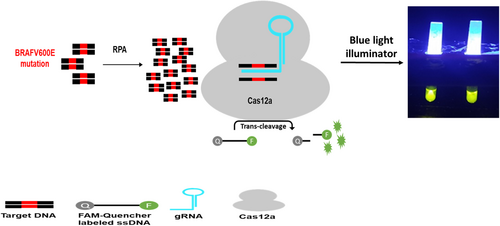
BRAF, the v-raf murine sarcoma viral oncogene homolog B, operates downstream of RAS and is essential in the mitogen-activated protein kinase (MAPK) signaling pathway. Mutations in the kinase domain have been detected in over 90% of cancer cells exhibiting BRAF activating mutations, specifically at amino acid valine 600, which is most often substituted to glutamic acid (V600E). Vemurafenib, a small molecule, demonstrates selective binding toward the ATP binding site in BRAF V600E kinase and acts as an ATP-competitive molecule, thus inhibiting the kinase activity [1-3]. The BRAF V600E mutations are highly prevalent driver mutations detectable in a wide range of cancer types, including but not limited to melanoma [4-7], hairy cell leukemia [8-10], papillary thyroid carcinoma [11-14], and ganglioglioma [15, 16]. The BRAF V600E accounts for 70%–88% of all BRAF mutations [17, 18]. Considering the importance of BRAF V600E mutation in various forms of cancers, precise identification of the mutation status within a tumor can play an influential role in selecting an optimal treatment approach, thereby helping to make the treatment more specialized and personalized.
It is necessary to utilize an appropriate technique with high efficiency to ascertain the status of molecular mutations in cancerous cells. A cost-effective and rapid method with high specificity and sensitivity may provide significant advantages in identifying mutations in affected individuals. The advent and development of the clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein (Cas) system has presented novel opportunities for low-cost and portable diagnostic techniques [19-22]. The CRISPR/Cas system has been recognized as an immune mechanism in bacteria that enables protection against bacteriophages [23]. This system entails the Cas endonuclease being guided to the target sequence through the assistance of a gRNA molecule. The CRISPR/Cas system can be combined with electrochemical, optical, and nanopore-based techniques to create a DNA sensor. This sensor has a reduced detection time and improved sensitivity and selectivity for specific nucleic acid sequences [24]. The identification of trans-cleaving activity in Cas12, Cas13, and Cas14, which belong to class 2 of Cas proteins, has resulted in the expansion of methodologies. This unique feature enables Cas12a to cleave single-stranded DNA (ssDNA) nonspecifically, whereas Cas13 can cleave nontarget, single-stranded RNA [25, 26]. The Cas12a protein is able to cleave the connecting ssDNA sequences between the fluorophore and the quencher of the reporter. Thus, fluorescent light is emitted if the Cas protein recognizes the target sequence in the samples with the guide of specific gRNA. A diagnostic system of this nature will greatly assist in advancing the quick and accurate detection of cancer mutations and viral infections, two areas that require immediate detection and treatment [27].
Increased sensitivity can be accomplished through the integration of a preamplification phase before CRISPR/Cas detection, like the utilization of PCR, recombinase polymerase amplification (RPA), and loop-mediated isothermal amplification (LAMP) [28-31]. The last two approaches enable a rapid and efficient isothermal amplification of low copies of target DNA without using a thermocycler [32]. The RPA method presents a unique advantage by being a single-temperature method that operates at 37°C–42°C; it can be performed simultaneously with the CRISPR/Cas reaction. This feature reduces detection time, and the entire reaction occurs within a microtube [33].
In this study, we successfully optimized a method to detect the BRAF V600E (1799T > A) mutation in clinical samples based on the single-nucleotide specificity of the CRISPR/Cas12a system coupled with RPA. For this purpose, multiple parameters, such as the volume of the amplification product, gRNA concentration, and reaction buffer compositions, were analyzed. In the following, we examined 32 clinical samples to determine their BRAF mutation status. We compared the results of real-time PCR with our optimized method and the Sanger sequencing technique.
2 Materials and Methods
2.1 Clinical Samples Collection
This study was approved by the Ethics Committee of the School of Medicine, Tehran University of Medical Sciences (No. IR.TUMS.MEDICINE.REC.1400.1113). A total of 32 formalin-fixed, paraffin-embedded (FFPE) tissues were obtained from the Genetics Laboratory of Imam Khomeini Hospital. All procedures strictly adhered to the protocols established by the Medical Ethics Committee. DNA was extracted and real-time PCR (The “Easy BRAF” Kit, India), was conducted to identify the BRAF V600E mutation status in these 32 clinical samples.
2.2 Preparation of Mutant DNA Template
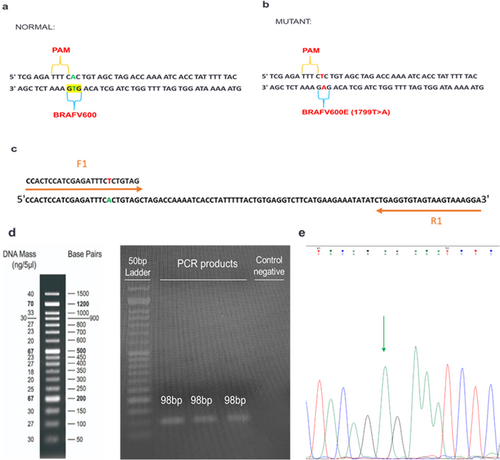
To generate sequences harboring BRAF 1799T > A mutation, PCR mutagenesis was conducted with specified primers (Table 1, F1, R1), with the forward primer comprised of the mutant sequence. 465 ng of normal genomic DNA was mixed with 0.4 μM of each primer (synthesized by Sinaclon, Iran), 1 × PCR buffer (Parstous, Iran), 80 nM dNTPmix (Parstous, Iran), 1.5 mM MgCl2 (Parstous, Iran), 0.04 U Apta Taq DNA polymerase (Parstous, Iran), and H2O, subsequently subjected to amplification through a thermal cycler. After a 4 min incubation at a temperature of 94°C, a series of 35 amplification cycles were conducted at 94°C, 56°C, and 72°C, each for 30 s, and ending at 72°C for 5 min. The PCR products were put through electrophoresis analysis using 2% agarose gel, followed by visualization under UV light.
| Oligonucleotide | Sequence (5′ – 3′) |
|---|---|
| F1 primer | CCACTCCATCGAGATTTCTCTGTAG |
| R1 primer | TCCTTTACTTACTACACCTCAG |
| F2 primer | GCCTCAATTCTTACCATCCAC |
| F3 primer | TACTGTTTTCCTTTACTTACTACACCTCAG |
| R3 primer | GACAACTGTTCAAACTGATGGGACCCACTC |
| gRNA | UAAUUUCUACUAAGUGUAGAUUGUGUAGCUAGACCAAAAU |
2.3 gRNA Designing
A gRNA was designed utilizing CRISPOR [34] and CHOPCHOP v3 [35] tools. After careful consideration of both efficiency and off-target scores, a specific gRNA was selected with the intent to target the BRAF 1799T > A mutation. The gRNA exhibits an extra single-nucleotide alteration within its synthetic sequence, alongside the mutated nucleotide, to increase the specificity of detection. The sequence of the utilized gRNA can be observed in Table 1.
2.4 RPA Assay
RPA assay was performed in 20 min utilizing the TwistAmp Basic kit (Twist Dx, UK). All recommended instructions outlined in the guidelines for primer design were followed. A total of 32 clinical DNA samples were amplified utilizing the forward and reverse primers (Table 1, F3R3) in the RPA reaction, with some minor alterations made to the manufacturer's protocol. The system consisted of an RPA solution comprising primer-free rehydration buffer, both forward and reverse primer, nuclease-free water, and magnesium acetate (MgOAc), which was subsequently mixed with TwistAmp Basic reaction pellets.
2.5 CRISPR/Cas12a-Mediated Target DNA Detection
The protocol utilized for detecting nucleic acids based on LbCas12a was performed at 37°C for 120 min in a 20 μL volume consisting of 2 μM fluorescent reporter (6-FAM/TTATT/BHQ-1), 1 × NEBuffer 2.1, 150 nM gRNA, 100 nM Cas12a, and amplification products (1 μL of PCR products or 3 μL of RPA products). NEBuffer 2.1 and EnGen LbCas12a were ordered from New England Biolabs, while fluorescent reporter and gRNA were synthesized by Metabion. The Cas12a protein cut the ssDNA-FQ probe by nonspecific trans-cleavage activity in the presence of specific gRNA and amplified target DNA, thereby releasing the fluorescent light (Figure 1). Rotor-Gene Q real-time PCR cycler was employed to quantify the fluorescent signal dynamically. Imaging the tubes was achieved through a blue-light illuminator to facilitate visual detection.
2.6 Assessment of the Limit of Detection (LOD)
To determine the LOD of the CRISPR/Cas12a assay, the amplified DNA fragments of the artificially generated mutant sequences were combined with the amplified DNA fragments of the normal sequences to create a serial dilution of samples containing the BRAF V600E sequence.
2.7 Statistical Analysis
GraphPad Prism 8 software (Graph-Pad Software Inc., San Diego, CA, USA) was used to perform the statistical analysis of fluorescent signals. Error bars indicate mean ± S.D.
3 Results
3.1 Optimizing the Quantity of PCR Product Used for the BRAF V600E Detection
PCR products amplified by mutant primers with a length of 98 bp were successfully obtained and they were verified via Sanger sequencing (Figure 2). The determination of the volume of the PCR product employed is crucial for enhancing assay sensitivity. PCR amplification was carried out for 35 cycles, and 1, 3, and 5 μL of PCR product were subsequently utilized for CRISPR/Cas12a-based detection. Our findings illustrate that 1 μL PCR product considerably increased fluorescence absorbance, whereas 5 μL PCR product hinders the diagnostic reaction and reduces fluorescence absorption (Figure 3).
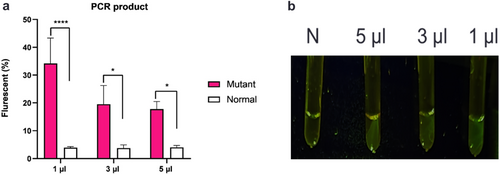
3.2 Fluorescent Signal Enhancement Through the Manipulation of Buffer Compositions
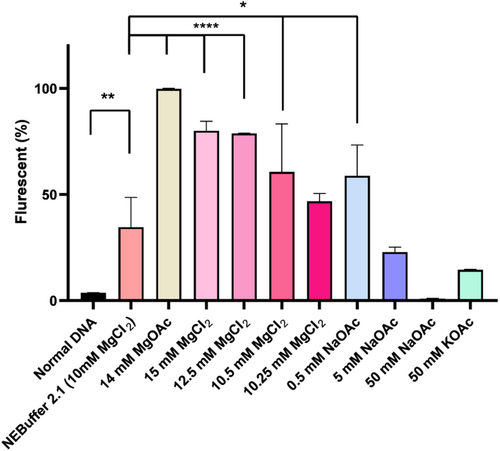
To optimize the reaction buffer for Cas12a, a comparative analysis was conducted on several salts for incorporation into the reaction buffer. The experimental results presented in Figure 4 indicated that the CRISPR/Cas12a system is highly sensitive to changes in buffer conditions. Different concentrations of MgCl2 from 10 to 15 mM were utilized, and our data demonstrated a significant signal intensity increase as the concentration increased. 0.5 M sodium acetate (NaOAc) resulted in a notable increase in the fluorescent signal. Conversely, a concentration of 5 M NaOAc led to a decrease in the signal intensity, while 50 M NaOAc strongly inhibited the reaction. Moreover, our findings revealed that 14 mM MgOAc exhibited superior performance compared to all other analyzed salts (Figure 4).
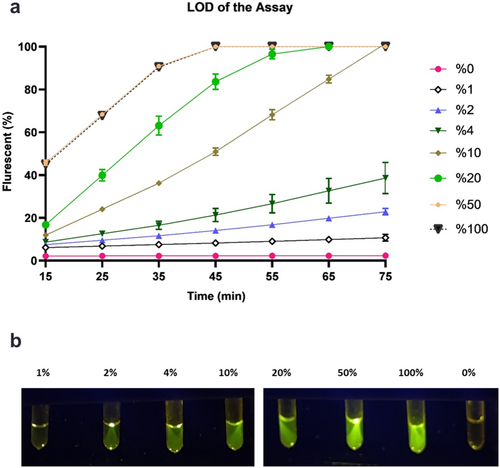
3.3 Evaluation of the LOD of the CRISPR/Cas12a Diagnostic Method
Different concentrations of mutant PCR products were prepared. One microliter of each sample was subjected to the CRISPR diagnostic method with additional MgOAc. After a 75 min incubation period, samples that contained 100% and 50% PCR products of the mutant sequences achieved the maximum fluorescence absorption in only 45 min. Visual detection using a blue-light illuminator showed that these samples emitted fluorescent green light, except for samples that contained 1% or 0% of mutant samples. The presented data demonstrated that our method can identify specimens with at least 2% mutated sequences in a 75 min timeframe (Figure 5).
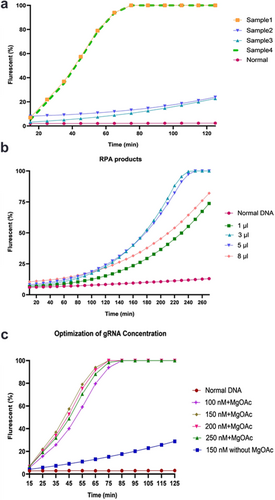
3.4 Assay Optimization for RPA Products
Four clinical samples in which the V600E mutation was validated through real-time PCR were subjected to RPA and evaluated in our optimized CRISPR/Cas12a assay. Our data showed that due to the heterogeneity of the mutation and the variance in minor allele frequency, the fluorescence intensity is different in distinct samples. Therefore, the sample with the lowest amounts of fluorescent signal was chosen for test optimization (Figure 6a). Different RPA product quantities were used as targets, and a volume of 3 μL was selected (Figure 6b). To enhance the sensitivity of CRISPR/Cas12a detection, the concentration of gRNA was analyzed in 100, 150, 200, and 250 nM. MgOAc was simultaneously added to the reaction buffer. The findings revealed that the inclusion of 14 mM MgOAc, in combination with 150 nM concentrations of the gRNA, has been positively correlated with a decrease in detection time to approximately 75 min. When considering 20 min for RPA, the total detection time decreased to 95 min. Notably, the increase in fluorescence absorption did not lead to false positives in the normal samples (Figure 6c).
3.5 Clinical Samples Analysis
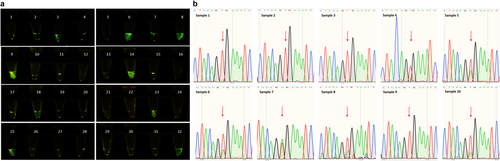
Among a set of 32 clinical samples, real-time PCR identified 10 samples as mutant BRAF V600E. Twenty-two samples did not exhibit BRAF V600E mutation. Additionally, one sample was identified as BRAF V600K, while another sample exhibited the BRAF V600D mutation. Our optimized CRISPR/Cas12a diagnostic approach was implemented to identify BRAF V600E mutation status in these 32 clinical samples. Initially, samples were employed as templates in the RPA reaction and subsequently subjected to CRISPR/Cas12a testing in a blinded manner. The results indicated that nine samples carrying the BRAF V600E mutation were visualized in green, whereas the nonmutant samples exhibited a colorless appearance when subjected to illumination with blue light as well as samples with other V600 mutations (Figure 7a). Consequently, the CRISPR/Cas12a assay could detect BRAF V600E mutation with 90% sensitivity and 100% specificity as indicated in Table 2. To compare our methodology with Sanger sequencing, PCR was performed with the F2R1 primer pair (Table 1), followed by sequencing of the products. Five samples, previously identified as BRAF V600E mutants by the real-time PCR, exhibited no mutation in the Sanger sequencing results, as demonstrated in Figure 7b.
| Confirmed by real-time PCR | |||
|---|---|---|---|
| Tested by CRISPR/Cas12a assay | BRAFV600E mutation | Non-BRAFV600E mutation | |
| Positive | 9 (True Positive, TP) | 0 (False positive, FP) | |
| Negative | 1 (False Negative, FN) | 22 (True Negative, TN) | |
| Sensitivity = TP/(TP + FN) = 90% | Specificity = TN/(TN + FP) = 100% | ||
4 Discussion
The role of BRAF signaling in cell division and differentiation is crucial [36]. Activated BRAF mutations lead to uncontrolled growth and tumorigenesis, thus presenting an appropriate target for precision cancer therapy [36, 37]. FDA-approved BRAF inhibitors such as dabrafenib and vemurafenib are used in the BRAF-targeted therapy [38, 39]. Due to the significance of BRAF mutation (V600E) in various cancer types, accurate knowledge of the status of mutations in a tumor is essential for treatment decisions.
The diagnostic platforms based on CRISPR/Cas exhibited significant potential for widespread clinical applications. They can be a diagnostic standard in the future, attributed to their easy programming, cost-effectiveness, and ability to search for the target sequence with elevated levels of sensitivity and specificity [40]. Multiple CRISPR-based tools were designed for accurate and timely detection of viral infections and cancer-related mutations in clinical samples [41-43]. Despite the significant potential of CRISPR-based methods, they face challenges concerning their universal applicability and specificity. However, recent studies have addressed limitations in nucleic acid detection using the CRISPR/Cas12a system. A gene detection platform has been developed to solve the problem of protospacer-adjacent motif (PAM) sequences without restriction for seed sequences as well as significantly improve the specificity for single-nucleotide mutation detection [44]. Furthermore, a genotyping method has been proposed, which involves the insertion of PAM sequences, and the introduction of an additional mismatch in crRNA to improve the signal-to-noise ratio [45]. Another study screened a 2-aminopurine probe as a reporter in the CRISPR/Cas12a system, increasing sensitivity for ssDNA detection and highly sensitive detection of viruses [46].
Various molecular methods were used for BRAF V600E mutation detection, including DNA-sequencing procedures such as Sanger sequencing, as well as PCR-based methods like real-time PCR and digital droplet PCR (ddPCR). Indeed, the LOD of Sanger sequencing typically ranges from approximately 20% – 25% of mutant alleles, while its sensitivity is 80% – 93% and is susceptible to the quality of DNA [47, 48]. In contrast, the Cobas 4800 BRAF Mutation Test, which utilizes a real-time PCR technique, can detect BRAF V600E mutations at ≥ 5% mutation level while exhibiting an enhanced sensitivity range of 98%–100% [48, 49]. Moreover, ddPCR can serve as a BRAF V600E detection method owing to the superior sensitivity (100%) and reduced LOD of the mutant allele (0.001) [50]. However, using these techniques requires an expert technician and expensive equipment, and consumes a substantial amount of time and resources. Currently, thermocycler-dependent approaches utilize the trans-cleavage capability of CRISPR/Cas for sensitively identifying BRAF V600E mutations. Although these methods are simple and fast, they require a thermocycler device [44, 51].
In this study, we compared three distinct methodologies to detect BRAF V600E mutations in clinical samples, including real-time PCR, Sanger sequencing, and an optimized CRISPR-based strategy that we have developed in our laboratory, which is thermocycler independent [52]. To evaluate the specificity, it would be necessary to investigate other closely related genetic variants using the CRISPR/Cas12a assay. We examine one sample with BRAF V600K mutation, as well as one sample with BRAF V600D. Both samples did not exhibit green color when subjected to blue light illuminator, indicating that our designed gRNA is specific for BRAF V600E mutation detection (Figure 7a, Samples 20, and 27). Furthermore, according to real-time PCR results in 32 clinical samples, specific samples were positive for mutations in KRAS and NRAS, while they were negative for BRAF V600E. These samples were accurately identified as negative for BRAF V600E mutation by our CRISPR/Cas12a assay. Our data confirmed the real-time PCR results with 100% specificity and 90% sensitivity (Table 2, and Figure 7a). Sequencing data demonstrated that the effectiveness of Sanger sequencing in diagnostic testing is hindered by the requirement of a significant proportion of tumor cells within the sample, which may not always be feasible (Figure 7b).
To achieve the most favorable diagnostic conditions for mutation detection, we conducted experiments on the critical parameters that influence the efficiency of the CRISPR/Cas12a assay. Specific gRNA design, the buffer compositions, the quantity of gRNA, and the volume of template DNA can significantly impact method sensitivity. The design of the gRNA is particularly crucial as it not only influences specificity but also Cas efficiency, thus enhancing diagnostic sensitivity. To improve the specificity for gRNA binding, a unique gRNA was designed, featuring a secondary nucleotide alteration adjacent to the original mutation, which poses a challenge for binding to normal sequences. However, this sequence has an affinity to the mutated sequence of DNA, activating the CRISPR/Cas system and releasing the fluorescent dyes. Previous researchers have also designed this type of gRNA to increase specificity [53-55]. Modifying the reaction systems by optimizing buffer conditions is an alternative approach to improve detection efficiency [56, 57]. The present study tested different salts that affect sensitivity, like MgOAc, NaOAc, KOAc, and MgCl2. Our findings indicated that the presence of 14 mM MgOAc has the most impact on the detection efficiency of Cas12a. The proportion of target DNA, gRNA, and Cas12a can potentially impact Cas12a efficiencies significantly [57]. In this study, it was observed that the utilization of 150 nM gRNA, coupled with a reduction in the volumes of PCR and RPA products to 1 μL and 3 μL, respectively, resulted in enhanced sensitivity and decreased detection time.
Herein, we have developed an optimized detection methodology for BRAF V600E (1799T > A) by coupling RPA with CRISPR-Cas12 assay with increased sensitivity and a low LOD.
This approach exhibits high specificity and sensitivity while it is rapid and simple to operate, which could facilitate the effective management of BRAF V600E (1799T > A). While this method demonstrates notable advantages, several limitations necessitate careful consideration. Additional validation may be required to ascertain the suitability of this approach for analyzing complex clinical samples with diverse genetic backgrounds. Future studies could utilize the CRISPR/Cas12a assay in a comparative framework in conjunction with other established methods for BRAF V600E mutation detection on a larger sample size. These would yield valuable insights into assay reliability and applicability in a range of clinical situations. Continuous research efforts are important for addressing remaining challenges and contributing to the improvement and validation of the assay.
5 Conclusion
The present investigation aimed to examine the optimized condition for CRISPR/Cas-based assay coupled with RPA to identify BRAF V600E (1799T > A) mutations in clinical samples with fluorescent signals. This was achieved by carefully optimizing the buffer compositions, the quantity of the amplified targets, and gRNA. The fluorescence intensity was evaluated using the real-time PCR device and blue-light illuminator. It was ascertained that a reduction in PCR and RPA products to 1 and 3 μL, respectively, coupled with the utilization of 14 mM MgOAc, resulted in a simultaneous increase in the maximum fluorescence absorption and reduction in detection time. It should be noted that the optimized CRISPR/Cas12a assay reported in this study can act as an efficient method for detecting BRAF 600E with 2% LOD. This assay has great potential as a fast and cost-effective mutation detection technique, especially in less-developed areas. The fusion of CRISPR/Cas technology with RPA enhances mutation detection sensitivity, making it a practicable choice for efficient molecular diagnostics in different clinical settings.
Acknowledgments
The authors would like to thank the staff of the Cancer Research Center of Imam Khomeini Hospital for their contribution to the preparation of clinical samples.
Ethics Statement
This study was approved by the Ethics Committee of the School of Medicine, Tehran University of Medical Sciences (No. IR.TUMS.MEDICINE.REC.1400.1113).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.




