Glucose Promotes a Pro-Oxidant and Pro-Inflammatory Stromal Microenvironment Which Favors Motile Properties in Breast Tumor Cells
ABSTRACT
Chronic inflammation and metabolic reprogramming have been proposed as hallmarks of cancer development. Currently, many of the functional clues between these two phenomena are studied under the integrative view of functional stroma–epithelia interaction. It has been proposed that stromal cells, due to their abundance and avidity for glucose, are able to modify the metabolic behavior of an entire solid tumor. In the present study, using a mammary stromal cell line derived from healthy tissue subjected to long-term culture in low (5 mM) or high (25 mM) glucose, we found that the hyperglycemic condition favors the establishment of a pro-inflammatory and pro-oxidant environment characterized by the induction of the COX-2/PGE2 axis. In this condition, epithelial migration was stimulated. Moreover, we also found that stromal-derived PGE2, acting as a stimulator of IL-1 epithelial expression was one of the factors that promote the acquisition of motile properties by epithelial cells and the maintenance of a COX-2/PGE2-dependent inflammatory condition. Overall, our work provides experimental evidence that glucose stimulates a tumor inflammatory environment that, as a result of a functional cross-talk between stroma and epithelia, may be responsible for tumor progression. J. Cell. Biochem. 118: 994–1002, 2017. © 2016 Wiley Periodicals, Inc.
There is growing recognition that chronic inflammation and oxidative stress are strongly associated with the development of cancer [Balkwill et al., 2005; Belousov et al., 2006; Berrino et al., 2014]. In many types of tumors, it has been proposed that chronic and subclinical levels of inflammation precludes oncogenic development and, eventually, can constitute a therapeutic target [Caldefie-Chezet et al., 2005]. Under inflammatory stimulus, normal and tumor-associated cells are able to mount a transcriptional response that enhances the expression of specific pro-inflammatory genes such as cyclooxygenase-2 (COX-2). Increased expression of COX-2 has been reported in many types of human cancers including breast cancer. Several epidemiologic studies indicate that regular use of non-steroidal anti-inflammatory drugs (NSAIDs) which inhibit COX-2, reduce the incidence of at least some types of human cancers like sporadic and familial colon cancer, pancreatic cancer, melanoma, and breast cancer [Cirri and Chiarugi, 2012; Chocarro-Calvo et al., 2013]. The specific role of COX-2 expression in breast cancer and its value as prognostic factor has not been fully elucidated. However, numerous studies have proposed that COX-2 expression is associated with worse survival of patients [Curiel-Lewandrowski et al., 2011]. The majority of studies have evaluated the expression of COX-2 by the epithelial tissue, without considering the role of stromal cells in its regulation. Nonetheless, recent studies have shown that COX-2 expression by stromal cells was associated with worse survival compared to those found for the tumor epithelial component [de Pedro et al., 2015].
Although there is strong evidence that genetic modifications in tumor cells undoubtedly initiate and drive malignancy, it is also clear that tumor progression occurs in close association with non-epithelial cellular components or tumor stroma [Dowling et al., 2012]. Particularly, it has been widely demonstrated that the interplay between breast tumor cells and cancer-associated fibroblasts (CAFs), the most abundant cellular component of the tumor, largely determines tumor proliferation rate and expression of malignant traits in many experimental models [Eligini et al., 2009; Fiaschi et al., 2012]. With this concept in mind, it is conceivable to expect that epithelial cells and CAFs, in a cooperative manner and through the expression of soluble factors, can determine the expression and/or maintenance of an inflammatory microenvironment prone to tumor progression. Inflammatory cytokines, like interleukin 1β (IL-1β), constitute a good example of this cellular cross-talk. IL-1β is often present in the inflammatory milieu of many tumors and its abundance has been significantly correlated with relapse and advanced disease [Gatenby et al., 2006].
Recent data support the concept that the metabolic behavior of tumor stroma can influence the tumorigenic potential of the epithelial compartment. This hypothesis posits that CAFs are induced by reactive oxygen species released by cancer cells to switch on aerobic glycolysis secreting lactate and pyruvate that, in turn, can serve as oxidative fuel for cancer cell proliferation [Hanahan and Coussens, 2012]. In the context of a tumor, fibroblasts behave as avid glucose consumers that supply energy to epithelia in the form of soluble lactate or pyruvate [Hirst et al., 2008]. It is interesting to notice that an inflammatory signal is present both at the onset of this heterotypic metabolic process [Howe, 2007] and during its maintenance. Recent work has provided evidence that metabolic reprogramming in stromal component generates an environment conducive to inflammation that maintains a pro-tumorigenic status of stromal cells that ensures epithelial cell malignancy [Hu et al., 2002].
Results from our group have demonstrated that soluble epithelial factors stimulate an oxidative response in mammary stromal cells that depend on NADPH4 activity [Kuperwasser et al., 2004]. Thus, in the context of a reciprocal epithelial/stromal cross-talk, we propose that an epithelial-induced stromal metabolic reprogramming, through the establishment of an oxidative environment, can be the starting point for an inflammatory reaction. In this regard, it has been proven that oxidative stress is capable of stimulating the expression of COX-2 [Li et al., 2012].
Epidemiological data indicate that hyperglycemia, insulin resistance, and obesity increase the risk of breast cancer [Lisanti et al., 2013]. It has been proposed that diabetes can promote cell growth and cancer acting through the insulin growth factor receptor signaling, which has led authors to relate cancer development with hyperinsulinemia more than hyperglycemia [Martinez-Outschoorn et al., 2011]. However, more recent data demonstrate that glucose levels may directly modulate cancer-related signaling pathways which are especially relevant given the increased-glucose-consumption characteristic of tumors [Pantschenko et al., 2003]. Moreover, it has been proposed that in conditions of persistent hyperglycemia, such as diabetes, glucose flux through the glycolytic pathway exerts an electron pressure on the mitochondrial electron transport chain to ameliorate this pseudohypoxic condition that produces an electron leakage that yields superoxide generation [Perrier et al., 2009].
In the present study, we report that human mammary stromal cells subjected to a prolonged culture in high (25 mM) glucose conditions mounted a pro-inflammatory response that promotes epithelial migration when it is compared to cells cultured in low (5 mM) glucose. This differential response seems to originate in the capacity of stromal cells, cultured at high glucose to induce a change in redox status and to activate the COX-2-PGE2 axis. IL-1β produced by epithelial cells appears as a candidate to stimulate, in a paracrine manner, a stromal inflammatory response. Also, we have found that PGE2 is able to stimulate epithelial migration. In the case of MDA-MB 231 cells (more migratory), we propose that this migratory stimulus depends on IL-1 binding to the membrane receptor. Our results suggest that mammary stromal cells cultured in high glucose induce the functioning of a feedback circuit in which IL-1 β enhances stromal COX-2 expression and PGE2 production which, in turn, may enhance epithelial IL-1 production, would be the final responsible for epithelial migration.
MATERIALS AND METHODS
CELLS
Human mammary cell lines MCF-7 and MDA MB-231 were obtained from ATCC (Manassas, VA) and were cultured in DMEM/F12 medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT) and maintained in a humidified atmosphere of 37°C, 5% CO2. RMF-EG stromal cells were kindly provided by Dr. Charlotte Kuperwasser (Tufts University, Boston, MA) and cultured in DMEM medium (Invitrogen) supplemented with 10% FBS [Pisani, 2008]. These cells were maintained in high (25 mM) or low (5 mM) glucose concentrations for 4 weeks before each experiment.
CELL IMAGING
To evaluate the cellular redox state in living cells, we used the FRET nanosensor Hyper [Radisky and Radisky, 2007] codified in a commercial vector (Evrogen, Moscow, Russia) and incorporated in adenoviral particles. Hyper is a fusion protein that acts as a type of FRET nanosensor and is composed by a regulatory domain (OxyR) from Escherichia coli built with two cysteine residues able to be oxidized only by hydrogen peroxide to form a disulfide bond. The conformational change induced by the oxidative reaction is transmitted to the fluorescence motives that, depending of their closeness, can transfer energy among them and change the fluorescence spectrum. Thereby, according to oxidative state of sulfhydryl groups, changes in emission spectra of Hyper from the blue (cyano-fluorescent CFP) to yellow protein (YPF), report the presence of hydrogen peroxide fluorescence. Imaging was carried out in a Nikon Ti microscope with a 40× objective equipped with a monochromator (Cairn Research, Favershan, UK), which allows dual excitation at 420 and 490 nm, whereas emitted light was collected over 525 nm with a long pass filter. Images were digitalized using a CCD camera (ORCA3, Hamamatsu, Japan) and data were expressed as ratio between emitted light at 490 and 420 nm (490/420). Experiments were conducted 48–72 h after infection at room temperature (24–26°C) in KRH buffer (in mM: 140 NaCl, 4.7 KCl, 20 Hepes, 1.25 MgSO4, 1.25 CaCl2, pH 7.4) supplemented with 5 mM of glucose. We applied two consecutive pulses of H2O2 of 50 and 500 μM; the last concentration saturating and useful to check the dynamic range of the biosensor.
CELL MIGRATION ASSAY
MCF-7 and MDA-MB231 cell migration was studied using a 6.5-mm Transwell chamber with a pore size of 8 mm (Corning, Corning, NY). The Transwell membranes were coated with 10% FBS in culture media for 2 h at 37°C only on the underside. MCF-7 and MDA-MB 231 cells (5 × 104) were re-suspended in serum-free medium and seeded on the upper compartment of the chamber. RMF-EG cells derived from low- and high-glucose culture conditions that were untreated or pre-treated with NS-398 were used to induce migration of epithelial cells. For this, 4 × 104 RMF-EG cells, suspended in each culture media enriched with 1% FBS, were incubated with 10 μM NS-398 for 24 h and were then washed with PBS twice, before placing the insert with migrating MCF-7 cells. In experiments in which migration was stimulated with PGE2, MCF-7 and MDA MB-231 cells were pre-incubated with PGE2 (10 pg/ml) for 24 h in serum-free media and the drug was maintained during migratory assay. In another set of experiments, epithelial cell migration was stimulated to migrate by media conditioned by stromal RMF-EG cells derived from high- and low-glucose cultures in the presence and the absence of 5 μM PGE2 receptor (EP4) inhibitor L-1 61,982. Migration was allowed to occur for 24 h (MCF-7) or 6 h (MDA-MB 231), against a gradient of 2% FBS. After this, cells of the upper membrane surface were removed by a cotton swab and repeatedly washed with PBS. Migration values were determined by counting five (×20) fields per chamber after fixing the membrane in methanol and staining the migratory cells on the lower side of the membrane with 0.2% crystal violet [Kuperwasser et al., 2004].
WESTERN BLOT AND ANTIBODIES
The expression of COX-2 and IL-β protein in RMF-621 and epithelial MCF-7 and MDA-MB 231 cells was evaluated by Western-blot. Briefly, cells were lyzed in lysis buffer (50 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.4, 150 mM NaCl2, mM MgCl2, 2 mM ethyleneglycol-bis (aminoethylether)-tetraacetic acid, 1% Triton X–100, and 10% glycerol) supplemented with complete protease inhibitors (Roche, Mannheim, Germany). Protein concentration of cell lysates was determined using a Pierce BCA Protein Assay kit (Thermo, Rockford, IL). Protein extracts were heat denatured in sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis loading buffer 4× (240 mM Tris–HCl, pH 6.8, 8% SDS, 40% glycerol, and 20% 2-mercaptoethanol). Equal amounts of protein from different treatments were resolved by SDS–polyacrylamide gel electrophoresis in 10% acrylamide gels and electrotransferred to polyvinylidene difluoride membranes using a buffer containing 24 mM Tris, 194 mM glycine, and 20% methanol. Proteins were further analyzed using the Pierce ECL Western Blotting Substrate (Thermo, Rockford, IL). The immunoreactions were achieved by incubation of the membranes, previously blocked with a solution containing 5% bovine serum albumin in tris-buffered saline and 0.05% Tween 20 (Sigma, St. Louis, MO), with anti COX-2 rabbit polyclonal antibody (sc-1745), anti IL-1β goat monoclonal antibody (sc-7884) both from Santa Cruz (Santa Cruz, CA), mouse anti-beta actin (A5441) and anti-tubulin (T5168) from Sigma (St. Louis, MO).
QUANTITATIVE PCR
Total RNA was isolated with Trizol (Ambion, Carlsbad, CA) from RMF-621 and epithelial MCF-7 and MDA-MB231 cells according to manufacturer instructions. Reverse transcription to complementary DNA was performed with 1 µg of RNA from each sample using M-MLV reverse transcriptase and oligo-dT (Promega, Madison, WI) as primer, according to manufacturer protocol. Messenger RNA (mRNA) expression was assessed by real-time PCR using a Light Cycler instrument (Roche, Mannheim, Germany). The reaction was performed using 100 ng of complementary DNA and LightCycler® FastStart DNA Master SYBR Green I kit (Roche, Mannheim, Germany) in a final volume of 20 µl. All reactions were performed in duplicate and negative controls were included. The primers used were as follows: COX-2 (forward), 5′ TTCAAATGAGATTGTGGGAAAATTGCT 3′, COX-2 (reverse), 5′AGATCATCTCTGCCTGAGTATCTT 3′; IL-1β (forward), 5′AATCCCCAGCCCTTTTGTTG 3′, IL-1 β (reverse), 5′AAATGTGGCCGTGGTTTCTG 3′; GPDH (forward), 5′ CAAAATCA AGTGGGGCGATGC TG 3′, GPDH (reverse), and 5′TGTGGTCA TGAGTCCTTCCACGAT 3′.
RESULTS
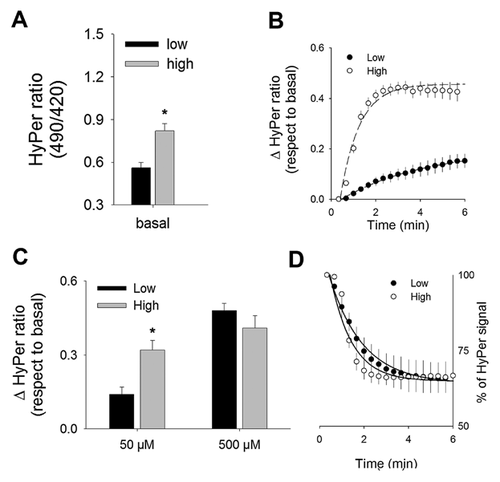
MAMMARY STROMAL CELLS CULTURED IN HIGH GLUCOSE ELICIT INCREASED BASAL LEVELS OF H2O2 ALONG WITH AN EXACERBATED RESPONSE TO MILD OXIDATIVE CHALLENGE
To evaluate the impact of high glucose on the cellular redox homeostasis of RMF-EG cells, we introduced a biosensor specific for hydrogen peroxide (HyPer) at cytoplasm of cells subjected to a long-term culture at low (5 mM) and high (25 mM) glucose. According to HyPer recordings, cells pre-cultured at high glucose showed significant higher of HyPer signal at basal level than those pre-cultured at low glucose (Fig. 1A). Next, to evaluate how cytoplasmic environment reacted upon a transient and mild oxidative challenge of H2O2 (50 µM), we analyzed HyPer signal rising in cells derived from low- and high-glucose conditions. As Figure 1B shows, low glucose-derived cells elicited a gradual increase in ratio signal during the pulse of H2O2 (50 µM), with an estimated initial velocity of 3.5 ± 0.4/min. On the contrary, cells cultured in high glucose showed significant higher rate of increase estimated in 53 ± 9/min. Biosensor response to 50 µM H2O2 culminated with significantly different values at this new equilibrium, probably as a consequence of different cytoplasmic environment and not due to changes in biosensor sensitivity, in fact, dynamic range of biosensor was similar in cells cultured at low- and high-glucose conditions based on maximal response obtained with the 10-fold more concentrated H2O2 pulse (Fig. 1C). With a similar purpose, we evaluated the capacity of the intracellular environment to restore the biosensor signal toward its basal condition analyzing the recovery rate just after removal of the H2O2 500 µM pulse. Kinetic parameters estimated from recovery curves obtained from cells cultured at low and high glucose did not show significant differences, suggesting that the reductive machinery is similar in cells cultured in low and high glucose (Fig. 1D).
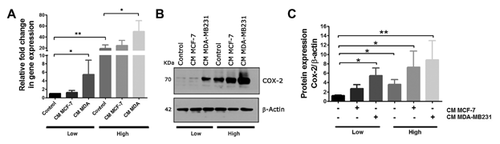
GLUCOSE MODULATES EPITHELIAL STIMULUS ON THE EXPRESSION OF COX-2 IN HUMAN MAMMARY STROMAL CELLS
To test whether glucose content and soluble factors derived from epithelial cells affect the expression of pro-inflammatory markers, RMF cells derived from low- and high-glucose conditions were pre-treated with 30% media conditioned by a mild invasive (MCF-7) and a strongly invasive mammary epithelial cell line (MDA-MB2311) and the expression of COX-2 was further evaluated by q-PCR and western blot. As Figure 2A and B show, stromal cells cultured in high glucose expressed a higher level of COX-2 at basal level and respond to epithelial stimuli in a dose-dependent manner. Media conditioned by the more invasive MDA-MB231 cells were more effective in the stimulation of COX-2 under both glucose conditions.
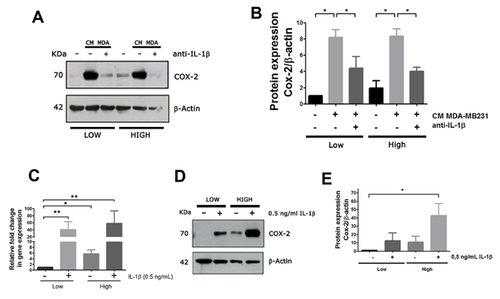
IL-1β PRESENT IN EPITHELIAL CONDITIONED MEDIA STIMULATES COX-2 EXPRESSION BY STROMAL CELLS
IL-1β and α have been proposed as soluble factors produced by tumor cells that could play a relevant role in tumor progression [Sicking et al., 2014]. To investigate the contribution of these cytokines in the stimulus to COX-2 stromal expression, we performed two types of experiments. On the one hand, we analyzed the stimulus of media conditioned by epithelial cells on stromal COX-2 expression as above, but in the presence of a blocking antibody to IL-1. As epithelial stimulus to COX-2 expression, we choose the media conditioned by MDA-MB231 cells that had previously shown high stimulating activity. Figure 3A shows that the presence of the blocking antibody almost completely eliminated epithelial stimulus on stromal COX-2 expression. A densitometry analysis of three separate replicates is shown in Figure 3B. Additionally, we analyzed the effect of IL-1 β on COX-2 expression. To do this, we incubated RMF-621 cells with 0.5 ng/ml of IL-1 β for 48 h and evaluated COX-2 production by qPCR (Fig. 3C) and western blot (Fig. 3D and E). These results confirm that IL-1β represents a specific epithelial stimulus to stromal COX-2 expression.
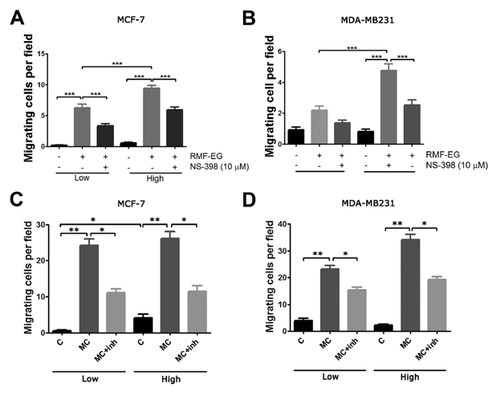
STROMAL-DERIVED PROSTAGLANDIN E2 STIMULATES EPITHELIAL MIGRATION
To test whether stromal PGE2, whose production was enhanced by glucose pre-treatment, modulates the capacity of these cells to stimulate epithelial migration, we performed migration/invasion experiments using MCF-7 (weakly migratory) and MDA-MB231 (highly migratory) cells using the Transwell cell migration system. Two types of experiments were carried out. (1) We evaluated whether glucose pre-treatment modulated stromal capacity to stimulate epithelial migration and whether COX-2 activity played a role in these stimuli. The results illustrated in Figure 4A, show that RMF-EG cells pre-cultured in high glucose and plated in the lower chamber of Transwell, constituted a more potent stimulus for epithelial migration (MCF-7 cells) and invasion (MDA MB-231 cells) than their counterpart cultured in low glucose. On the other hand, we found that COX-2 inhibitor NS-398 was able to inhibit epithelial migration in both conditions. (2) Further, we reasoned if EPG2 generated by stromal cells constituted a stimulus to epithelial migration, the blockade of EPG2 receptor signaling would eliminate this stimulus. To do this, we used an EP4-specific PGE2 receptor antagonist, L-161,982, that blocks the stromal stimulus represented, in this case, by conditioned medium originated in RMF-EG cells derived from high- and low-glucose cultures. As Figure 4C shows, the presence of L-161,982 during the assay provoked a blockade of stromal stimulus on epithelial migration/invasion.
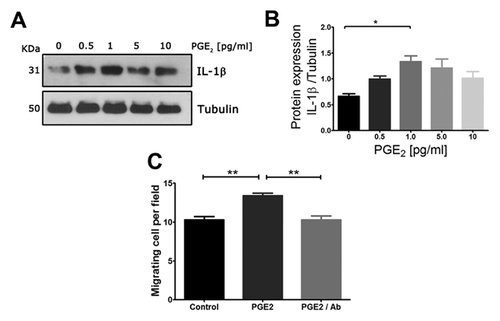
PRODUCTION OF EPITHELIAL IL-1β IS STIMULATED BY PGE2 AND IS PARTIALLY RESPONSIBLE FOR ITS MIGRATORY STIMULUS
Previous results strongly suggested that PGE2 played a role in epithelial migration. To test whether epithelial IL-1 β production is controlled by PGE2 presumptively generate by stromal cells and to assay whether this stimulus has a consequence in epithelial motility, we performed two different experiments: (1) first, we subjected MDA-MB231 cells, that behave as high IL-1β producers, to increasing concentrations of PGE2 and analyzed C production by western blot; and (2) we interfered the PGE2 stimulus on MDA MB231 cells migration with a polyclonal antibody that inhibits the binding of IL-1 to its receptor. Figure 5A shows that IL-1β production is stimulated in a bell-shape manner by PGE2. On the other hand, using 1 pg/ml (the optimal concentration of PGE2 to stimulate IL-1β production), we showed that PGE2-dependent migratory stimulus was eliminated by the presence of the IL-1 blocking antibody in the migration assay (Fig. 5C). These results strongly suggested that the PGE2-dependent stimulus on cell migration was mediated by IL-1 production and represents an autocrine response to the inflammatory stimulus.
DISCUSSION
There is increasing evidence supporting the notion that tumor development and progression are strongly dependent on cellular interactions with the stromal component of the tumor [Sieri et al., 2012]. Breast tumors belong to a family of stiffed neoplastic lesions characterized by a high proportion of stromal components (mainly fibroblasts) that may allow and support tumor survival, outgrowth, and invasion [Sotgia et al., 2011]. It has been proposed that cancer-associated fibroblasts shift into the Warburg metabolism increasing glucose uptake generating lactate for epithelial consumption [Terzic et al., 2010]. In desmoplastic tumors, it is possible that this metabolic adaptation has drastic consequences, substantially modifying the metabolic microenvironment of the tumor by increasing glucose withdrawal and accumulating lactate with the consequent extracellular space acidification. Moreover, it has been proposed that this condition predisposes the acquisition of migratory behavior in cancer cells [Tobar et al., 2010].
With these concepts in mind, it is possible to propose that the hyperglycemic environment of a tumor may be associated with a worse outcome. This hypothesis is consistent with the fact that high circulating glucose and insulin resistance appears to be strongly associated with an increased risk of breast cancer [Tobar et al., 2014]. Long-term epidemiological studies have shown reliable evidence that hyperglycemia and insulin resistance increase the risk of breast cancer [Martinez-Outschoorn et al., 2011]. Moreover, emerging evidence suggests that the anti-diabetic drug metformin, a commonly used biguanide derivative, may also be used for the prevention and treatment of some forms of cancer [Tobar et al., 2016].
Exposure to high glucose can induce changes in a variety of cancer-associated signaling pathways by amplifying their normal responses [Pantschenko et al., 2003]. In the context of the tumoral environment, it has been proposed that epithelial substrate availability results from the establishment of a stromal glucose metabolic reprogramming that determines tumorigenic potential of the tumor epithelial compartment [Hanahan and Coussens, 2012]. Recent findings of our laboratory suggest that stromal cells exposed to long-term culture in high glucose adjust the lactate transport between stromal and epithelial cells establishing a cooperative connection that favor epithelial motility [Urban et al., 2014]. In many cases, this glucose metabolic reprogramming phenomenon triggers an inflammation process in the stromal component, generating signal-soluble factors that increase or maintain epithelial malignancy [Hu et al., 2002].
Cellular mechanisms of chronic hyperglycemia are complex but have been suggested to be involved in the production of reactive oxygen species and oxidative stress. In a hyperglycemic state, more glucose will flux through the glycolytic pathway yielding more pyruvate and acetyl-CoA, leading to more NADH production. As NADH is an electron carrier, excessive amounts of it will cause an electron pressure on the mitochondrial electron transport chain (complex I) leading to a proportional increase in electron leakage that will partially reduce oxygen to yield superoxide [Valencia et al., 2014]. Data presented here showed that mammary stromal cells derived from high-glucose cultures generated a more oxidative environment, a condition favored by less-efficient machinery for H2O2 disposal, compared to cells cultured at low glucose. These findings open the possibility that this redox modification is the starting point of an inflammation phenomenon that stimulates epithelial invasion.
Our data also showed that both glucose and tumor cell-derived epithelial factors induced the expression of stromal COX-2, a key enzyme in prostaglandin synthesis whose activity has been associated with the worsening of cancer progression. Among the soluble epithelial factors responsible for this phenomenon, we suggest that IL-1β could play a relevant role. Moreover, stromal factors derived from cells cultured in high glucose are a more potent stimulus to IL-1β expression in MDA-MB231 cells. IL-1β has been shown to upregulate a variety of processes that contribute to higher angiogenesis, tumor growth, and progression in breast cancer. It is considered a strong and causative pro-malignancy factor whose expression is associated with advanced disease [Gatenby et al., 2006].
It has been proposed that in tissues with high adipose abundance, such as the breast, the primary role of IL-1 in inflammation is the induction of leptin [Walker, 2001]. Therefore, taking into account that leptin (1) is predominantly expressed in obesity; (2) stimulates the proliferation of cancer cell lines [Williams et al., 1999]; and (3) is not expressed in healthy breast tissue [Yan, 2014]. We can suggest that the interplay between both “adipocytokines” may play a significant role in the development of breast cancer.
Our results suggest that high glucose generates an oxidative and inflammatory stromal environment characterized by a stimulus on the expression of the COX-2/PGE2 axis that predisposes epithelial motility. We demonstrated the existence of a glucose-dependent stromal stimulus, mediated by PGE2, on epithelial IL-1 expression that seems to be responsible for epithelial migration. Taken together, findings imply the existence of a two-component feedback mechanism initiated in a stromal glucose-enriched environment that, through an IL-1-dependent phenomenon, enhances cell migration. These data contribute to a new understanding of the complex association between tissue inflammation and human cancer progression.
ACKNOWLEDGMENTS
The present work was supported by grants #1120187 and #1120201 from Fondo Nacional de Investigación Científica y Tecnológica of Chile, FONDECYT to JM and OP.