The role of platelet parameters for the diagnosis of preeclampsia among pregnant women attending at the University of Gondar Comprehensive Specialized Hospital antenatal care unit, Gondar, Ethiopia
Abstract
Background
Preeclampsia (PE) is a pregnancy-related illness characterized by high blood pressure (BP) and proteinuria after the 20th gestational week (GW). Platelet (PLT) parameter changes are the common hematological abnormalities observed in PE patients. The main aim of this study was to assess the role of PLT parameters for PE diagnosis among pregnant women.
Methods
A comparative cross-sectional study was conducted at the University of Gondar Specialized Hospital. A total of 126 pregnant women (63 normotensive [NT] and 63 PE) were recruited using a convenient sampling method. Three milliliter blood was collected from each participant, and PLT parameters were determined using Sysmex XS-500i analyzer. An independent t-test supplemented with receiver-operating characteristics (ROC) were used for comparisons and diagnostic value of PLT parameters between the study groups.
Results
Platelet count (PC) was significantly lower in the PE group compared to that in the NT group, whereas mean platelet volume (MPV), platelet large cell ratio (P-LCR), and platelet distribution width (PDW) were significantly higher in PE. MPV had the largest area under the curve (AUC) [0.91: 95% CI; 0.85–0.96] followed by PC [0.79: 95% CI; 0.72–0.87]. MPV can differentiate PE patients from NT pregnant women at cut-off value ≥12.10 fl (84.1% sensitivity and 87.3% specificity) while PC can indicate PE at a cut-off value ≤176.5 × 109/L (65.1% sensitivity and 87.3% specificity).
Conclusion
A decreased PC and an increased MPV, P-LCR, and PDW can be used as a simple, cost-effective, quick, and reliable method of PE screening. Of them, MPV is the best indicator of PE.
1 BACKGROUND
Platelets are small anucleated cell fragments that are generated from megakaryocytes and normally circulate in the blood as an inactive state.1, 2 They play a central role in regulating hemostasis through primary hemostasis, support of coagulation, and even antifibrinolytic effects.3, 4 PC and PLT indices such as MPV, P-LCR, PDW, and plateletcrit (PCT) are among the PLT parameters obtained from an automatic complete blood count (CBC) test.5, 6 Alterations of PLT parameters are one of the most commonly identified hematological changes.
in PE, and these parameters have been identified as good candidates for the diagnosis of PE.7, 8
Preeclampsia is a pregnancy-specific disorder characterized by the onset of hypertension and either proteinuria or end-organ dysfunction after 20th GW.9 It is characterized by the abnormal vascular response to placentation, which is associated with increased systemic vascular resistance, enhanced PLT aggregation, activation of the coagulation system, and endothelial cell dysfunction.10 This disorder is a major cause of maternal and fetal morbidity and mortality.11 Around 3%–5% of all pregnancies is complicated by PE globally.12-14 It also affects 1.8%–16.7% of pregnancies in developing countries.15 Likewise, in Ethiopia, the prevalence of PE ranges from 3.99% to 5.49%.16
Abnormal placentation due to inadequate fetal trophoblast invasion of uterine tissue to remodel the uterine spiral arteries and then the development of placental ischemia or inadequate perfusion of the placenta followed by progressive ischemia or hypoxia are the postulated mechanism of pregnancy-related PE pathogenesis.8, 17 The ischemic placenta may release reactive oxygen species, which further contributes to placental oxidative stress and placental dysfunction. This hypoxic state also releases chemokines, proinflammatory cytokines, and antiangiogenic factors into the maternal circulation that contribute to endothelial damage.18-20
When endothelial damage occurs, PLTs adhere to the injured endothelium and become activated.21 The activated PLT secrets and releases constituents of alpha and dense granule, which contribute to PLT aggregation.22 Moreover, the damaged vascular endothelium releases tissue factor, which initiates the process of coagulation.23 The activation of the coagulation system, along with PLT aggregation, leads to reduced organ perfusion and multisystem dysfunction in PE patients.8
Early diagnosis of PE is important to start preventive therapy for high-risk women.24, 25 However, there is no available test that can accurately and reliably distinguish women developing PE at the early stage of pregnancy.26 MPV is the surrogate marker of platelet activation and is associated with inflammatory conditions including nasal polyps,27 diabetes mellitus,28 obesity,29 rheumatoid arthritis,30 and infections.31 Similarly, PDW has been reported to be associated with inflammatory diseases such as diabetes mellitus type 2,32 autoimmune conditions,33 and irritable bowel disease.34 Therefore, early diagnosis of PE could be facilitated by indirect measurement of PLT production and activation.7
Increased consumption of PLTs due to abnormal coagulation system in PE causes thrombocytopenia, which can be utilized as an important sign of this disorder.35 Moreover, the increased consumption and destruction of PLTs in PE obligated the bone marrow to produce and release young and large PLTs, resulting in increased MPV, PDW, and P-LCR.7, 36 Thus, the main aim of this study is to compare PLT parameters between preeclamptic patients and NT pregnancy and to assess the diagnostic role of those parameters for the diagnosis of PE.
2 MATERIALS AND METHODS
2.1 Study area, study design, and period
A hospital-based comparative cross-sectional study was conducted at the University of Gondar Comprehensive Specialized Hospital antenatal care (ANC) unit, Gondar, Northwest Ethiopia from February to May 2021. The hospital is located in Gondar town, Central Gondar Zone, Amhara regional state, Ethiopia. Gondar town is located 727 km away from Addis Ababa, the capital city of Ethiopia, and 180 km far from Bahir dar city, the capital city of Amhara regional state in the northwest direction. The town is situated at a latitude and longitude of 12°36’N 37°28’E with an elevation of 2133 m above the sea level. Currently, the hospital has been serving around 7000 people from Central, North, and Western Gondar Zone. The hospital provides medical services, including internal medicine, pediatrics, surgery, gynecology, psychiatry, ophthalmology, and maternal and child care. The ANC clinic is one of the units under the department of Gynecology and Obstetrics serving around 17,000 pregnant women per year. Currently, there are 16 gynecologists and 27 full times midwives working at the ANC unit.
2.2 Eligibility criteria
Definition of PE was based on the recommendation of the American College of Obstetricians and Gynecologists task force on hypertension in pregnancy as an elevated BP of ≥140/90 mmHg after 20th GW with proteinuria ≥1+ in urine dipstick.12 Age and GW-matched NT pregnant women who attended the hospital for routine obstetric care were used as control.
Patients with a known history of hypertension, renal disease, liver disease, thyroid disease, diabetes mellitus, heart disease, thromboembolism or known thrombophilic disease, recurrent miscarriage, preterm labor, intrauterine growth restriction, intrauterine fetal death, coagulation disorder, hematological malignancy, and women with recent major surgery or trauma, morbid obesity or body mass index (BMI) ≥40 kg/m2, inflammatory diseases, anticoagulant treatment (aspirin, heparin, and warfarin), and antihypertensive drug user were excluded from the study. The exclusion was accomplished by reviewing their medical records using an exclusion criteria checklist. Moreover, target populations who refuse to participate in the study were excluded.
2.3 Sample size determination and sampling technique
Therefore, the initial sample size in each group was 57. After a 10% nonresponse rate was added, a total of 126 pregnant women (63 preeclamptic and 63 NT who were age and GW match) were enrolled using the convenient sampling technique.
2.4 Data collection
2.4.1 Sociodemographic, clinical, and anthropometric data
After informed consent was obtained from each study participant, data related to sociodemographic characteristics such as age, residence, educational status, and occupation were collected through a face-to-face interview using a pretested structured questionnaire. The interview was conducted by a senior midwife using a questionnaire written in Amharic (the local language) that was translated from the English version. Clinical data including parity, gravidity, and GW were extracted from patients’ medical chart using a predesigned data collection format.
For the BP measurement of pregnant women, they were first asked to sit and take rest for 5 min if they were exerted. Excess clothing was then removed, and the participants were requested to sit upright with their right arm positioned on the bench. Finally, the BP was measured with conventional adult cuffs and a standard sphygmomanometer, with the stethoscope bell softly placed over the brachial artery. The average systolic blood pressure (SBP) and diastolic blood pressure (DBP) were taken in mmHg after two readings recorded with 5- to 10-min intermission.
Stadiometer (Infiniti Med Lab Pvt. Ltd.) was used to measure the height of the participants. Participants were instructed to stand erect on the floorboard of the stadiometer with their backs to the vertical backboard. The heels of the feet are placed together with both heels touching the base of the vertical board. Feet were pointed slightly outward at a 60° angle. During the height measurement, the participant's shoes and hats were removed. The height was measured to the nearest 0.1 cm without shoes and a hat.39
The weight of the participants was measured using a weight measurement scale (Infiniti Med Lab Pvt. Ltd.). The weight scale was set to zero before starting the weight measurement. Participants were asked to remove extra layers of clothing, shoes, jewelry, and any items in their pockets and then stand with their weight evenly distributed between both feet, arms hanging freely by the sides of the body, palms toward thighs, and heads up and facing straight ahead. Weights were finally recorded to the nearest 0.1 kg.39 All the height and weight measures were performed twice, with the average being used. Finally, the BMI of each participant was calculated by dividing weight in (kg) by height squared in meters (m2) to screen body fat ratio.39
2.4.2 Laboratory sample collection and analysis
A blood sample was collected from each participant who completed the questionnaire and agreed to give blood during ANC visit and/or at the time of an emergency arrival of PE complication. A total of three milliliters of venous blood sample was collected into an ethylene diamine tetraacetic acid (EDTA) sample tube and adequately mixed. The CBC test was then performed within 2 h of blood collection using a Sysmex XS-500i automated hematology analyzer (Sysmex) for the determination of PLT parameters (PC, MPV, PDW, P-LCR, and PCT). The performance of the instrument for the CBC test was monitored using low-, medium-, and high-value control materials. Moreover, all procedures were conducted according to the manufacturers’ instruction manual and following standard operating procedures (SOPs).
A urine specimen was also collected using a clean dry leak-proof urine cup to detect proteinuria. Proteinuria was detected by Cromatest® Linear URS-10 chemical strip (Linear Chemicals S.L, 08390 Montgat), which is a semiquantitative test. The presence of protein is indicated by the appearance of any green color on the strip after 1 min of immersing and removing the strip from a cup of urine. Colors range from yellow for "Negative" reactions to yellow-green, green, and green-blue for "positive" reactions that can be graded as +1, +2, and +3 reactions, respectively.
2.5 Data analysis and interpretation
The collected data were entered into Epi-data 4.6 software and then exported into a statistical package for social science statistical software version 20 (SPSS 20) (SPSS Inc.) for analysis. Data distribution was checked by the Kolmogorov–Smirnov normality test. Comparison of normally distributed data between the two groups was done by independent t-test, and the results are expressed as mean ± standard deviation (SD).
For those PLT parameters that showed significant differences between the PE and NT groups, ROC curve analysis was performed to determine AUC, sensitivity, and specificity for PE diagnosis. Youden's index was calculated as “sensitivity+specificity−1” to determine the optimal cut-off values of PLT parameters that showed best combinations of sensitivity and specificity for diagnosis of PE. The largest AUC was considered as the best marker for the detection of PE. For all statistical analysis, p < 0.05 was considered as statistically significant.
3 RESULTS
3.1 Sociodemographic and clinical characteristics of study participants
A total of 126 pregnant women were enrolled in this study (63 PE and 63 NT). Out of the included participants, 88 (69.8%) were urban residents, and 39 (31%) had no formal education. Occupationally, most of them were housewives 46 (36.5%) followed by farmers 36 (28.6%). The majority of the study participants were married, 121 (96%). The study participants’ age ranged from 18 to 39 years old with a mean age of 27.8 ± 4.68 (28.1 ± 4.61 years old for PE and 27.5 ± 4.77 years old for NT) (Table 1). There were no significant variations in GWs, gravidity, and parity between the control and PE groups. On the other hand, BMI was found lower in the PE group compared to that in the NT group (80.9 ± 7.8 vs. 111.7 ± 6.1) (p < 0.05) (Table 2).
| Variable type | Category | Study group | Total | p-value | |
|---|---|---|---|---|---|
| NT group | PE group | ||||
| Age: mean ± SD | No | 27.5 ± 4.77 | 28.1 ± 4.61 | 27.8 ± 4.68 | 0.449 |
| Residence: N (%) | Urban | 46 (73) | 42 (66.7) | 88 (69.8) | 0.437 |
| Rural | 17 (27) | 21 (33.3) | 38 (30.2) | ||
| Educational status: N (%) | No formal education | 19 (30.2) | 20 (31.7) | 39 (31.0) | 0.956 |
| Primary | 12 (19) | 11 (17.5) | 23 (18.3) | ||
| Secondary | 19 (30.2) | 17 (27) | 36 (28.6) | ||
| Diploma and above | 13 (20.6) | 15 (23.8) | 28 (22.2) | ||
| Marital status: N (%) | Married | 59 (93.7) | 62 (98.4) | 121 (96) | 0.171 |
| Single | 4 (6.3) | 1 (1.6) | 5 (4) | ||
| Occupation: N (%) | Housewife | 26 (41.3) | 20 (31.7) | 46 (36.5) | 0.703 |
| Farmer | 16 (25.4) | 20 (31.7) | 36 (28.6) | ||
| Government employee | 11 (17.5) | 11 (17.5) | 22 (17.5) | ||
| Private | 10 (15.9) | 12 (19) | 22 (17.5) | ||
Note
- p-value by Pearson chi-square for categorical variable and by independent t-test for continuous variable.
- Abbreviations: ANC, antenatal care; NT, normotensive; PE, preeclampsia; SD, standard deviation.
| Variable | Study group | p-value | |
|---|---|---|---|
|
NT group (n = 63) (mean ± SD) |
PE group (n = 63) (mean ± SD) |
||
| Gravidity | 2.29 ± 1.5 | 2.57 ± 1.5 | 0.298 |
| Parity | 1.1 ± 1.2 | 1.5 ± 1.4 | 0.082 |
| GW (weeks) | 33.8 ± 4.8 | 34.1 ± 4.4 | 0.743 |
| BMI (kg/m2) | 22.9 ± 2.7 | 21.8 ± 2.1 | 0.009 |
| SBP (mmHg) | 103.7 ± 10.2 | 145.4 ± 8.6 | 0.000 |
| DBP (mmHg) | 69.5 ± 7.5 | 94.5 ± 6.1 | 0.000 |
| Proteinuria | 0.1 ± 0.3 | 1.8 ± 0.6 | 0.000 |
Note
- p-value by independent t-test.
- Abbreviations: ANC, antenatal care; BMI, body mass index; DBP, diastolic blood pressure; GW, gestational week; NT, normotensive; PE, preeclampsia; SBP, systolic blood pressure; SD, standard deviation.
3.2 Platelet parameters among study groups
We found that PC was significantly lower in PE patients compared to that in NT pregnant women (164.90 ± 54.87 × 109/L vs. 229.87 ± 54.26 × 109/L). However, MPV, PDW, and P-LCR were significantly elevated in the PE group (p < 0.001). The mean difference in PC was lower by 64.97 × 109/L (95% CI; 45.7–84.2) in PE patients than the NT group, whereas MPV, PDW, and P-CR showed an increased mean difference of 2.20 fl (95% CI; 1.78–2.62), 1.74 fl (95% CI; 0.86–2.62), and 6.10% (95% CI; 2.99–9.21) in PE patients, respectively. Even though there was no statistically significant difference in PCT among the two groups, a slight decrement in the PE group (0.21 ± 0.08) was observed compared to that in the controls (0.24 ± 0.07) (Table 3).
| Variable | Study group | p-value | ||
|---|---|---|---|---|
| NT group (n = 63) (mean ± SD) | PE group (n = 63) (mean ± SD) | Mean difference (95% CI) | ||
| PC (109/L) | 229.87 ± 54.26 | 164.90 ± 54.87 | −64.97 (−84.2, −45.7) | <0.001 |
| MPV (fl) | 11.2681 ± 0.92 | 13.47 ± 1.43 | 2.20 (1.78, 2.62) | <0.001 |
| PDW (fl) | 14.37 ± 2.02 | 16.11 ± 2.88 | 1.74 (0.86, 2.62) | <0.001 |
| P-LCR (%) | 36.15 ± 7.42 | 42.26 ± 9.98 | 6.10 (2.99, 9.21) | <0.001 |
| PCT (%) | 0.24 ± 0.07 | 0.21 ± 0.08 | 0.02 (0, 0.05) | 0.067 |
Note
- p-value by independent t-test.
- Abbreviations: ANC, antenatal care; MPV, mean platelet volume; NT, normotensive; PC, platelet count; PCT, plateletcrit; PDW, platelet distribution width; PE, preeclampsia; P-LCR, platelet large cell ratio; PLT, platelet; SD, standard deviation.
3.3 Diagnostic values of platelet parameters for preeclampsia diagnosis
The diagnostic values such as AUC, cut-off value, sensitivity, and specificity were determined for those PLT parameters that showed significant differences between the PE and NT groups using ROC curve analysis and calculation of Youden's index. The result showed that MPV had the largest AUC (0.91: 95% CI; 0.85–0.96) and can distinguish PE patients from NT pregnant women at a cut-off value of ≥12.10 fl with a sensitivity of 84.1% and specificity of 87.3%. At this cut-off value, the positive predictive value (PPV) and negative predictive value (NPV) were 86.88% and 84.61%, respectively.
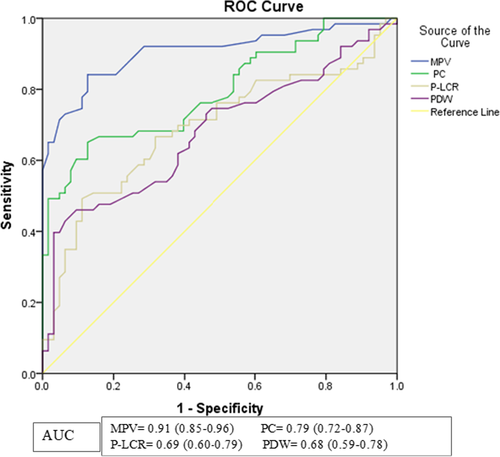
The result demonstrated that PC had the second-largest AUC (0.79: 0.72–0.87) and can differentiate PE patients from NT pregnant women at a cut-off value of 176.5 × 109/L with a sensitivity of 65.1% and specificity of 87.3%. At this cut-off point, it has a test accuracy of 76.19%, and the PPV and NPV were 83.67% and 71.42%, respectively. Moreover, P-LCR and PDW are also indicated as a marker for the diagnosis of PE with sensitivity and specificity of 49.2% and 88.9%, and 42.9% and 93.7% at cut-off values of ≥43.85% and ≥16.75 fl, respectively (Table 4; Figure 1).
| PLT parameter | AUC (95% CI) | Cut-off point | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy | Youden's index |
|---|---|---|---|---|---|---|---|---|
| MPV (fl) | 0.91 (0.85–0.96) | ≥12.10 fl | 84.1 | 87.3 | 86.88 | 84.61 | 85.71 | 0.714 |
| PC (109/L) | 0.79 (0.72–0.87) | ≤176.5 × 109/L | 65.1 | 87.3 | 83.67 | 71.42 | 76.19 | 0.524 |
| P-LCR (%) | 0.69 (0.60–0.79) | ≥43.85 | 49.2 | 88.9 | 81.57 | 63.63 | 69.05 | 0.381 |
| PDW (fl) | 0.68 (0.59–0.78) | ≥16.75 fl | 42.9 | 93.7 | 87.09 | 62.1 | 68.25 | 0.365 |
- Abbreviations: ANC, antenatal care; AUC, area under the curve; MPV, mean platelet volume; NPV, negative predictive value; PC, platelet count; PDW, platelet distribution width; PE, preeclampsia; P-LCR, platelet large cell ratio; PLT, platelet; PPV, positive predictive value.
4 DISCUSSION
The identification of pregnant women with an increased risk of PE is a critical goal in modern obstetrics.40 Obstetricians are increasingly relying on laboratory techniques to diagnose PE and manage pregnant women.41 PLT parameters such as a decrease in PC and an increase in MPV, P-LCR, and PDW have been proposed as useful markers for PE development that cause coagulation system activation.42, 43
In the current study, PC was found significantly decreased in the PE group (164.90 ± 54.87 × 109/L) compared to that in the NT group (229.87 ± 54.26 × 109/L) by 64.97 × 109/L (95% CI; 45.7–84.2). This finding was in line with previous studies done by Tesfay et al.,7 Alkholy et al.,44 and Annam et al.41 This could be related to endothelial damage in PE, which causes an increase in PLT aggregation and turnover.8 However, a significant difference was not observed in studies conducted by Abass et al.,38 Thalor et al.,45 and Kurtoglu et al.46 The major reason for the disagreement with a study done by Thalor et al.,45 probably be due to the differences in sample size. This discrepancy can also be explained by the variation in the mean period of GW in the PE group at the time of sample collection as the PC decreases with the progression of gestation.47 The participants of the PE group in their study were at the early GW (24 weeks), whereas it was 35 weeks in our study.
The current study showed that MPV was significantly higher in the PE group (13.47 ± 1.43 fl) compared to that in the NT group (11.2681 ± 0.92 fl) by 2.20 (95% CI; 1.78–2.62). This result is in agreement with previous studies conducted by Sitotaw et al.,48 Alkholy et al.,44 and Han et al.,49 that showed a significant increase of MPV in PE patients. The increment in the MPV value in PE patients might be due to the presence of large PLTs in the peripheral circulation. In patients with PE, the bone marrow is obligated to produce and release young PLTs with large sizes to compensate for the accelerated consumptions and destruction of PLTs due to aggregation within the damaged endothelium.8, 36 In contrast, a study conducted by Amita et al.,50 reported that there was no significant difference in MPV value between the PE and NT groups. This discrepancy is most likely due to the MPV measuring time. It is known that MPV increases over time when exposed to EDTA.51
In the current study, we observed statistically significant elevated PDW in the PE group as compared to the control group (p < 0.05) with a mean difference of 1.74 fl (95% CI; 0.86–2.62). Similar results were reported in the previous studies done by Tesfay et al.,7 Sitotaw et al.,48 and Dadhich et al.47 The increased PDW in PE patients could be due to PLT activation as a result of endothelial damage, which may cause a change in shape from discoid to spherical to obtain a larger surface, resulting in an increased PDW in PE.36, 43 On the other hand, our result was contrary to studies done by Doğan et al.,8 and Gogoi et al.,52 which reported as there is no syntactically significant difference in PDW between the two groups. This variation in PDW values might be due to the differences in equipment and method of the automated hematologic analyzer.53 A study done by Doğan et al., used LH780 Beckmann-Coulter8 and a study done by Gogoi et al.,52 used ABX Micros ES 60 ABX Micros ES 60, whereas we used Sysmex XS-500i. Moreover, it might be due to the retention time of the blood sample that was exposed to EDTA before analysis.51
The current study demonstrated that P-LCR increased significantly in the PE group (42.26 ± 9.98) compared to that in the NT group (36.15 ± 7.42) by 6.10% (95% CI; 2.99–9.21). This was in agreement with previous findings of studies conducted by Sitotaw et al.,48 Abass et al.,38 and Alisi et al.54 The rise in P-LCR levels in the PE group could be explained by an increase in PLT turnover following the decrease in PLT survival time as a result of an increase in PLT activation. It could be a sign of increased bone marrow activity.41
In the present study, PCT did not show a statistically significant difference between the study groups (mean difference = 0.02: 95% CI; 0–0.05). This result was comparable with previous research reports done by Abass et al.,38 Gogoi et al.,52 and Chen et al.55 Furthermore, a study that was done by Freitas et al.,56 considered PCT to be ineffective in the diagnosis of PE.
The ROC curve analysis showed that MPV had the largest AUC (0.91; 95% CI; 0.85–0.96) indicating that it is the best PLT parameter to distinguish PE patients from NT pregnant women at a cut-off value of 12.1 fl with 84.1% sensitivity, 87.3% specificity, and 85.71% test accuracy. This finding was in line with previous works conducted by Tesfay et al.,7 and Alkholy et al.,44 as they found the largest AUC on the ROC curve (0.85 and 0.88) and described it as the best marker for PE diagnosis at cut-off point 9.45 fl and ≥9.3, respectively. Moreover, studies conducted by Dadhich et al.,47 Doğan et al.,8 and Chen et al.,55 indicated MPV at cut-off 8.5, 9, and 10 fl, respectively, for PE diagnosis. The modest variation in MPV cut-off values between studies could be due to the use of different hematological analyzers that might produce up to 40% different MPV results.57 For instance, a study conducted by Doğan et al.,8 used an LH780 Beckmann-Coulter hematology analyzer, whereas we used the Sysmex XS-500i, which has its reference range.
The ROC curve in the current study showed that PC had the second-largest AUC (0.79; 95% CI; 0.72–0.87), allowing it to differentiate PE patients from NT pregnant women at a cut-off value 176.5 × 109/L with a sensitivity of 65.1%, specificity of 87.3%, and test accuracy of 76.19%. This value is nearly similar to the findings of studies done by Chen et al.,55 Doğan et al.,8 and Alkholy et al.,44 as they reported a diagnostic cut-off point of 177, 190, and 198 × 109/L, respectively, to predict PE development.
The ROC curve analysis also demonstrated that P-LCR had an AUC of 0.69 (95% CI; 0.60–0.79) and can distinguish PE patients from NT pregnant women at a cut-off value of ≥43.85% with 49.2% sensitivity, 88.9% specificity, and 69.05% test accuracy. Similarly, a study by Tesfay et al.,7 revealed that P-LCR at a cut-off >26.2% to distinguish PE patients from NT pregnant women with 81.0% sensitivity and 35.0% specificity.
Furthermore, the ROC analysis in this study showed that PDW had the smallest AUC (0.68; 95% CI; 0.59–0.78). Despite having the shortest AUC, PDW can distinguish PE patients from NT pregnant women with 42.9% sensitivity, 93.7% specificity, and 68.25% test accuracy at a cut-off value of ≥16.75 fl. This suggests that PDW could be utilized as a marker to predict PE. Similarly, studies that were done by Tesfay et al.,7 and Yang et al.,58 showed that the PDW might be used as a candidate marker for predicting PE with AUC of 0.63 and 0.62 at cut-off points 10.85 and 13.5 fl, respectively. The small variation in the cut-off points of P-LCR and PDW with the previous studies could be attributed to the differences in automated hematology analyzer.53
4.1 Strengths and limitations of the study
The study's strength is that the preeclamptic patients and the NT pregnant women were age- and GW-matched. The main limitation of this study is that it is a single-center study, which limits the generalizability of the findings. Another drawback of this study is that the sample size is not large, which may limit the statistical power of the study, and it was a nonlongitudinal study. Furthermore, exclusion of the participants was made by reviewing their medical records for the presence of conditions, which could only rule out pre-existing conditions.
5 CONCLUSIONS AND RECOMMENDATIONS
Based on the current findings, we can conclude that a decreased PC and an increased MPV, P-LCR, and PDW from the specified cut-off value can be used for the diagnosis of PE development among pregnant women. Of them, MPV had the largest AUC, which makes it the best indicator of PE development at a cut-off value of 12.1 fl. More importantly, the determination of PLT parameters such as PC, MPV, PDW, and P-LCR can be considered as a simple, rapid, and cost-effective procedure in the diagnosis of PE because they can be easily acquired from a routine CBC test. Therefore, we advise clinicians to use PLT parameters to diagnose PE development in pregnant women. Furthermore, multicenter longitudinal studies with a large sample size are required to verify the role of PLT parameters in the diagnosis of PE and to evaluate their role at various GW of pregnancy.
ACKNOWLEDGMENT
We appreciate the University of Gondar Comprehensive Specialized Hospital for permitting and creating a favorable condition to conduct this study. We would like to thank all study participants who volunteered to participate in this study. Our appreciation further goes to ANC clinic staff and diagnostic laboratory staff for their cooperation throughout the time of data collection.
AUTHOR CONTRIBUTIONS
Muluken Walle conceived the idea of this study. Muluken Walle, Zegeye Getaneh, Yemataw Gelaw, and Fikir Asrie were involved in the study designing, data organization, and data analysis, result interpretation, and drafting, write-up, reviewing, and editing of this manuscript. All authors approved the final manuscript.
COMPETING INTERESTS
The authors declare that they have no competing interests.
CONSENT FOR PUBLICATION
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.




