A Rapid Manual Solid Phase Peptide Synthesis Method for High-Throughput Peptide Production
Funding: This work was supported by the National Institutes of Health and National Science Foundation.
ABSTRACT
Solid phase peptide synthesis (SPPS) techniques are critical for developing and using peptides in various biomedical applications. However, typical synthesis routes used in SPPS are either resource-intensive (e.g., with automated synthesis or commercial services) or time-consuming (e.g., with manual benchtop synthesis). Here, a rapid manual synthesis method was developed to produce up to 8 peptides with fast cycle times simultaneously. Peptides synthesized manually were of equivalent or superior quality to those produced by in-house microwave-assisted automated peptide synthesis, with higher average crude purity of 70% compared to 50%. The method significantly reduced synthesis time, enabling the parallel coupling of up to 8 amino acids simultaneously in 15–20 min, as opposed to traditional benchtop peptide synthesis, which requires 80–150 min per amino acid. This approach offers an intermediate throughput between milligram-scale libraries and gram-scale single peptide synthesis, enabling rapid iteration for novel peptide designs without the need for expensive automated systems. As a result, peptide modifications, including incorporation of unnatural amino acids, can be explored, accelerating the development of peptides for a wide range of applications.
1 Introduction
Solid phase peptide synthesis (SPPS) is a powerful and versatile technique for rapidly synthesizing precisely defined peptides from amino acid precursors [1-3]. SPPS enables access to nearly any peptide sequence and has applications in immunology, biomaterials, biotherapeutics, and more [4-7]. Substantial commercial development has led to widespread availability of specialized reagents needed for SPPS at relatively low cost. Commercially available peptide synthesis services are reasonably priced. Still, the turnaround time can be lengthy, up to weeks or months, which can present challenges for tuning peptide composition or reaction conditions. In-laboratory synthesis of novel peptides is useful, as novel peptides are prone to synthetic difficulty or low solubility, which can be resolved via sequence or reaction condition modifications. However, peptide synthesis via solid phase methods is unfamiliar to many laboratories, and specialized equipment to facilitate peptide synthesis can be costly. Room-temperature manual benchtop synthesis requires little investment in equipment. Still, the lack of heating necessitates using highly reactive reagents prone to side reactions [8] and the process is labor-intensive and slow. Automated laboratory SPPS methods can produce high-quality peptides quickly with far less user time required, but require substantial investments in equipment, training, and maintenance, making the costs prohibitive for most laboratories. Considering the multifaceted complications of current methods for in-laboratory SPPS, advancements in low-cost, high-speed SPPS methods would enable low-resource laboratories to expand investigations into novel peptides.
We present a simple, low-cost, manual, parallel SPPS method. This method allows the simultaneous synthesis of multiple high-quality peptides with parallel amino acid deprotection and addition cycles. Due to heating, cycle times are 15 min or less compared to the hour-long cycle times of typical room-temperature manual peptide synthesis. Key aspects enabling this facile method are the use of reaction vessels submerged in a heating bath to accelerate reactions and glass-fiber-backed poly(tetrafluoroethylene) (PTFE) syringe filters as drainage check valves. This method can be implemented with a manifold made from commonly available silicone tubing and polypropylene fittings or 3D-printed polypropylene. Performing the reactions at elevated temperatures also allows inexpensive carbodiimide chemistry, which abrogates racemization typical for uronium-based chemistry and does not require additional reagents and steps such as Hydroxybenzotriazole (HOBt) and reagent preactivation [2]. We have found this method to be suitable for a variety of peptides, including the integrin binding peptide RGDS, an enzymatically degradable peptide for hydrogel crosslinking, and the tartrate-resistant acid phosphatase (TRAP) binding peptide for nanoparticle targeting [9-12]. Mass spectroscopy and chromatography results confirm that the synthetic purity of peptides produced by this method is equivalent or superior to those synthesized on a commercial automated microwave-assisted peptide synthesizer (CEM Liberty 1). This methodology may also be amenable to other solid-phase chemical synthesis methods that use stepwise reactions, such as oligonucleotide synthesis.
2 Materials and Methods
All solvents were purchased from Sigma-Aldrich unless otherwise specified and used without further purification. Peptide reagents were, unless otherwise specified, purchased from Aapptec and used without further purification. All materials unique to the peptide synthesizer apparatus, along with sources and costs, are listed in Table 1. A vacuum source, temperature-controlled heated stir plate, suitable water bath container, a 1 L filtering flask or similar waste container, and a chemical fume hood are also required.
| Item | Vendor | Catalog number | Quantity | List price ($) |
|---|---|---|---|---|
| 3 mm ID × 4 mm OD, 3 m silicone tubing | Amazon | B08BR4Z4RS | 1 | $8 |
| 4 mm ID × 6 mm OD, 3 m silicone tubing | Amazon | B08BR8MQSR | 1 | $9 |
| 3/16” barbed polypropylene T-fitting, 10 pk | Amazon | B095YR8NKT | 1 | $12 |
| 1/8″–1/4″ tube clamps, 5 pk | Amazon | B08LTKPPT5 | 1 | $8 |
| Syringe filters w/glass fiber prefilter-PTFE, 25 mm, 0.2 μm, 200 pk | Environmental express | SF120T | 1 | $390 |
| 10 mL disposable polypropylene reaction vessel with Frit, 50 pk | Torviq | SF-1000 | 1 | $70 |
| 3D printed manifold | Self-manufactured or service | N/A | 1 | Varies |
2.1 Reaction Vessel Assembly
Two versions of the parallel peptide synthesizer are described here, one using entirely commercially available components (Figure 1) and another using a 3D-printed manifold (Figure 2). Comments on materials selection are provided to guide substitutions and further development, providing a flexible basis for other designs.
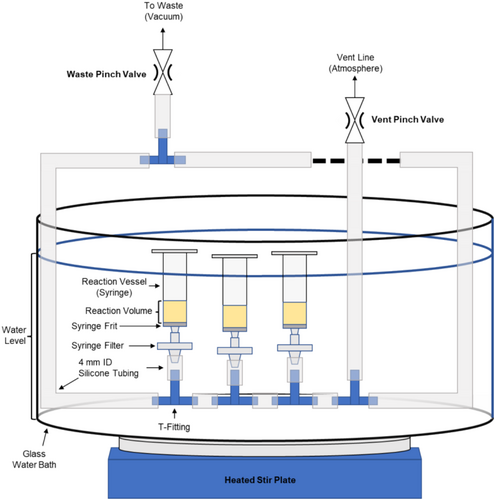
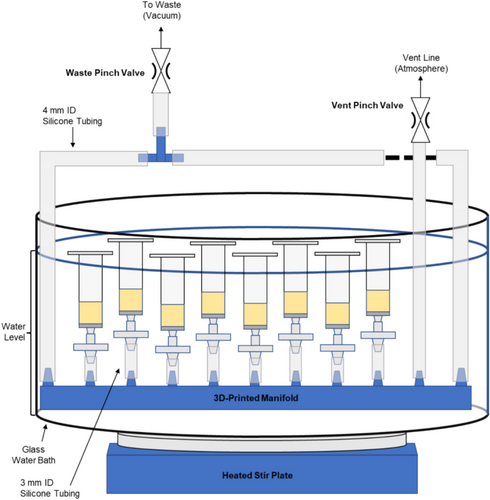
2.2 Materials Selection
SPPS typically uses solvents such as dimethylformamide (DMF), tetrahydrofuran (THF), N-methyl-2-pyrrolidone (NMP), and methanol (MeOH) as well as highly reactive agents such as carbodiimides or amines. The use of these solvents restricts the materials that can be used to handle the reagents during synthesis. Common laboratory materials identified that can resist chemical attack from all reagents are glass, polypropylene (PP), polytetrafluoroethylene (PTFE), and silicone. While other materials may be compatible with the reagents used for SPPS, they were not tested in this approach.
2.3 Manifold Design
The key components enabling the manual technique herein are the vacuum manifold and 0.22 μm glass-fiber prefiltered PTFE syringe filters. The vacuum manifold comprises silicone tubing and either PP t-fittings or, optionally, a PP 3D-printed manifold which increases syringe packing density. These are arranged to allow two independent flow paths to distribute flow and vacuum to all syringes evenly. The materials given in Table 1 for tubing sizes and fitting sizes are guidelines based on successful tests, but scaling up or down may be possible. It is critical that the vacuum lines do not collapse due to vacuum; 4 mm ID × 6 mm OD silicone tubing was found to collapse after several uses at elevated temperatures, suggesting larger diameters will require thicker walled tubing.
The other key component is 0.22 μm glass-fiber prefiltered PTFE syringe filters as one-way valves. Using fritted syringes (Torviq SF-1000) is also beneficial to containing the resin in the reaction chamber. However, tests without fritted syringes still produced high-quality peptides in which resin was relegated to the reaction vessel by the filter media itself. The principle of operation is that the low surface energy (hydrophobic) PTFE prevents the solvents from passing through the 0.22 μm pores until sufficient vacuum is applied. This allows the reagents to remain in the syringe with the resin without requiring U-bends or mechanical valves for each syringe, maintaining a small dead volume. The filter can contain the resin instead of a frit in the syringe, but recovery is more difficult due to resin packing. The glass fiber pre-filter greatly increased draining speed over non-prefiltered PTFE filters, likely due to the increased effective surface area for vacuum draining. Compared to the 0.22 μm PTFE filters, 0.45 μm filters leaked without vacuum, making them unsuitable for this apparatus. Other pore sizes and prefilter combinations were not tested. Note that poly(vinylidene difluoride) (PVDF), polyethersulfone (PES), and nylon filters are unsuitable for this method due to incompatibility with DMF.
2.4 Manifold 3D-Printing
A 9-port 3D-printed manifold was used as an alternative to PP T-fittings. The manifold enables a more space-efficient design (e.g., less vertical height and greater syringe density) and forms a rigid base for the apparatus. The versions used in this work were printed in polypropylene using fused filament fabrication (FFF), the typical method used in standard desktop 3D printers. An STL file for the design is available in the supplemental information. On a Prusa i3 MK3, the following printing parameters were used: 270°C nozzle temperature, 0.4 mm steel nozzle, a 100°C bed temperature, 0.2 mm layer height, and 40 mm/s perimeter speed. Slicing of the STL model was performed in PrusaSlicer 2.4.1 with 8 top and 8 bottom layers, 15% infill, and no support structures. Printing was performed on PP packing tape carefully applied to the print bed; the adhesive was occasionally lifted during printing, which resulted in a slightly distorted manifold. Minimizing warping was challenging due to the considerable shrinkage of the semicrystalline PP during solidification. However, manifolds were vacuum-tight for multiple (up to 5) uses, even when warped. Selective laser sintering (SLS), as offered by many commercial services, may produce a similar quality manifold with less warping and may be more convenient for most laboratories, but post-processing (drilling) of the small holes for syringe attachments may be necessary. Stereolithography (SLA) printing, often referred to as resin printing, may also be suitable as the resulting printed material is typically highly cross-linked and likely solvent resistant. However, testing is required to ensure chemical compatibility before any SLA-printed manifold is used for SPPS.
2.5 General SPPS Procedure
2.5.1 Reaction Conditions
All reactions were performed at 75°C in a water bath. Unless otherwise specified, all reagents were dissolved in dimethylformamide (DMF), and washes were performed with DMF. All reaction conditions and volumes specified here are for 100 μmole scale peptide synthesis. Draining was performed by opening the waste valve and closing the vent valve, while venting was accomplished by closing the waste valve and opening the vent valve. The water level was maintained above the level of the reaction volume inside the reaction vessels to ensure consistent heating of the reagents. All steps were performed in a chemical fume hood (see Figure 3).

For all coupling reactions, the amino acid precursors were protected with 9-fluorenylmethyloxycarbonyl (Fmoc), which was removed by reaction with piperidine or piperazine. Amino acid precursor sidechains were protected orthogonally from Fmoc with either tert-Butyl ether (tBu), triphenylmethyl (Trt), tert-Butyloxycarbonyl (Boc), or 2,2,4,6,7-pentamethyl-2,3-dihydrobenzofuran-5-sulfonyl (Pbf).
A diagram of the cycles of reagent additions is shown in Figure 4. Note that when performing SPPS, amino acids are added to the N-terminus of the resin-bound amino acids. As peptides are commonly written from N-terminus to C-terminus, SPPS cycles are executed in reverse order compared to the standard written form.
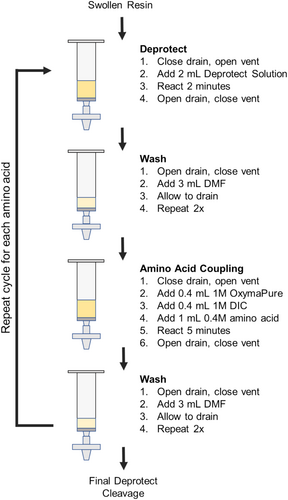
2.5.2 Resin Swelling
In the first step, dry resin (Fmoc-Gly-Wang resin, Aapptec RGW101) was loaded into the reaction vessel before submerging the assembly into the water bath. Once the resin had been loaded and the assembly submerged, the waste valve was closed, and the vent valve was opened. The resin was allowed to swell for 15 min using 4 mL DMF before draining by opening the waste valve and closing the vent valve.
2.5.3 Fmoc Deprotection
While the vent remained open and the waste valve closed, 2 mL of 10% mass/volume of piperazine in 90/10% v/v DMF/ethanol was added into each reaction vessel using a repeating pipettor. The reaction was allowed to proceed for 2 min before draining.
2.5.4 Resin Washing
Between each Fmoc deprotection and amino acid coupling cycle, resin was washed with DMF. In the washing step, 3 mL of DMF was added to each drained reaction vessel while the vent line was closed and the waste line was open. Care was taken to ensure the walls of the reaction vessel were rinsed well during this process. Washes were repeated twice for three total washes between each deprotection/coupling cycle, with complete draining of DMF between each wash. After washing, the manifold was vented. A repeating pipettor or squirt bottle was used for this step.
2.5.5 Amino Acid Coupling
After performing Fmoc deprotection and rinsing, 0.4 mL 1 M OxymaPure (Ethyl (hydroxyimino)cyanoacetate, Aapptec CXZ021) was added, followed by 0.4 mL 1 M N,N-diisopropylcarbodiimide (DIC, Sigma-Aldrich 38,370). The addition of OxymaPure caused the resin to become a bright yellow. Next, 1 mL 0.4 mM of Fmoc-protected amino acid precursor was added to each reaction vessel and the mixture was allowed to react for 5 min. Throughout the reaction, the yellow color of the resin faded and after 5 min, the vessels were drained. To double couple difficult amino acids, such as Fmoc-Arginine(Pbf), the addition of OxymaPure, DIC, and amino acid was repeated in the same volumes and reacted for 5 min. Double coupling was also used whenever a missing amino acid was detected from a previous synthesis. The resin was drained and rinsed following the “resin washing” procedure above before performing a subsequent Fmoc deprotection.
2.5.6 Final Amino Acid Coupling
A standard Fmoc deprotection was performed after the coupling step was completed for the final amino acid of the sequence. The resin was rinsed once with DMF and then twice with dichloromethane. The waste bottle was replaced before the dichloromethane wash to minimize halogenated waste volume. The resin was allowed to dry before being removed from the reaction vessel and proceeding with cleavage.
2.5.7 Peptide Cleavage and Deprotection
The resin was transferred from the reaction vessels to 20 mL glass scintillation vials for cleavage. For typical amino acid protecting groups (tBu, Trt, Boc), 5 mL of the common cleavage cocktail 92.5%/2.5%/2.5%/2.5% v/v trifluoroacetic acid (TFA, Sigma-Aldrich 8.08260), water, triisopropylsilane (TIPS, Sigma-Aldrich 233,781), and 3,6-dioxa-1,8-octanedithiol (DODT, TCI Chemicals D2649) were used for each peptide (100 μmole scale). After adding the cleavage cocktail, the reaction proceeded at room temperature for 2 h. For amino acids containing Pbf protecting groups, such as arginine, 2.5% v/v thioanisole was added to the above mixture and reacted for 4 h at room temperature. Other protecting groups, such as Alloc, require unique deprotection conditions [1, 2]. Cleavage and deprotection were monitored using mass spectrometry.
2.5.8 Peptide Precipitation
After cleavage was completed, resin was separated from the peptide within the cleavage cocktail by vacuum filtration. Per peptide, 45 mL of ice-cold diethyl ether was added to a 50 mL polypropylene conical tube (VWR 21008-951) followed by dropwise addition of the cleavage cocktail/peptide filtrate, resulting in an immediate white precipitate. Tubes were capped and, if separate phases were present, the tube was carefully inverted to mix. The tubes were centrifuged at 2000 RCF for 5 min. The supernatant was carefully decanted from the tubes before adding more cold diethyl ether and centrifuging twice more for a total of three ether washes. After the final decanting, the peptide was dried in a chemical fume hood until it formed a loose solid, then transferred to dry further under house vacuum overnight, leaving a dry white powder crude peptide product. Note: air drying is critical for peptide isolation, as immediate placement in a vacuum chamber results in rapid ether vaporization and physical loss of peptide.
2.6 Analysis
2.6.1 Peptide Mass Determination
Peptide masses were determined via matrix-assisted laser desorption-ionization time-of-flight mass spectroscopy (MALDI-ToF MS) (Shimadzu Axima Confidence). Briefly, peptides were dissolved at 1 mg/mL in a 1:1 v/v mixture of water and acetonitrile with 0.1% TFA v/v (MALDI solvent). The matrix, α-Cyano-4-hydroxycinnamic acid (CHCA) was dissolved separately at 10 mg/mL in the MALDI solvent. 5 μL of sample was mixed with 5 μL of matrix solution before 1 μL of the mixture (per replicate) was placed on the MALDI sample plate (Shimadzu DE1271TA) and allowed to dry. Standards (Sigma-Aldrich MSCAL1) were prepared similarly. Reflection mode was used for all samples. For the Shimadzu Axima Confidence used in this study, a laser power of 50–70 was typically used.
2.6.2 HPLC
Crude peptide purity was assessed by high-performance liquid chromatography (HPLC) on a Shimadzu 20A Prominence analytical system (LC-20 AD Solvent Delivery Unit, CBM-20A System Control Unit, CTO-20A Column Oven, DGU-20A Inline Degasser, SIL-20A HT Autosampler, SPD-20AV UV–Vis Detector) equipped with a Kromasil Eternity-5-C18 column (4.6 × 50 mm). A gradient of water (A) and acetonitrile (B), both containing 0.05% v/v trifluoroacetic acid, was used as the mobile phase at a flow rate of 0.5 mL/min, and analytes were monitored by UV detection at 214 nm. The gradient method was as follows: isocratic hold at 7% B for 2 min, gradient from 7% to 25% B over 10.5 min, gradient from 25% to 95% B for 2 min, isocratic hold at 95% B for 2 min, gradient from 95% to 7% B for 1 min, isocratic hold at 7% B for 4 min. Crude peptide samples were dissolved in phosphate-buffered saline (PBS) at approximately 0.8 mg/mL and filtered through a 0.45 μm polyvinylidene difluoride (PVDF) syringe filter before analysis.
3 Results and Discussion
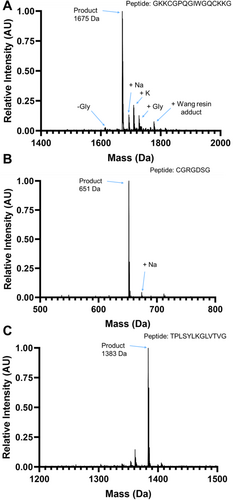
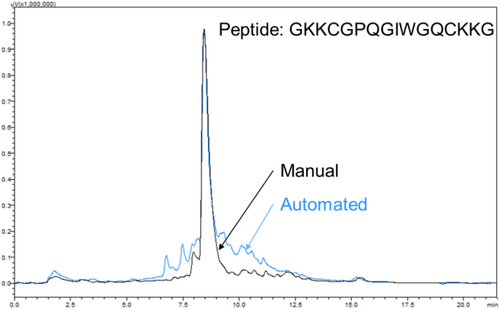
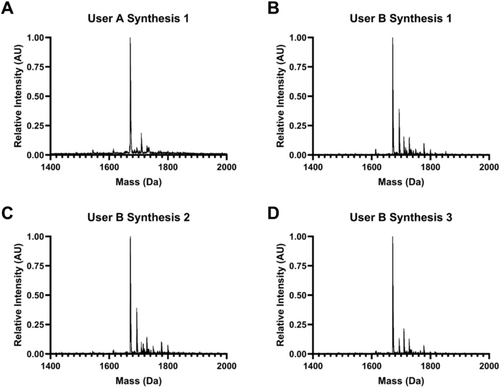
Rapid manual synthesis was successful in the simultaneous production of up to 8 peptides with rapid cycle times. The described method yielded crude peptides without deletions, as observed by the expected mass peaks dominating the MALDI spectra (Figure 5). Nevertheless, additional peaks were observed as well, including peptide plus salts including sodium (Na+) and potassium (K+) and, for Figure 5A, small amounts of peptide with deleted or additional Glycine and Wang resin adducts. These contaminants and off-target products are common to solid-phase synthesis [22]. Manually synthesized peptides were of equivalent or better quality to a microwave-assisted automated peptide synthesizer (CEM Liberty 1), as measured by HPLC (Figure 6), with 70% versus 50% average crude purity. Low variability was observed between different multiple independent syntheses of the same peptide (GKKCGPQGIWGQCKKG) by two different users, as assessed by MALDI spectra (Figure 7), indicating high process repeatability.
Using the methods described here, a full amino acid coupling and Fmoc deprotection cycle was completed simultaneously for 8 peptides in approximately 15–20 min. In comparison, the manual synthesis of a single peptide at a time requires 10–15 min per residue, resulting in a total of 80–150 min to add an amino acid to 8 peptides individually. Therefore, using the apparatus designed here to couple 8 amino acids simultaneously, the average synthesis time per peptide was reduced to timescales that rival high-speed sequential peptide synthesizers.
Rapid manual synthesis also offers an intermediate scale of peptide synthesis throughput between small, milligram-scale libraries and large, gram-scale single peptide synthesis. A batch of up to 8 crude 15-mer peptides can be synthesized every 2 days by a single user, enabling experiments utilizing between 10 and 100 unique peptides at ~100 mg scales. Rapid manual in-house SPPS allows iteration of synthesis procedures and sequences. When synthesizing novel peptides, it can be challenging to predict which amino acids may require double coupling or alternative reaction conditions or which peptides may have poor solubility and require sequence modification. Typical commercial peptide synthesis services typically have turnaround times of weeks. In the case of unexpectedly difficult syntheses requiring reaction condition or sequence changes, time may increase to months. Rapid manual synthesis allows laboratories without automated SPPS systems to iterate reaction conditions for novel peptides of interest on timescales of a day. Additionally, any amino acid or reagent can be added as the user desires, enabling the facile investigation of unique modifications or unnatural amino acids in peptides.
However, even rapid parallel manual synthesis requires considerable time block demands. The fast cycles make multitasking difficult for the user, as the most prolonged period of inactivity needed is 5 min. As a result, careful scheduling and collaboration of synthesis duties for peptides exceeding 10 amino acids in length are recommended. Clear and concise checklists for each synthesis step are necessary for individual and shared use to minimize human errors. A representative checklist has been included in the supplemental information.
4 Conclusions
Methods were developed for rapid manual parallel solid-state peptide synthesis as a practical, low-cost approach for quickly synthesizing peptides up to 16 amino acids long. An apparatus was designed to execute eight parallel reactions, and methods were demonstrated for synthesizing over 50 standard peptides with a range of functions, including adhesive peptides, crosslink hydrogels, and functionalized nanoparticles for tissue targeting or reduced protein adhesion. This simple method provides an opportunity to greatly expand access to peptides for low-resource settings.
Acknowledgments
Funding for this study was provided by the National Institutes of Health (NIH) F31AR076874 (CO), T32GM118283, R01DE018023, R01AR056696, R01EY033192, R21AR081063, R21EY027834, S10OD030302, the National Science Foundation (NSF) CBET-1450897 and DMR-2103553, and Schlumberger Faculty for the Future (to EAS). The content is solely the authors' responsibility and does not necessarily represent the official view of the National Institutes of Health or the National Science Foundation.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




