Peimine inhibits the growth and motility of prostate cancer cells and induces apoptosis by disruption of intracellular calcium homeostasis through Ca2+/CaMKII/JNK pathway
Abstract
Prostate cancer (PC) is one of the most common malignant tumors in man. Peimine (PM) is a bioactive substance isolated from Fritillaria. Previous studies have shown that PM could inhibit the occurrence of a variety of cancers. However, the roles of PM in PC and its related mechanism have not been elucidated. Calcium (Ca2+) is an important intracellular messenger involved in a variety of cell processes. In this study, we found that the appropriate doses of PM (2.5, 5, and 10 μM) significantly inhibited the growth of PC cells (DU-145, LNCap, and PC-3), but has no significant effect on normal prostate cells (RWPE-1). In addition, PM treatment inhibited the invasion and migration of PC-3 cells and blocked the epithelial-mesenchymal transition process. These effects were exhibited a dose-dependent manner. Furthermore, the current results also showed that PM treatment significantly increased the Ca2+ concentration, the increased Ca2+ promoted the phosphorylation of Ca2+/calmodulin-dependent protein kinase II (CaMKII) and c-Jun N-terminal kinase (JNK), further inhibited the growth and invasion of PC-3 cells, and induced its apoptosis. Ca2+ chelator BAPTA-AM (1 μM) could counteract the increase of intracellular Ca2+ concentration. Similarly, JNK pathway inhibitor SP600125 (10 μM) also inhibited cell growth and invasion and induced apoptosis. In addition, experiments in nude mice showed that PM inhibited tumor formation through Ca2+/CaMKII/JNK signaling pathway. In conclusion, our results show that PM inhibits the growth and motility of prostate cancer cells and induces apoptosis by disruption of intracellular calcium homeostasis through Ca2+/CaMKII/JNK pathway.
1 INTRODUCTION
Prostate cancer (PC) is a common malignant tumor in the world.1 A total of 1 094 916 cases of PC patients were diagnosed worldwide in 2012, of which 307 481 died.2 Although PC has several promising treatments, the 5-year survival rate is still only about 27%. Treatment of castrated drug-resistant cancer may increase the microvessel density of tumors.6, 7 In addition, PC mortality is very high due to the high metastatic nature of tumor cells and their resistance to chemotherapy.8 As drug resistance increases, the survival rate is frustrating regardless of which drug is used. Therefore, innovative and novel treatment play an important role in the treatment of PC.
Peimine (PM), also known as verticine, is an alkaloid extracted from Fritillaria, such as Fritillaria cirrhosa D. Don, Fritillaria thunbergii Miq., and Fritillaria unibracteata Hsiao and K.C.Hsia.9, 10 PM, as an active component of Fritillaria, plays an important role in human health, such as antitussive, antiasthmatic, expectorant, analgesic, and anti-inflammatory Besides, previous studies have also shown that PM was associated with a variety of cancers, such as breast cancer,16, 17 leukemia,18 gastric cancer,19 RAW264.7 macrophages and HMC-1 cells.20, 21 However, the role and molecular mechanism of PM in PC remain unclear.
Intracellular calcium (Ca2+) signaling is involved in various physiological processes of the cell, including mitochondrial homeostasis, cell survival, and cell death.22 The endoplasmic reticulum (ER) is an important Ca2+ repository. Consumption of Ca2+ in ER could inhibits proliferation of transformed cells and induces apoptosis.23 The release of intracellular Ca2+ into the cytoplasm activates apoptosis pathway. Extensive studies suggested that elevated level of mitochondrial Ca2+ was associated with apoptosis in a variety of cancers, such as ovary cancer,24 liver cancer,25 and osteoblast cancer.26, 27 In addition, Ca2+ also induced caspase-12 activation, as well as caspase-9 and -3 cleavage, ultimately accelerating apoptosis. ER stress caused by Ca2+ homeostasis also induced apoptosis by inhibiting the expression of c-Jun N-terminal kinase (JNK) protein.
Simultaneously, wide-ranging evidence displayed that Ca2+ was involved in cellular regulation through the Ca2+/CaMKII/JNK pathway. Previous studies have shown that IL-1β promoted the expression of Fas death receptor by activating Ca2+/CaMKII/JNK pathway, thereby promoting the expression of MMP9.28, 29 In addition, Gao et al30 reported that angiotensin II (Ang II)-induced insulin resistance in adipocytes was regulated by TRPM2 through the Ca2+/CaMKII/JNK pathway. Furthermore, curcumin activated phosphorylated of Ca2+/calmodulin-dependent protein kinase II (CaMKII) and JNK under the stimulation of Ca2+.31 However, However, it is unclear whether Ca2+ can regulate PC via CaMKII/JNK pathway.
To date, the role of PM in human PC cells and its possible molecular mechanism are unclear. In this study, we first discuss the role of PM in PC cells and further investigate the underlying molecular mechanisms in vivo and in vitro. In short, we first provide direct evidence that PM may be used as a promising PC medicine.
2 MATERIAL AND METHODS
2.1 Cell culture and treatment
The immortalized prostate-derived cell line RWPE-1 and human PC cell lines (DU-145, LNCap, and PC-3) were purchased from American Type Culture Collection (Manassas, VA). RWPE-1 cells were maintained in K-SFM, while DU-145, LNCap, and PC-3 cells were maintained in minimal essential medium, Roswell Park Memorial Institute 1640 medium, and F-12K, respectively. All mediums were supplemental with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. (Invitrogen, Grand Island, NY) Cells were cultured at 37°C under 5% CO2. PM (molecular weight, 431.65 g/mol; Cas. 23496-41-5; purity over 98%) was obtained from Abcam (Cambridge, UK) and dissolved in dimethyl sulfoxide. All the cells were treated with different doses of PM (0, 0.1, 0.25, 1, 2.5, 5, 10, 25, 50, 100, and 200 μM) for 24 hours.
2.2 Cell viability
Cell Counting Kit-8 (CCK-8; Dojindo, Kumamoto, Kyushu, Japan) was used to monitor the cytotoxicity induced by PM according to the manufacturer's protocol. The cells were inoculated in 96-well plat at a density of 1500 cells per well and treated with PM (0, 0.1, 0.25, 1, 2.5, 5, 10, 25, 50, 100, and 200 μM) for 24 hours.17 Subsequently, the cells were incubated overnight in an incubator at 37°C and 5% CO2. 10 μL CCK-8 solution was added to each pore and cultured for 2 hours under the same conditions. The absorbance of each well at 450 nm was measured by a multifunctional microplate reader SpectraMax M5 (Molecular Devices, Sunnyvale, CA). All experiments were in triplicate.
2.3 BrdU staining
PC-3 cells were treated with different concentrations of PM (2.5, 5, and 10 μM) and cultured at 37°C with 5% CO2 for 24 hours. Then the cells treated with PM were inoculated into 96-well plate and cultured until the cell density was 50% to 60%. After adding 10% Bromodeoxyuridine (BrdU) for 4 hours, the cells were fixed with 4% paraformaldehyde for 20 minutes, permeated with 0.2% Triton X-100 for 10 minutes, and cocultured with BrdU antibody (ab6326; Abcam) overnight at 4°C (Frdbio, Wuhan, China). Finally, the cells were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (P36931; ProLong Gold Antifade Mountant with DAP; Thermo Fisher Scientific, Waltham, MA) and photographed with fluorescence microscopy (×200 microscope; Olympus, Corporation, Tokyo, Japan). All experiments were in triplicate.
2.4 Western blot assay
Protein samples were extracted with Nuclear and Cytoplasmic Protein Extraction Kit (P0028, Beyotime, China). The protein samples were separated by 8% sodium dodecyl sulfate polyacrylamide gel and transferred to polyvinylidene fluoride membrane (IPFL00010; Millipore, Billerica, MA). After blocking with blocking buffer, the proteins samples were incubated with primary antibodies and secondary antibodies (sc-516102; 1:1000; Santa Cruz Biotechnology, Delaware, CA) and detected with an enhanced chemiluminescent substrate kit (#6883; Cell Signaling Technology, Danvers, MA). The primary antibodies specific to Ki-67 (sc-23900, 1:1000), vascular endothelial growth factor (VEGF) (sc-7269; 1:1000), proliferating cell nuclear antigen (PCNA) (sc-71858; 1:1000), E-cadherin (sc-8426; 1:1000), vimentin (sc-80975, 1:1000), N-cadherin (sc-8424, 1:1000), caspase-3 (sc-271759; 1:1000), caspase-9 (sc-81650; 1:1000), CaMKII (sc-5306; 1:1000), p-CaMKII (sc-32289; 1:1000), JNK (sc-7345; 1:1000), p-JNK (sc-293136; 1:1000), and glyceraldehyde 3-phosphate dehydrogenase (sc-66163; 1:1000) were all from Santa Cruz Biotechnology. The relative content of protein was analyzed by the ImageJ software (National Institutes of Health, Bethesda, MD). All experiments are in triplicate.
2.5 Flow cytometry
The cells treated with PM (2.5, 5, and 10 μM) were digested with trypsin and fixed with 70% ethanol overnight at 4°C. Then 10 mg/mL RNaseA and propidium iodide (PI) were added to stain overnight at 4°C. Apoptosis rates were monitored by flow cytometry (Becton Dickinson, San Jose, CA) by use of the annexin V-FITC Apoptosis Detection kit (KeyGEN Biotech, Jiangsu, China) according to the manufacturer's instructions. Annexin V-fluorescein isothiocyanate (FITC)−/PI− (lower left) are normal cells, annexin V-FITC+/PI− cells (lower right) are early apoptotic cells, and annexin V-FITC+/PI+ (upper right) are late apoptotic cells. Annexin V−/PI+ (upper left) is necrotic. All experiments are in triplicate.
2.6 Transwell assay
PC-3 cells treated with PM (2.5, 5, and 10 μΜ) were starved in serum-free medium for 24 hours. The cell suspension was collected and added to the upper chamber (200 mL) covered with matrigel. The medium (600 μL) containing 20% FBS was added to the lower chamber and incubated at 37°C for 48 hours. Then the upper chamber was taken out and the residual cells in the upper membrane were removed by PBS and cotton bud. The cells were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. The cells were photographed under a microscope (Leica Microsystems, Wetzlar, Germany). All experiments were in triplicate.
2.7 Wound-healing assay
Mitomycin C (10 μg/mL) was added to the cell culture medium to inhibit the proliferation of cells.32 PC-3 cells treated with PM were cultured in an incubator with 5% CO2 at 37°C to form monolayer cells. The monolayer cells of PC-3 cells were torn open by the tip of aseptic microtubule, and the isolated cells were photographed by inverted microscope (Olympus IX70; Olympus Corporation) at 0 and 24 hours, respectively. The percentage of wound closure was estimated on the basis of the previous formula.33 All experiments were in triplicate.
2.8 Immunofluorescent assay
The nuclear metastasis of mesenchymal marker protein vimentin in PC-3 cells was detected by immunofluorescence. PC-3 cells treated with PM were fixed with 4% paraformaldehyde for 30 minutes, permeabilized with 0.5% Triton X-100 for another 30 minutes, and blocked with Tris-buffered saline with Tween 20 containing 5% bovine serum albumin (Affymetrix, Cleveland, OH) overnight at 37°C. After pretreatment with Vimentin primary antibody (1:50; ab92547; Abcam) overnight at 4°C, the cells were incubated with Alexa Fluor 594 secondary antibody (1:2000; catalog no. Z-25307; Thermo Fisher Scientific) and stained with DAPI (1:1000; catalog no. D9564; Sigma-Aldrich, St. Louis, MO) at room temperature for 3 hours. Subsequently, the cells were washed with PBS and examined by confocal microscopy (LSM 510 Meta; Zeiss, Oberkochen, Germany). Vimentin spots were counted from more than 10 microscopic views. Microscopic images were processed as described.34 All experiments were in triplicate.
2.9 Determination of intracellular Ca2+ concentration
The intracellular Ca2+ concentration was determined by Rhod-2/AM. The cells were cultured in Ca2+-free medium, treated with different concentrations of PM (0, 2.5, 5, and 10 μM) for 24 hours and loaded with Rhod-2/AM (5 mM) at 37°C for 45 minutes in the dark. Then, the cells were gently washed with the Hank-D solution (Ca2+-free medium) and analyzed by flow cytometry through FL2 channel. In addition, PC-3 cells were precultured in medium containing BAPTA-AM (1 μM) for 0.5 hours and then treated with PM (10 μM) for 24 hours. Intracellular Ca2+ concentration was measured by flow cytometry. All experiments were in triplicate.
2.10 Animal models
All animal experiments were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by Qingdao University. A total of 40 BALB/c nude mice (male, 4-week old) were obtained from the Animal Center of Qingdao University and housed in a controlled environment at 25 ± 3°C, humidity 60%, in a 12-hour light/dark cycle with free access to food and water. PC-3 cells were injected into the prostate to form orthotopic tumors.8 After successful modeling, the mice were randomly divided into four groups with ten in each group: control group, PM (10 mg), PM (20 mg), and PM (50 mg). The PM (10 mg) group, tumor-bearing mice were given 10 mg PM; the PM (20 mg) group, tumor-bearing mice were given 20 mg PM; and the PM (50 mg) group, tumor-bearing mice were given 50 mg PM. Tumor weight were measured after 25 days. After 25 days, the mice were euthanized by intraperitoneal injection of pentobarbital sodium (200 mg/kg body weight). Tumors were harvested for the following experiments. All experiments were in triplicate.
2.11 Terminal deoxynucleoitidyl transferase mediated nick-end labeling assay
The terminal deoxynucleoitidyl transferase mediated nick-end labeling (TUNEL) assay (C1098; Beyotime, Jiangsu, China) was used to detect the apoptosis of tumor tissues according to the manufacturer's instruction. The stained tissue was observed under a fluorescence microscope. All experiments were in triplicate.
2.12 Immunohistochemistry
Immunohistochemistry (IHC) assay was performed as previously described.35 Paraffin sections were separated in xylene and rehydrated in gradient ethanol. After the antigen was extracted in 10 mM citric acid buffer, the tissue sections were incubated in 3% H2O2 for 10 minutes and sealed at room temperature for 1 hour. The tissue sections were then incubated overnight with anti-Ki-67 (#12202; 1:400; CST) and anti-VEGF (#9698; 1:16; CST). The corresponding second antibody (#8114; CST) was incubated at room temperature for 1 hour. The images were observed under an optical microscope. All experiments were in triplicate.
2.13 Statistical analysis
The statistical analysis was undertaken with SPSS 21.0 (SPSS, Inc, Chicago, IL). Measurement data were presented as mean ± SD (x ± s), and data consistent with the normal distribution were analyzed by t test. Multiple sets of data were analyzed by one-way analysis of variance attended by the Bonferroni post hoc test. Enumeration data were available in a percentage or ratio and verified with the χ2 test. P < 0.05 was considered statistically significant.
3 RESULTS
3.1 Low concentration of PM inhibited the viability of prostate cancer cells
Cell viability was detected by CCK-8 assay. As shown in Figure 1B, the toxic effect of PM on PC cells and normal prostate cells exhibited a dose-dependent manner. When the doses were lower than 10 μM, PM significantly inhibited the viability of PC cells (DU-145, LNCap, and PC-3), but had no significant effect on the normal cells (RWPE-1). Moreover, of the three cell lines, PC-3 cells were the most sensitive to PM treatment. When the doses were higher than 10 μM, PM showed toxic effect on both types of cells. Therefore, the most sensitive PC-3 cell line and the nontoxic doses of PM (2.5, 5, and 10 μM) to normal cells were selected as follow-up studies.
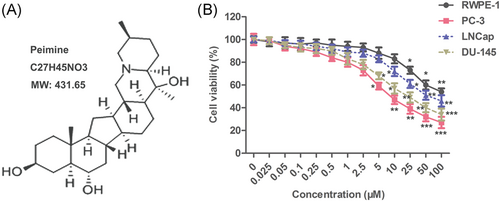
Low concentration of PM inhibited the viability of prostate cancer cells. RWPE-1, DU-145, LNCap, and PC-3 cells were treated with PM (0, 0.1, 0.25, 1, 2.5, 5, 10, 25, 50, 100, and 200 μM) for 24 hours. A, Structure of the PM molecule. B, Cell viability was monitored by CCK-8 assay. The results are presented as mean ± SD and represent three individual experiments. CCK-8, cell counting kit-8, PM, peimine. *P < 0.05 vs control, ** < 0.01 vs control, and ***P < 0.001 vs control
3.2 Peimine inhibited the proliferation of PC-3 cells and induced apoptosis in a dose-dependent manner
BrdU staining was used to evaluate the proliferation of cells. As shown in Figure 2A, different doses of PM (2.5, 5, and 10 μM) significantly inhibited the proliferation of PC-3 cells. The expression of proliferation marker protein Ki-67 and PCNA was detected by Western blot. As shown in Figure 2B, different doses of PM (2.5, 5, and 10 μM) decreased the levels of Ki-67 and PCNA in PC-3 cells in a dose-dependent manner. Apoptosis was detected by flow cytometry. As shown in Figure 2C, the apoptotic rates were 7%, 18%, and 29%, respectively, after treated with different doses of PM (2.5, 5, and 10 μM), which were significantly higher than those of untreated PC-3 cells (3.0%). In addition, the expression of apoptosis marker proteins (cleavage-caspase-3 and -9) also increased in a dose-dependent manner after PM treatment (Figure 2D). These results suggest that PM inhibited the proliferation of PC-3 cells and induced apoptosis in a dose-dependent manner.
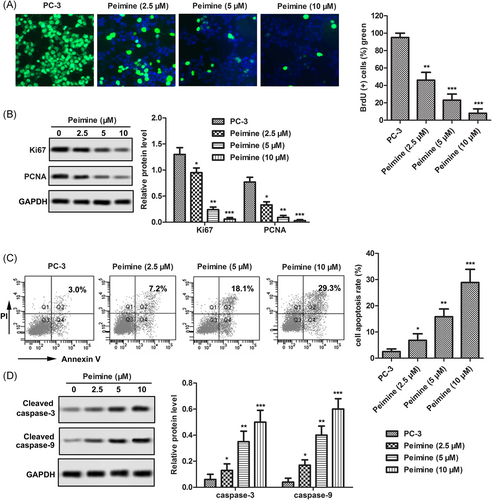
Peimine (PM) inhibited the proliferation of PC-3 cells and induced apoptosis in a dose-dependent manner. PC-3 cells were treated with different doses of PM (0, 2.5, 5, 10 μM) for 24 hours. A, Proliferation capacity was monitored by BrdU staining; ×200 magnification. B, Expression of proliferation marker proteins Ki-67 and PCNA were measured by Western blot. C, Apoptosis rate was monitored by flow cytometry. D, Expression of apoptosis marker protein cleaved caspase-3 and cleaved caspase-9 were monitored by Western blot. The results are presented as mean ± SD and represent three individual experiments. BrdU, Bromodeoxyuridine; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; PCNA, proliferating cell nuclear antigen. *P < 0.05 vs control, **P < 0.01 vs control, and ***P < 0.001 vs control
3.3 Peimine restrained invasion, migration, and epithelial-mesenchymal transition in PC-3 cells
Transwell assay was used to detect the invasiveness of PC-3 cells, while wound-healing assay was used to detect the ability of cell migration. As shown in Figure 3A,B, invasive and migrative abilities of PC-3 cells were decreased significantly after PM treatment compared with the control group. Western blot analysis was used to detect the expression of marker proteins in the process of epithelial-mesenchymal transformation (EMT). As shown in Figure 3C, compared with the control group, after treatment with PM, the levels of interstitial marker protein (N-cadherin) and angiogenesis marker protein VEGF in PC-3 cells were decreased significantly, while the level of epithelial marker protein (E-cadherin) increased. The effect of PM on the expression of vimentin was detected by immunofluorescence. As shown in Figure 3D, PM treatment significantly decreased the expression of vimentin in the nucleus of PC-3 cells, and the inhibitory effect of PM on vimentin expression increased with the increase of PM concentration. These results suggest that PM restrained invasion, migration, and EMT in PC-3 cells.
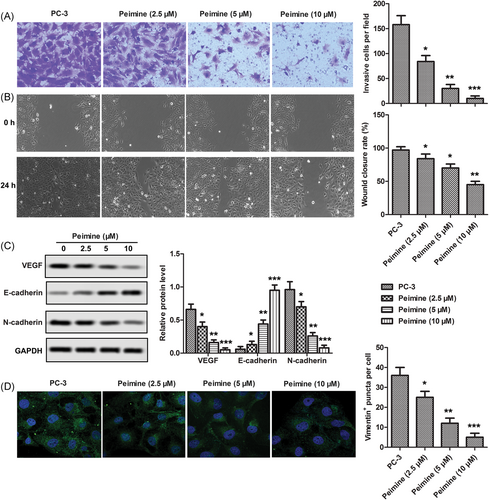
Peimine restrained invasion, migration, and epithelial-mesenchymal transition (EMT) in PC-3 cells. PC-3 cells were treated with various dosages of peimine (0, 2.5, 5, and 10 μM) for 24 hours. A, Invasion ability was monitored by transwell assay; ×200 magnification. B, Migration ability was monitored by wound-healing assay. C, Levels of EMT and angiogenesis marker proteins were measured by Western blot. D, Expression of vimentin was analyzed by immunofluorescence assay; ×200 magnification. The results are presented as mean ± SD and represent three individual experiments. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; VEGF, vascular endothelial growth factor. *P < 0.05 vs. control, **P < 0.01 vs control, and ***P 0.001 vs control
3.4 Peimine inhibited the growth and motility of PC-3 cells and induced apoptosis by regulating Ca2+/CaMKII/JNK pathway in vitro
As an important signaling molecule, Ca2+ plays an important role in the occurrence and development of tumor. In this study, intracellular Ca2+ concentration was measured by flow cytometry. As shown in Figure 4A, intracellular Ca2+ level was increased significantly after treatment with different doses of PM (2.5, 5, and 10 μM). After incubation with Ca2+ chelator BAPTA-AM (1 μM), the intracellular Ca2+ content was decreased from 55% to 43.68% (Figure 4B). In addition, the increase of Ca2+ concentration in cytoplasm significantly inhibited the growth and motility of PC-3 cells and induced apoptosis, while BAPTA-AM (1 μM) showed the opposite effect. (Figure 4C-E) Western blot analysis was used to detect the effect of PM on the phosphorylation of CaMKII and JNK in PC-3 cells. As shown in Figure 4F,G, exposure to different doses of PM (2.5, 5, and 10 μM) significantly increased the phosphorylation levels of CaMKII and JNK. In addition, after adding JNK inhibitor SP600125, the phosphorylation level of JNK was significantly lower than that of PM alone. The increase of JNK phosphorylation significantly inhibited the growth and motility of PC-3 cells and induced apoptosis. SP600125 showed the opposite effect (Figure 4H-K). In general, the results of this study showed that PM significantly increased the intracellular Ca2+ concentration, and the increased Ca2+ concentration activated the Ca2+/CaMKII/JNK pathway, thus inhibiting the growth and motility of PC-3 cells and inducing apoptosis.
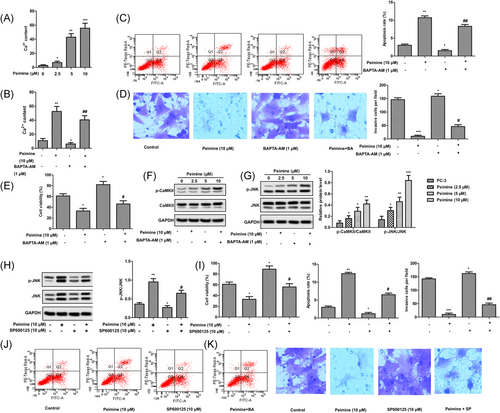
Peimine (PM) inhibited the growth and motility of PC-3 cells and induced apoptosis by regulating Ca2+/CaMKII/JNK pathway in vitro. PC-3 cells were treated with different doses of PM (0, 2.5, 5, and 10 μM) for 24 hours. A, Intracellular Ca2+ content was measured by flow cytometry. P < 0.05 vs control, **P < 0.01 vs control, and ***P < 0.001 vs control. B, PC-3 cells were treated with PM (10 μM) for 24 hours or BAPTA-AM (1 μM) for 0.5 hours and in combination with BAPTA-AM (1 μM). Intracellular Ca2+ content was measured by flow cytometry. *P < 0.05 vs control, **P < 0.01 vs control, and ##P < 0.01 vs peimine group. C, Apoptosis rates. D, Invasive ability. × 200 magnification. E, Cell viability. F, Phosphorylation level of CaMKII was monitored by Western blot analysis. G, Phosphorylation level of c-Jun N-terminal kinase (JNK) was monitored by Western blot analysis. *P < 0.05 vs control, **P < 0.01 vs control, and ***P < 0.001 vs control. H, PC-3 cells were treated with PM (10 μM) for 24 hours or SP600125 (10 μM) for 1 hour and in combination with SP600125 (10 μM). Phosphorylation level of JNK was monitored by Western blot analysis. Glyceraldehyde 3-phosphate dehydrogenase was used as a protein-loading control. I, Cell viability. J, Apoptosis rates. K, Invasive ability. ×200 magnification. The results are presented as mean ± SD and represent three individual experiments. *P < 0.05 vs control, **P < 0.01 vs control, and #P < 0.05 vs peimine group
3.5 Peimine inhibited tumor growth and induced tumor cell apoptosis by activating Ca2+/CaMKII/JNK pathway in vivo
PC-3 cells were injected subcutaneously into the flanks of nude mice to establish tumor-bearing mice model. After successful modeling, tumor-bearing mice were divided into four group with ten in each group and treated with different doses of PM (0, 10, 20, and 50 mg). TUNEL and IHC were used to detect the inhibitory effect of PM on prostate tumor in tumor-bearing mice, and Western blot analysis was used to detect its molecular mechanism. As shown in Figure 5A, the tumor weight in the PM treatment group was significantly lower than that in the control group. The results of TUNEL and IHC showed that PM could induce apoptosis of tumor cells and inhibit the expression of Ki-67 and VEGF in tumor tissues. (Figure 5C) Mechanically, Western blot showed that PM treatment significantly increased the phosphorylation of CaMKII and JNK in a dose-dependent manner (Figure 5B). This study suggests that PM inhibits tumor growth and induces tumor cell apoptosis by activating Ca2+/CaMKII/JNK pathway in vivo.
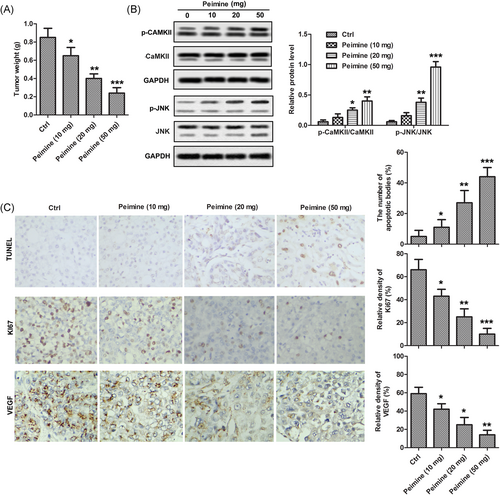
Peimine (PM) inhibited tumor growth and induced tumor cell apoptosis by activating Ca2+ group: control group, PM (10 mg), PM (20 mg), PM (50 mg) A, Tumor weight in each group. B, Phosphorylation levels of CaMKII and JNK were monitored by Western blot analysis. C, Apoptosis cells were monitored in tumor sections by TUNEL assay and Ki-67 and VEGF were detected by IHC assay. The results are presented as mean ± SD and represent three individual experiments. IHC, immunohistochemistry; JNK, c-Jun N-terminal kinase; TUNEL, terminal deoxynucleoitidyl transferase mediated nick-end labeling; VEGF, vascular endothelial growth factor. *P < 0.05 vs control, **P < 0.01 vs control, and ***P < 0.001 vs control
4 DISCUSSION
Fritillaria is an important Chinese herbal medicine plant, which has a variety of pharmacological effects, such as antitussive, expectorant, antiasthmatic, anti-inflammation, and antitumor.13, 36 Fritillaria contains a variety of bioactive components, such as peimine, peiminine, and sipeimine. Peimine and peiminine have significant inhibitory effect on 4T1 breast cancer cells, which regulated the tumor microenvironment by inhibiting the release of inflammatory related factors.17 Besides, peimine inhibited the cell viability and induced apoptosis of multidrug resistant human leukemia cell line K562/A02 by inducing reactive oxygen species (ROS) burst and decreasing Glutathione GSH level.37 Guo et al38 found that peiminine inhibited bleomycin-induced pulmonary inflammation and pulmonary fibrosis injury by reducing the level of circulating interferon-γ and inhibiting the signal transduction pathway. Lyu et al39 reported that peiminine inhibited the proliferation and growth of colorectal cancer cell line HCT-116 by inducing autophagy death. Similarly, Zheng et al40 found that peiminine induced apoptosis and autophagy by regulating PI3K/AKT/mTOR pathway, thus inhibiting the proliferation of colorectal cancer cells. In addition, Tang et al41 believed that peiminine could be used as an adriamycin chemosensitizer for gastric cancer to improve the sensitivity of gastric cancer to doxorubicin. Zhao et al42 found that peiminine significantly inhibited the proliferation of GBM cells, blocked cell cycle, blocked autophagy flux, and inhibited the growth of U251 glioma in vivo. Furthermore, Chang et al43 reported that Matrine suppressed PC via activating unfolded protein response/ER stress signaling and inhibiting EMT. In the present study, the results found that PM inhibited the growth and motility, and induced apoptosis of prostate cancer PC-3 cells in vivo and in vitro. Additionally, numerous studies demonstrated that JNK could be activated by CaMKII.29, 30 Likeness, the present study indicated that PM accelerated phosphorylation of CaMKII, and promoted the activation of JNK in a dose-dependent manner. The addition of JNK inhibitor SP600125 could alleviate the above increase. In conclusion, we believe that CaMKII, as a signal intermediate molecule, binds Ca2+ signal to JNK to inhibit the tumorigenesis of PC-3 cells. These results suggest that PM destroys intracellular Ca2+ homeostasis through Ca2+/CaMKII/JNK signaling pathway and plays a key role in anticancer.
Furthermore, numerous studies have shown that PM inhibited the growth and induce apoptosis in various cancers. As reported by Chen et al,16 PM significantly inhibited the proliferation of breast cancer cells, induce apoptosis and block cell cycle. Further studies have found that the inhibitory effect of PM on breast cancer was mainly achieved by inhibiting the release of inflammatory related factors and regulating the tumor microenvironment.17 More importantly, PM inhibited the viability and induces apoptosis of multidrug resistant leukemia K562/A02 cells by inducing ROS burst and increasing GSH content.18 Consistent with the above results, our study showed that PM significantly inhibited the proliferation of PC-3 cells by regulating the expression of proliferation-related proteins, and induced apoptosis by increasing Ca2+ concentration in cytoplasm to activate ER stress pathway in vitro and in vivo.
The study of pharmacokinetics is of guiding significance for the clinical use of PM. After oral administration of 4.25 g/kg Fritillaria thunbergii Miq. extract in SD rats, the blood concentration of PM in rat blood was detected at different time points. The results showed that the lower limit of quantification of PM and PMI was 2.0 ng/mL in skin and urine samples, while the lower limit of quantification in other tissues was 1.0 ng/mL. In addition, the excretion of PM and PMI in plasma of male rats was slower than that of female rats, and there were significant gender differences in pharmacokinetic parameters. The drug concentration in blood and tissue of male rats was significantly higher than that of female rats, indicating that the absorption of PM and PMI in male rats was higher and the therapeutic dose needed was higher.44 In addition, Wang et al45 gave male Beagle dogs oral administration of 1 g/kg Fritillaria ussuriensis Maxim and F. thunbergii Miq, respectively. The blood concentration was measured at different time points. The results showed that the two drugs showed a slow absorption and elimination process in Beagle dogs.45 Consistent with these results, in this study, the oral doses of PM (10, 20, and 50 mg) in rats is reasonable and safe.
As a crucial intracellular messenger, Ca2+ affects plenty of physiological processes, such as apoptosis.31 Apoptosis is an important way of cell death in the body. At present, the main apoptosis pathways of eukaryotic cells are external apoptosis mediated by death receptor, internal mitochondrial pathway, B-granulase-mediated apoptosis pathway and endoplasmic reticulum stress pathway. The apoptosis pathway involved in this study might be endoplasmic reticulum stress pathway. External stimulators could lead to the release of Ca2+ from ER into cytoplasm, such as curcumin31 and fibronectin.46 Increased Ca2+ in cytoplasm further activated CaMKII and then induced apoptosis31, 47 Similarly, the present study exerted that PM treatment increased the concentration of Ca2+ in cytoplasm, led to the destruction of intracellular Ca2+ homeostasis, caused ER stress, activated caspase-12 to cleave caspase-9, and further cleave caspase-3. In addition, ER stress further activated JNK protein and accelerated apoptosis.
In conclusion, our research reveals a new mechanism of PM in PC. In this study, we demonstrated that PM (2.5, 5, and 10 μM) could inhibit the proliferation and motility of PC-3 cells and induce apoptosis in a dose-dependent manner by increasing Ca2+ concentration in cytoplasm and promoting the phosphorylation of CaMKII and JNK. These results conclude that PM has antitumor activity by disruption of intracellular calcium homeostasis through Ca2+/CaMKII/JNK pathway in PC.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
HT and XW participated in the design of the study, HT, GZ, XY, and TJ performed the experiments, TJ and DS analyzed the data. HT and DS wrote the manuscript. HT and XW conceived and designed the study, analyzed the data and was involved in manuscript preparation. All authors read and approved the final manuscript.