lncRNA-POIR promotes epithelial–mesenchymal transition and suppresses sorafenib sensitivity simultaneously in hepatocellular carcinoma by sponging miR-182-5p
Abstract
Sorafenib (SOR) resistance remains a major obstacle in the effective treatment of hepatocellular carcinoma (HCC). A number of long noncoding RNAs (lncRNAs) are responsible for this chemoresistance. This study aimed to reveal the essential function of a recently defined lncRNA, lncRNA-POIR, in the epithelial–mesenchymal transition (EMT) and SOR sensitivity of HCC cells. SOR-induced cytotoxicity was analyzed via cell counting kit-8 and ethynyl-2'-deoxyuridine incorporation assays, whereas immunoblotting and confocal immunofluorescence were used to determine the expression levels of EMT markers. Furthermore, loss- or gain-of-function approaches were used to demonstrate the role of lncRNA-POIR/miR-182-5p on EMT and SOR sensitivity in HCC. The direct interaction between lncRNA-POIR and miR-182-5p was verified using a luciferase reporter assay. We found that knockdown of lncRNA-POIR sensitized HCC cells to SOR and simultaneously reversed EMT. As expected, miR-182-5p was confirmed as the downstream target of lncRNA-POIR. Moreover, miR-182-5p overexpression clearly reversed EMT and promoted SOR-induced cytotoxicity in representative HCC cells, whereas miR-182-5p downregulation played a contrasting role; miR-182-5p knockdown abolished the modulatory effects of lncRNA-POIR siRNA on EMT and SOR sensitivity. Together, these pieces of data suggest that lncRNA-POIR promotes EMT progression and suppresses SOR sensitivity simultaneously by sponging miR-182-5p. Thus, we proposed a compelling rationale for the use of lncRNA-POIR as a promising predictor of SOR response and as a potential therapeutic target for HCC treatment in the future.
1 INTRODUCTION
Liver cancer is the sixth most commonly occurring malignancy, and the fourth leading cause of cancer-related death globally, with an annual incidence of 840,080 new cases diagnosed and a death rate of 781,630 people.1 Arising from hepatocytes, hepatocellular carcinoma (HCC) accounts for approximately ninety percent of all primary hepatic malignancies in adults.2 Surgical resection, liver transplantation, and radiofrequency ablation are the recommended therapeutic strategies for early-stage HCC, despite the fact that a significantly high proportion of the patients are diagnosed with the disease at an advanced stage.2, 3 The multikinase inhibitor—sorafenib (SOR)—is the first-line systemic therapeutic option for patients with advanced disease, and has been reported to significantly prolong the overall survival in patients from either the Western or Asia-Pacific region. Unfortunately, the benefits of SOR are only observed in one-third of the patients, and acquired drug resistance always develops within half a year, resulting in disease progression and an unfavorable prognosis.4, 5 Therefore, efforts are now underway for the development of novel bio-targets aiming to overcome SOR resistance in HCC.
Epithelial–mesenchymal transition (EMT)—a vital and reversible cellular program by which tumor cells transform from polarized epithelial phenotypes to motile mesenchymal phenotypes—was believed to play a crucial role in the tumorigenesis and progression of malignancies. The consensus view seems to be that tumor cells would generally acquire multi-drug resistance during this particular process.6, 7 Alternatively, our previous studies had revealed the presence of close correlations between EMT and doxorubicin chemoresistance in HCC.8, 9 In addition, growing evidence indicates that EMT and concomitant molecules are critical determinants in the resistance of HCC to SOR treatment.10-13 Hence, elucidation of the molecular mechanism underlying EMT induction may eventually help potentiate the antitumor effect of SOR.
Long noncoding RNAs (lncRNAs) are a newly identified category of limited protein-coding transcripts with a length of over 200 nucleotides.14 Till date, numerous lncRNAs have been shown to be implicated in diverse cellular signaling pathways in human cancer, making them novel potential biomarkers and therapeutic targets in cancer therapy.15, 16 Meanwhile, the aberrant expression profiles of lncRNAs in HCC have recently been annotated.17 Intriguingly, some of the abnormally expressed lncRNAs are involved in the triggering or inhibition of EMT during hepatocarcinogenesis, whereas some are likely to be responsible for inducing SOR chemoresistance in HCC.17-20 However, the potential underlying mechanisms have not been investigated thoroughly.
In this study, we aimed to validate the role of a critical lncRNA, human periodontal ligament stem cell (hPDLSC) osteogenesis impairment-related lncRNA (lncRNA-POIR),21 in EMT and SOR chemosensitivity. lncRNA-POIR was first discovered in hPDLSCs and was shown to facilitate the osteogenic differentiation of hPDLSCs via the inhibition of TCF-4 for β-catenin and subsequently the canonical Wnt pathway.21 However, we revealed the biological functions of lncRNA-POIR as an aberrantly expressed lncRNA in HCC cells involved in modulating EMT and SOR sensitivity. Specifically, knockdown of lncRNA-POIR potently reversed EMT and sensitized HCC cells to SOR in vitro. Moreover, mechanistic studies demonstrated that lncRNA-POIR modulates EMT and SOR sensitivity in malignant cells by serving as a competing endogenous RNA (ceRNA) for miR-182-5p. Our findings provide a convincing rationale for lncRNA-POIR as a novel therapeutic target and a predictor of SOR response in clinical HCC treatment.
2 MATERIALS AND METHODS
2.1 Cell culture
All HCC cell lines were obtained from the Shanghai Institute for Biological Sciences. Huh7 cells were cultured routinely in Dulbecco's modified Eagle's medium (high-glucose; Gibco) containing 10% fetal bovine serum (FBS; Gibco) at 37°C in humidified 5% CO2 atmosphere. Hep3B, SNU-387, and SNU-449 cells were incubated under the same conditions in MEM (Gibco), RPMI-1640 (Gibco), and RPMI-1640, respectively. All cells were used within 3 months of resuscitation, and cell passage was conducted when cells reached 80–90% confluence.
2.2 Reagents and antibodies
Sorafenib was purchased form BioVison (Milpitas), dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich), and utilized at the concentrations indicated. The final concentration of DMSO in any experiment was <0.5%. Primary antibodies against glyceraldehyde 3-phosphate dehydrogenase (GAPDH), E-cadherin, and vimentin were obtained from Abcam. The HRP-conjugated secondary antibody was purchased from the Beyotime Institute of Biotechnology.
2.3 Assessment of cell viability and proliferation
Cell viability was determined using a cell counting kit-8 (CCK-8), which was obtained from Dojindo. In brief, 5000 HCC cells were seeded in each of the 96-well microplates and incubated overnight. Afterwards, 10% FBS medium containing various concentrations of SOR (0, 1.25, 2.5, 5.0, 10.0, and 20.0 μM) was added for 48 h. Cells were then exposed to 10 µl of CCK-8 solution and incubated further for 2 h, according to the manufacturer's instructions. The optical density value at 450 nm was measured by a microplate reader. The relative cell viability was expressed as a ratio to the CCK-8 value of the control cells.
As for cell proliferation, cells were incubated with IC50 concentrations of SOR for 48 h and assayed by the utilization of the Click-iT EdU Imaging Kit (Invitrogen) in accordance with the usage instructions. Meanwhile, cells were counterstained with Hoechst 3344 for the visualization of all the living cells. Five random fields of vision were selected and observed under an Olympus inverted fluorescence microscope. Additionally, the percentage of proliferative cells was expressed as a relative value to the corresponding controls.
2.4 Quantitative real-time polymerase chain reaction
Total cell RNAs, including lncRNAs and miRNAs, were extracted using TRIzol reagent (Invitrogen) and reverse-transcribed using the Prime Script reagent RT Kit (Takara Biotechnology). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed using SYBR Premix ExTaq II kit (Takara) and ABI PRISM 7900HT (Applied Biosystems Inc). Primers for lncRNA-POIR were purchased from Takara (forward, 5′-CTCCTGTTTGGCCTGTTCAC-3′; reverse, 5′-CTCCTGTTTGGCCTGTTCAC-3′); the miR-182-5p RNAs were reverse-transcribed using a specific primer (RiboBio) according to the manufacturer's instructions. The expression levels of mRNA and miRNA were normalized to those of GAPDH and RNU6B, respectively, and determined using the 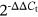 method.22 All experiments were repeated three times.
method.22 All experiments were repeated three times.
2.5 Cell transfection
Human lncRNA-POIR-specific siRNAs and negative controls (NCs) were synthesized by GenePharma Co., Ltd. MiR-182-5p mimics/inhibitors were also obtained from GenePharma. The transient transfection of lncRNA-POIR siRNA and miR-182-5p mimic/inhibitor was performed using Lipofectamine 2000 (Invitrogen) in accordance with the usage instructions. The sequences of lncRNA-POIR siRNAs, comprising three individual siRNAs, were as follows: ENST00000446358.1-homo-745 (sense, 5′-GCUGAGUCUAGUCCAAUCUTT-3′; antisense, 3′-AGAUUGGACUAGACUCAGCTT-5′), ENST00000446358.1-homo-651 (sense, 5′-CUUACCUCUUAUUGUUACUTT-3′; antisense, 3′-AGUAACAAUAAGAGGUAAGTT-5′), ENST00000446358.1-homo-575 (sense, 5′-GGAAUGACAUCUCCUGUUUTT-3′; antisense, 3′-AAACAGGAGAUGUCAUUCCTT-5′). The sequences of miR-182-5p mimic were 5′-UUUGGCAAUGGUAGAACUCACACU-3′ (sense) and 3′-UGUGAGUUCUACCAUUGCCAAAUU-5′ (antisense), and the sequence of the miRNA inhibitor was 5′-AGUGUGAGUUCUACCAUUGCCAAA-3′.
2.6 Confocal immunofluorescence microscopy
HCC cells were cultured in 24-well plates after transfected with the indicated siRNAs and miRNA inhibitors/mimics for 48 h. Cells then were fixed with either 4% paraformaldehyde or methanol/acetone for 15 min, incubated with 5% bovine serum albumin (BSA) for half an hour at room temperature, and the following primary antibodies were applied at 4°C overnight: mouse anti-human E-cadherin and anti-human vimentin (diluted 1:100; both Abcam). Subsequently, HCC cells were incubated with the corresponding FITC secondary antibody (Abcam) for 2 h, at a dilution of 1:200. Nuclei were visualized by 4ʹ,6ʹ-diamidino-2-phenylindole (1:10,000; Sigma-Aldrich) and measured under a Zeiss LSM800 Confocal Laser Scanning Microscope.
2.7 Immunoblot analyses
HCC cells were seeded and treated as described above, washed with 4°C phosphate buffered saline twice and collected in 50 μl of radioimmunoprecipitation assay buffer (Cell Signaling) which contains protease inhibitors. Protein concentrations were quantified using a bicinchoninic acid protein assay kit (Thermo Fisher Scientific). Immunoblotting was performed following standard protocols. Briefly, an equivalent amount of protein was fractionated on 10% tris-glycine polyacrylamide gels, transferred to polyvinylidene difluoride membranes (Millipore), blocked by 5% BSA, and subsequently incubated overnight with anti-E-cadherin/anti-vimentin/anti-GAPDH antibodies (all diluted 1:1000) at 4°C. Membranes were conventionally rinsed, incubated for 1 h with the corresponding secondary antibodies (diluted 1:2000), and developed via enhanced chemiluminescence. The immunoreactive bands were visualized by autoradiography. GAPDH was utilized as a loading control.
2.8 Luciferase reporter assay
The putative wild type (WT) and mutant (mut) miR-182-5p target binding sites to the lncRNA-POIR sequence were synthesized and cloned to Renilla luciferase vectors (Beyotime), respectively. lncRNA-POIR-WT/lncRNA-POIR-mut vectors were cotransfected with miR-182-5p/NC mimics to 293T cells. Luciferase activity was monitored by a dual-luciferase reporter gene assay kit (Beyotime) after 48 h of cotransfection.
2.9 Statistical analysis
Statistical calculations were performed in Prism 7 (GraphPad). All the experimental data are shown as mean ± SD or 95% confidence intervals (CI). One-way analysis of variance or the Student t test was conducted to assess the significance of the observed differences. p < .05 indicates statistical significance.
3 RESULTS
3.1 lncRNA-POIR expression is negatively correlated with SOR sensitivity in HCC cells
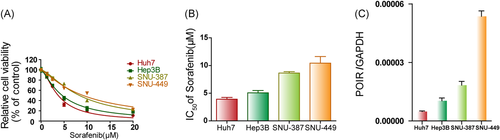
To investigate the cytotoxic effect of the multikinase inhibitor in vitro, dose-dependent cell viability was first determined after 48 h of SOR monotherapy utilizing CCK-8 assays (Figure 1A,B and Table S1). The mean half-maximal inhibitory concentration (IC50) values for SOR in Huh7, Hep3B, SNU-387, and SNU-449 cells were 3.731 μM (3.498–3.965 μM), 4.605 μM (4.281–4.928 μM), 8.348 μM (7.923–8.773 μM), and 9.15 μM (8.462–9.838 μM; 95% CI; n = 3), respectively. To determine the lncRNA responsible for SOR sensitivity, the expression levels of various novel lncRNAs were detected in SOR-treated HCC cells by PCR. Interestingly, treatment with SOR led to a significant upregulation of lncRNA-POIR expression in the HCC cell lines (Figure S1A). The relative expression levels of lncRNA-POIR in the aforementioned HCC cells were then measured through qRT-PCR. As revealed in Figure 1C, the expressions levels of lncRNA-POIR in SNU-449, SNU-387, Hep3B, and Huh7 cells went from high to low in that order, exhibiting an opposite trend relative to the sensitivity to SOR treatment. Therefore, lncRNA-POIR expression levels may be correlated to SOR sensitivity to an extent.
3.2 Knockdown of lncRNA-POIR sensitizes HCC cells to SOR
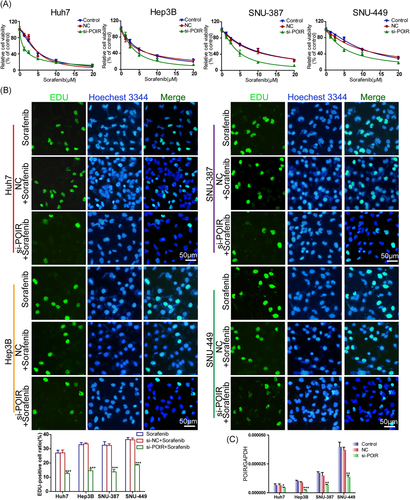
To further identify the potential role of lncRNA-POIR in the regulation of SOR sensitivity in HCC, loss-of-function experiments were performed with interfering oligonucleotides. As expected, the knockdown of lncRNA-POIR dramatically enhanced the strength of SOR-induced growth inhibition in Huh7 cells compared to NC siRNA-transfected cells, with a considerable decline in the mean IC50 value of 1.546 μM (1.339–1.753 μM; 95% CI; n = 3). Parallel results were observed in the other three cell lines (Hep3B, SNU-387, and SNU-449), accompanied by a significant reduction in the IC50 values, at 3.495 μM (3.191–3.799 μM), 4.111 μM (3.835–4.386 μM), and 5.634 μM (5.198–6.069 μM; 95% CI; n = 3), respectively (Figure 2A and Table 1).
| Cell lines | IC50 (μmol/L)a | ||
|---|---|---|---|
| Control | si-NC | si-POIR | |
| Huh7 | 4.299 (4.037–4.561) | 4.435 (4.018–4.851) | 1.546 (1.339–1.753) |
| Hep3B | 5.141 (4.678–5.604) | 4.749 (4.411–5.088) | 3.495 (3.191–3.799) |
| SNU-387 | 8.751 (8.143–9.358) | 8.221 (7.534–8.908) | 4.111 (3.835–4.386) |
| SNU-449 | 10.60 (9.891–11.30) | 10.18 (9.441–10.93) | 5.634 (5.198–6.069) |
- a IC50 values show sorafenib concentration (μmol/L, mean [95% confidence interval]). IC50, half-maximal inhibitory concentration.
In consideration of the failure of the CCK-8 assays in distinguishing between cytotoxicity and cell cycle arrest in non-quiescent cells, the EdU incorporation assay was conducted to assess cell proliferation in lncRNA-POIR siRNA-transfected cells incubated with SOR for 48 h. In agreement with the CCK-8 assay, lncRNA-POIR knockdown potentiated the proliferation inhibition of HCC cells induced by SOR compared to the empty vector-transfected groups. The EdU-positive cell ratios in the lncRNA-POIR knockdown cells were markedly lower than those in the control groups (Figure 2B). The efficiency of siRNA knockdown was validated by qRT-PCR (Figure 2C). Collectively, these data suggested that lncRNA-POIR knockdown restored the sensitivity of HCC cells to SOR.
3.3 lncRNA-POIR knockdown suppresses EMT
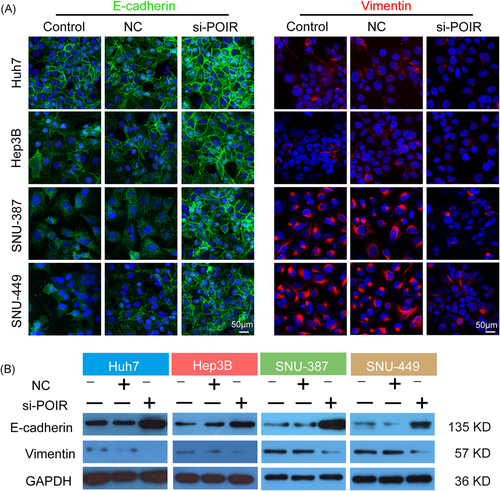
Considering the close correlation between EMT and concomitant molecules with SOR chemoresistance in HCC, we additionally investigated the effect of lncRNA-POIR knockdown on the EMT process. As revealed in Figure 3A, EMT marker characterization by confocal immunofluorescence analysis confirmed the diverse phenotypes of the aforementioned HCC cell lines. The degree of membrane localization of E-cadherin was intense in cells with the epithelial phenotype (Huh7 and Hep3B) but was extremely weak in those with the mesenchymal phenotype (SNU-387 and SNU-449). Conversely, HCC cells with a mesenchymal phenotype showed a higher intracytoplasmic vimentin expression level than those with the epithelial phenotype, in which the protein was hardly expressed. Nevertheless, the introduction of lncRNA-POIR siRNA observably enhanced E-cadherin fluorescence intensity and decreased vimentin expression in HCC cells, especially in cells with a mesenchymal phenotype, indicating EMT reversal (Figure 3A). The results of the immunoblot analyses were consistent with the immunofluorescence assay (Figure 3B), demonstrating an EMT promotion effect for lncRNA-POIR.
3.4 miR-182-5p is the downstream target of lncRNA-POIR and sensitizes HCC cells to SOR
Many lncRNAs are known to function as ceRNAs for specific miRNAs.23 Accordingly, lncRNA-targeted miRNAs were screened through the MicroInspector program online.24 Interestingly, the results appeared to suggest that miR-182-5p, a vital miRNA associated with drug resistance in cancer cells,25, 26 shared complementary bonds at 3′-untranslated regions of lncRNA-POIR (Figure 4A). Consistently, the expression levels of lncRNA-POIR were negatively associated with miR-182-5p expression, and those of miR-182-5p were remarkably enhanced in cells transfected with lncRNA-POIR siRNA compared to the empty vector-transfected cells (Figures 1C and S1B). Fluorescent in situ hybridization (FISH) assay was then performed, and lncRNA-POIR was found to be predominantly localized in the cytoplasm (Figure S1C), indicating the ceRNA function of lncRNA-POIR in HCC cells. To further confirm the direct interaction between lncRNA-POIR and miR-182-5p, luciferase reporter constructs were generated. As revealed in Figure 4B, the luciferase activity was notably decreased in the lncRNA-POIR-WT and miR-182-5p cotransfected group. However, the lncRNA-POIR-mut reporter was unaffected by miR-182-5p, indicating that miR-182-5p is directly targeted by lncRNA-POIR.
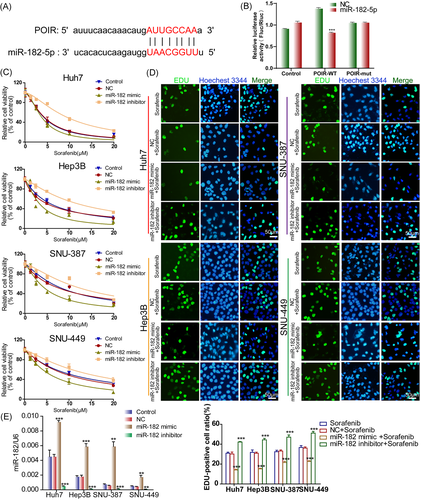
The role of miR-182-5p in SOR chemosensitivity was then examined. HCC cells were transfected with miR-182-5p mimic/inhibitor or the corresponding NC and subjected to the CCK-8 assay to validate whether miR-182-5p is implicated in SOR sensitivity. The overexpression of miR-182-5p robustly sensitized HCC cells to SOR, whereas its knockdown achieved an absolutely opposite effect (Figure 4C and Table S2). Consistently, lower proportions of EdU-positive cells were observed among miRNA mimic-transfected HCC cells, while miR-182-5p knockdown obviously increased the EdU-positive cell ratios compared with NC groups (Figure 4D). The efficiency of the miRNA mimic or inhibitor was detected using qRT-PCR (Figure 4E). These results implied that miR-182-5p is a downstream target of lncRNA-POIR and may be involved in SOR sensitivity in HCC cells.
3.5 miR-182-5p reverses EMT
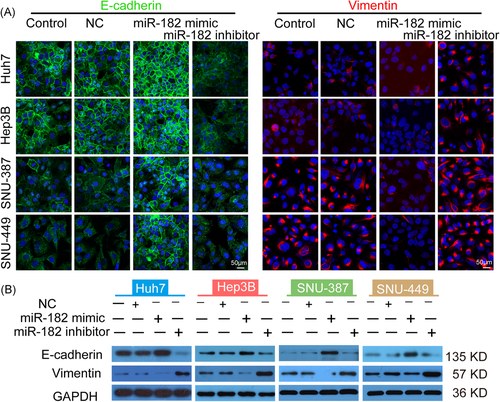
The impact of miR-182-5p on EMT was investigated to further illustrate the mechanism behind how this miRNA improves the in vitro sensitivity of HCC cells to SOR. As shown in Figure 5A, the introduction of miR-182-5p mimic led to a robust upregulation of E-cadherin expression and simultaneously downregulated the expression of vimentin, regardless of the cell phenotype. In contrast, the reduction in the level of miR-182-5p facilitated the mesenchymal feature formation in all of the HCC cell lines, especially in those with an epithelial phenotype (Huh7 and Hep3B; Figure 5A). Immunoblot analyses yielded consistent results with the confocal immunofluorescence assay (Figure 5B). Based on the aforementioned data, we hypothesized that miR-182-5p modulated SOR sensitivity in HCC via mediating the EMT process.
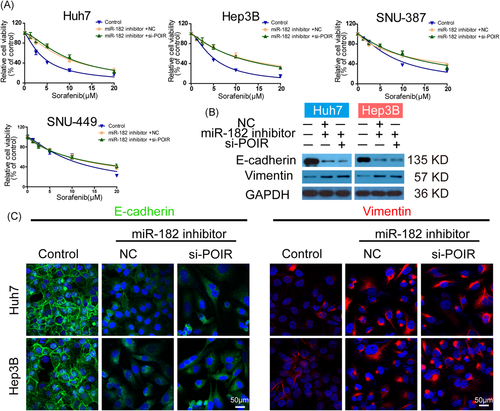
3.6 Knockdown of miR-182-5p abrogated the modulatory effect of lncRNA-POIR siRNA on SOR sensitivity and EMT
To verify whether lncRNA-POIR induced EMT and concurrently reduced SOR sensitivity in HCC cells in a miR-182-5p-dependent manner, miR-182-5p inhibitor + si-NC, miR-182-5p inhibitor + lncRNA-POIR siRNA or miRNA inhibitor NC + si-NC were cotransfected into HCC cells. In agreement with the aforementioned results, miR-182-5p-knockdown HCC cells seemed more resistant to SOR treatment. However, the inhibitory role of miR-182-5p in SOR sensitivity could scarcely be reversed by the further transfection of lncRNA-POIR siRNA (Figure 6A and Table S3). Meanwhile, lncRNA-POIR siRNA transfection failed to enhance the expression of E-cadherin and attenuate the intracellular abundance of vimentin in miR-182-5p-knockdown Huh7/Hep3B cells (Figure 6B). The immunofluorescence assay revealed results that were consistent with those of the immunoblot analyses (Figure 6C). Collectively, these results indicated the regulatory effect of the lncRNA-POIR/miR-182-5p axis on SOR sensitivity and EMT in HCC cells.
4 DISCUSSION
In this study, we found that miR-182-5p knockdown abolishes the modulatory effects of lncRNA-POIR siRNA on EMT and SOR sensitivity. SOR occupies a distinct position among the therapeutic approaches for HCC owing to its potential in conferring a survival benefit of nearly 3 months in patients with advanced disease.4, 5 However, the development of SOR resistance, regardless of whether it is primary or acquired, has notoriously emerged as a dominating obstacle. Thus, it is essential to clarify the mechanisms involved and identify predictive biomarkers of the response to SOR. In this study, we validated lncRNA-POIR as a tumor promoter and provided evidence that high expression levels of lncRNA-POIR are predictive of poor response to SOR treatment.
lncRNA-POIR, first discovered in hPDLSCs, was confirmed to positively regulate the osteogenic differentiation of the cells in an inflammatory microenvironment.21 However, the role of this particular lncRNA in carcinogenesis and tumor progression remains unclear. In this study, we propounded for the first time that lncRNA-POIR promotes the EMT process and suppresses SOR sensitivity simultaneously in HCC cells. Mechanistically, lncRNA-POIR acted as a molecular sponge to downregulate miR-182-5p, which was shown to be a negative regulator of EMT and SOR insensitivity. Most lncRNAs are stable and detectable in body fluids, rendering them potential biomarkers and therapeutic targets for cancer treatment.15 lncRNA-POIR appears to be a promising predictor of SOR response in HCC due to the negative correlation between its expression and SOR sensitivity in hepatoma cells, and clinical trials are planned for validating its clinical implication. In contrast, lncRNA-targeting approaches, such as antisense oligonucleotide (ASO) therapy, have recently emerged as an attractive choice in oncotherapy.16 Our results present early evidence for utilizing lncRNA-POIR siRNA to improve the sensitivity of HCC cells to SOR. In patients with HCC, especially whose tumors express elevated levels of lncRNA-POIR, the combination of SOR with pharmaceutical downregulation of lncRNA-POIR is expected to be a valuable strategy in the near future. However, further preclinical studies including in vivo experiments are urgently needed for its validation.
EMT is a pivotal biological program that was recently found to be associated with enhanced cell invasion capabilities and multi-drug resistance.6, 27 Particularly, there seem to be complicated interactions between EMT and SOR efficacy in HCC. A growing literature supports the view that SOR prevents EMT acquisition.28-30 SOR was reported to restrain polarized macrophage-induced EMT in HCC cells by blocking the hepatocyte growth factor-Met signaling pathway.28 This observation was further supported by the finding that SOR exerts an inhibitory effect on EMT by the suppression of MAPK signaling and subsequently downregulation of SNAI1 expression in HCC cells.30 On the other hand, EMT contributes to the development of SOR chemoresistance. Our research indicates that epithelial-like cells were more susceptible to the multikinase inhibitor whereas mesenchymal-like cells showed relative resistance (Figures 1A,B and 3A,B), in agreement with the findings of a previous study.29 In addition, αB-Crystallin was found to foster tumor progression by inducing EMT in HCC cells in a recent study, consequently counteracting the effect of SOR.10 In contrast, the reversal of EMT boosted SOR antitumor responses in either HCC cell lines or patient-derived tumor xenograft models.11, 31 These observations support the assumption that the lncRNA-POIR/miR-182-5p axis may inhibit SOR sensitivity in HCC via EMT induction, even though we only observed simultaneous changes in EMT markers and SOR sensitivity during loss- or gain-of-function experiments. Further investigation in this field is urgently warranted to validate these views. Thus, EMT-targeted therapy is now expected to be a promising strategy aimed at improving SOR sensitivity in HCC.
A number of lncRNAs were suggested to be implicated as regulators of SOR sensitivity in HCC through various cellular signaling pathways. Li et al.32 have proved that lncRNA SNHG1 facilitates SOR chemoresistance via activating the canonical Akt pathway. Not coincidentally, lncRNA THOR and lncARSR were recently indicated to be related to poor SOR response due to their β-catenin and STAT3 pathway targeting activities, respectively.33, 34 Besides this, functioning as ceRNAs, lncRNAs mediate the SOR sensitivity of HCC cells through sponging corresponding miRNAs.35, 36 In our study, FISH was performed to demonstrate the potential of lncRNA-POIR in functioning as a ceRNA through the determination of the subcellular localization. Additionally, bioinformatics analysis, luciferase reporter assay, and cotransfection experiments verified that miR-182-5p was the downstream target of lncRNA-POIR in the regulation of EMT and SOR sensitivity (Figures 4 and 6). Nevertheless, further research in this area may include the precise binding mechanisms and binding sites for lncRNA-POIR and downstream miR-182-5p. Furthermore, several miRNAs were reported to be involved in the response to SOR in HCC.37-40 miR-423-5p was demonstrated to be upregulated in SOR-treated HCC cells, and its increase was positively correlated with response to therapy.39 Moreover, miR-125a-5p was found to mediate the antiproliferative activity of SOR in HCC cells by suppression of sirtuin-7 and subsequently inducing p21/p27-dependent cell cycle arrest,38 offering another category of possible targets to overcome SOR resistance in HCC.
miR-182-5p is known as an oncomiR. Several research studies have demonstrated the promoting role of miR-182-5p in HCC progression, such as through cell proliferation, invasion, angiogenesis, and distant dissemination.41-43 Nevertheless, the functions of miR-182-5p we characterized in the present study seemed distinct. The manipulation of the expression level of miR-182-5p with a mimic or inhibitor moderately enhanced or attenuated the cytotoxic effect of SOR, and significantly affected the EMT phenotypes in HCC cells (Figures 4C–E and 5). Consistent with our study, miR-182-5p was also shown to sensitize breast cancer cells to the DNA damage induced by PARP inhibitors.26 Furthermore, Lai and colleagues reported that the miR-182-5p-mediated downregulation of RAD51 promoted sensitivity to sapacitabine in acute myelogenous leukemia.25 These findings reinforce the concept that miR-182-5p is a positive mediator of chemotherapy response in a wide variety of tumors. Besides this, overexpression of miR-182-5p resulted in the reversal of EMT in the current study (Figure 5). In agreement with our results, Qu et al.44 proved that miR-182-5p contributed to MET features in prostate cancer. Similarly, miR-182-5p was found to enable the epithelial-like state via blocking the Met/AKT/Snail signal pathway in lung cancer cells45; therefore, it is plausible to characterize it as an EMT inhibitor.
In conclusion, our data demonstrate that lncRNA-POIR promotes EMT progression and suppresses SOR sensitivity simultaneously by sponging miR-182-5p. This systematic study is the first attempt to preliminarily demonstrate the role of lncRNA-POIR in HCC. The combination of pharmaceutical downregulation of lncRNA-POIR along with the conventional targeted therapeutic agent SOR may serve as a novel treatment option for HCC.
ACKNOWLEDGMENT
The study was supported by the National Natural Science Foundation of China (No. 81602092).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Bryan Wei Chen, Yue Zhou, and Tao Wei conceived and designed the study. Yue Zhou, Liang Wen, Yi-bo Zhang, Shi-chao Shen, Lei Ni, and Yi Wang performed the experiments. Bryan Wei Chen, Tao Wei, and Jian Zhang analyzed the data. Bryan Wei Chen, Tao Ma, Wen Chen, and Xue-li Bai wrote and revised the manuscript. All authors have read the manuscript and agreed to the submission.