Artificially altered gravity elicits cell homeostasis imbalance in planarian worms, and cerium oxide nanoparticles counteract this effect
Alessandra Salvetti and Andrea Degl'Innocenti contributed equally to this study.
Leonardo Rossi and Gianni Ciofani contributed equally to this study.
Funding information: European Space Agency, Grant/Award Number: CORA-GBF-2017-001
Abstract
Gravity alterations elicit complex and mostly detrimental effects on biological systems. Among these, a prominent role is occupied by oxidative stress, with consequences for tissue homeostasis and development. Studies in altered gravity are relevant for both Earth and space biomedicine, but their implementation using whole organisms is often troublesome. Here we utilize planarians, simple worm model for stem cell and regeneration biology, to characterize the pathogenic mechanisms brought by artificial gravity alterations. In particular, we provide a comprehensive evaluation of molecular responses in intact and regenerating specimens, and demonstrate a protective action from the space-apt for nanotechnological antioxidant cerium oxide nanoparticles.
1 INTRODUCTION
Gravity is an environmental parameter that has constantly influenced the evolution of life on Earth, promoting the development of specific structures such as the skeletal, circulatory and muscle apparatuses, and affecting several biological processes including embryogenesis and organ physiology.1 Although frog (Xenopus laevis) embryos develop properly under altered gravity,2 mouse embryos do not,3 and newt (Pleurodeles walt) embryos develop with neural tube defects,4 regenerating abnormal curved tail.5 Microgravity stimulates cell growth,6 affects the beating frequency of cardiomyocytes,7 and enhances differentiation of mesenchymal stem cells.8-10 Hypergravity influences longevity in Drosophila,11 and increases proliferation and differentiation of bone marrow stromal progenitors.12 Moreover, several studies demonstrate that altered gravity affects signaling pathways13-16 and modifies the transcriptional profile of factors not exclusively associated with stress response.10, 17-22 The understanding of the biological effects produced by altered gravity conditions is a relevant issue for spaceflight, bearing in mind that – after long-term missions – astronauts experience a number of health problems, like atrophy of muscles, bone demineralization, cardiopulmonary, ocular and vestibular complications, and modifications in the nervous system connections.23-26 Special challenges are to be faced when attempting to study altered gravity on a whole organism, and therefore our knowledge about the effects of gravity perturbations in vivo is still scarce and fragmented. Using simple worms (planarians, Platyhelminthes) as a model system, we simulated altered gravity conditions by using a large diameter centrifuge (LDC) and a random positioning machine (RPM) at the European Space Research and Technology Centre of the European Space Agency (ESA-ESTEC) ground-based facilities, in Noordwijk, the Netherlands. Our aim is to gain insight about the potential effects of altered gravity on stem cell proliferation, differentiation and homing, as well as tissue and organ morphogenesis. Aspects making planarians apt for our study reside in their outstanding capacity of regeneration, a process in which planarian stem cells – the neoblasts – proliferate and accumulate beneath a wound surface producing a blastema, a region in which missing body structures are rebuilt through the activation of complex developmental processes.27, 28 The availability of numerous experimental techniques and cell- or tissue-specific molecular markers make planarians a sound system for in vivo studies.29-35
Recently, planarians have also been proposed as a model for space biology. A few papers report about how altered gravity influences planarian locomotion, asexual reproduction and transcriptional profile. In particular, the effects on tissue regeneration change in dependence of different experimental designs and conditions.36-38 Thus, a more systematic approach and additional investigations are needed to discover the impact of gravity on de novo tissue morphogenesis. In this respect, we provide comprehensive information on the biological response to simulated hyper- and microgravity in both intact and regenerating planarians, and propose a model in which reactive oxygen species (ROS) are the underlying cause of cell homeostasis unbalance produced by altered gravity. Indeed, pre-treatment of animals exposed to artificially altered gravity with cerium oxide nanoparticles (CONPs), biocompatible and long-lasting ROS scavengers that mimic endogenous antioxidants like catalase and superoxide dismutase,39, 40 counteracts such homeostasis unbalance in all tested altered gravity conditions.
2 MATERIALS AND METHODS
2.1 Animals
The asexual strain GI of the planarian Dugesia japonica (Tricladida Dugesiidae)41 was bred in artificial water (CaCl2 2.5 mM, NaHCO3 0.8 mM, MgSO4 0.4 mM, KCl 0.077 mM in distilled water) at 18°C.42 Planarians were transferred to ESA-ESTEC, in Noordwijk (The Netherlands), in boxes filled with artificial water at the temperature of about 22°C. They were used for experiments after 24 h of acclimation and 2 weeks of fasting. Planarians are non-cephalopod invertebrates, and all animal experiments were performed in agreement with Italian and European law, as well as with guidelines and recommendations of the Federation of European Laboratory Animal Science Associations (FELASA).
2.2 Nanoparticle characterization
A single source of CONPs (1406RE, Nanostructured & Amorphous Materials, Inc., Houston) was utilized throughout all experimental procedures. To characterize nanoparticle morphology, size and crystalline structure, micrographs of CONPs were taken via transmission electron microscopy (TEM), utilizing a JEM-1400Plus microscope with thermionic source (LaB6), operated at 120 kV, equipped with a Gatan CCD camera Orius 830 (2048 × 2048 active pixels). Images were collected with two different modalities, namely in bright field (BF) and in selected area aperture diameter (SAED, approximately 5.25 μm). Water suspensions of CONPs for TEM analyses were prepared as follows: CONPs were suspended in ultra-pure water, sonicated with ultrasound, loaded onto an ultrathin carbon film on Cu grid, and dried in low vacuum at room temperature.
X-ray photoelectron spectroscopy (XPS) analysis was carried out to evaluate the chemical state of the CONPs used in the experiments, with a particular focus on assessing the Ce3+ and Ce4+ contents. The specimen for XPS analysis was prepared by pressing a few milligrams of finely ground CeO2 powder onto a high purity indium pellet (Sigma-Aldrich). Measurements were conducted on a Kratos Axis UltraDLD spectrometer (Kratos Analytical Ltd.) using a monochromatic Al Kα source (hν = 1,486.6 eV) operated at 20 mA and 15 kV. Wide scans were collected at pass-energy of 160 eV and energy step of 1 eV, while high-resolution spectra were collected at pass-energy of 10 eV and energy step of 0.1 eV. The Kratos charge neutralizer system was used for all the measurements. Spectra were analyzed with CasaXPS software (Casa Software, Ltd., version 2.3.22).
To determine the size distribution profile of CONPs, nanoparticles were dispersed to 1 mg/ml, together with 1 mg/ml gum Arabic (GA, G9752, Sigma-Aldrich) in planarian artificial water, and gently sonicated. We then performed dynamic light scattering (DLS) and Z-potential measurements (at 20°C), using a Zeta-sizer NanoZS 90 instrument (Malvern Instruments Ltd.). Both polydispersity index (PDI) and Z-potential were measured as average ± standard deviation of three measurements, each consisting of 15 runs.
2.3 Cerium oxide nanoparticle treatment
CONPs used in this research underwent extensive characterization in previous works, for example,39 and were already used successfully in planarians without any particular adverse effects.43, 44 CONPs at 1 mg/ml were dispersed through a mild sonication with a probe sonicator (GM mini20 Bandelin Sonopuls, Bandelin), 5 min at 8 W, in a GA 1 mg/ml dispersion in artificial water. For controls, GA-only dispersions were prepared following the same procedures. GA is the vehicle in which CONPs are dispersed and it has no effect on planarian tissue regeneration and stem cell biology.44 CONP treatment was performed following the experimental conditions described in43, 44 at which its internalization in the cytoplasm of planarian cells has been demonstrated. Briefly, animals were immersed for 24 h in CONP or GA-only medium and then rinsed 3 times with 50 ml of artificial water to completely wash out CONPs and GA.
2.4 Simulated altered gravity experiments
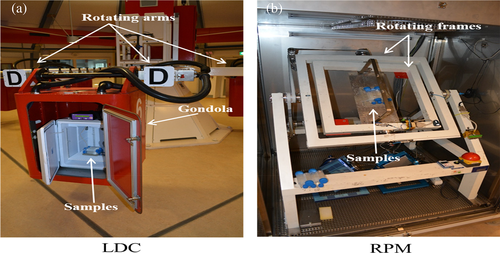
The large diameter centrifuge (LDC)45 (Figure 1(a)) was used at 10 g for simulated hypergravity experiments. Simulated microgravity was achieved via a random positioning machine (RPM; Dutch Space/EADS) at 30°/s (Figure 1(b)).46, 47 The RPM was used with a maximum random speed of 30°/s, random direction and interval. The samples were never more than 10 cm away from the center of rotation. Both kinds of experiments were invariably carried out at 18°C. For 1 g control, animals were simply left at 18°C for the appropriate amount of time in parallel with the LCD or RPM run. Groups of 10 planarians were put in 50 ml falcon tubes, filled out completely with artificial water. Those for RPM experiments were especially inspected to make sure that no visible air bubbles formed after caps were screwed on securely. Two independent runs (run 1 and run 2) were performed for each experimental condition at a distance of 2 days (experiments of exposition to altered gravity for 6 and 24 h) or 3 days (experiments of exposition to altered gravity for 60 h) (Figure 2).
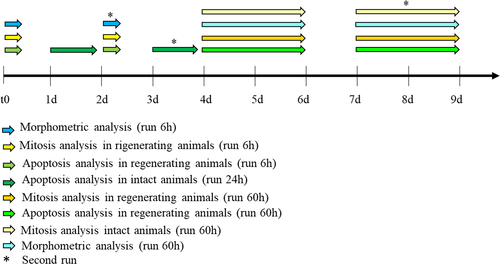
2.5 Morphometric analysis of blastema size
Immediately before to enter LDC or RPM, GA-, and CONPs-treated animals were cut between auricles and pharynx, producing head (tail-regenerating), and tail (head-regenerating) fragments. Fragments were then exposed to simulated altered gravity for 6 or 60 h and fixed as previously described48 at 60 h after cut, namely immediately at the end of a 60-h run or after 54 h from a 6-h run. Controls (1 g) were processed analogously. Samples were examined under a stereomicroscope (Stemi 305, Carl Zeiss Microscopy GmbH), equipped with a camera (Axiocam Erc 5 s, Carl Zeiss Microscopy GmbH). Digital images were quantified using Image J software.49 For each animal, blastema and total body area were measured for at least 15 regenerating animals for each experimental group. We considered as blastema the unpigmented region below the wound epithelium; blastemal margin was manually highlighted by the operator in blind. The total body area was approximated to the area of the rectangle drawn around the body margin.
2.6 Whole-mount in situ hybridization
DNA template for NB.21.11.e, a marker for early stem cell progeny,50 was produced as previously described.51 Digoxigenin (DIG)-labeled RNA probe was obtained by in vitro transcription of the purified template using DIG-labelling mix (Roche Applied Science). 60 h after exposure to altered gravity, intact animals were fixed and treated for whole-mount in situ hybridization (WISH) as previously described.52 After colorimetric development, samples were dipped in 50% glycerol/10% methanol, and analyzed under the stereomicroscope. NB.21.11.e signal was quantified as already described.53 Briefly, each animal was photographed at the same magnification and exposition; pictures were then converted into grayscale mode and inverted. The mean gray value (raw intensity/animal area) was recorded by the ImageJ software. Mean gray recorded from animals (n = 10) belonging to the same experimental class was utilized to obtain the mean gray value of the group.
2.7 Terminal deoxynucleotidyl transferase dUTP nick end labeling
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay was performed by using ApopTAg Red in situ apoptosis detection kit (EMD Millipore Corporation) as previously described.48 Stained animals were mounted in 80% glycerol, and analyzed using a confocal microscope (TCS SP8, Leica Microsystems CMS) by tile scan function and zeta-stack optical sectioning every 2 μm, respectively. Five planarians were analyzed for each experimental condition. A single composite image was taken for each specimen. The number of apoptotic cells in intact and regenerating (60 h of simulated altered gravity regime) animals was evaluated using the ''find maxima'' option of the Image J software and normalized versus the animal body area. In the case of regenerating animals exposed to artificially altered gravity for 6 h, the number of apoptotic cells was exclusively counted in 100 μm tissue strip from the amputation site.
2.8 Western blotting
Mitotic cells were quantified using the mitotic marker phospho-histone H3 antibody (H3P Ab, 06–570, Sigma-Aldrich) by Western blotting. Nine animals for each experimental class were amputated and immediately exposed to artificially altered gravity for 6 or 60 h. Nine intact animals were exposed to simulated altered gravity for 24 h. After exposition, three independent groups (each composed by three animals) were immediately frozen, and proteins were extracted by adding 1X Laemmli buffer (0.1% 2-mercaptoethanol, 0.0005% bromophenol blue, 10% glycerol, 2% SDS electrophoresis-grade, 63 mM Tris–HCl pH 6.8). Proteins were transferred onto nitrocellulose membrane using Mini Trans-blot electrophoretic transfer cell apparatus (Bio-Rad Laboratories Inc.) following the manufacturer's instruction. After blocking with 5% nonfat dry milk (Bio-Rad Laboratories Inc.) in PBST (0.05% Tween 20, Sigma, in PBS 1×), membranes were incubated with 1:500 of H3P antibody in 1% non-fat dry milk in PBST for 2 h. Anti-α-tubulin antibody, mouse monoclonal (T6199, Sigma-Aldrich) was used as loading control at 1:20000 dilution. Membranes were then washed in PBST and incubated with anti-mouse IgG horseradish peroxidase (HRP; 6,402–05, Biovision) or anti-rabbit IgG HRP (6401–05, Biovision) diluted 1:50000 in 1% nonfat dry milk in PBST for 1 h. The chemiluminescence detection was performed using the Immobilon Western chemiluminescent HRP substrate kit (Millipore Corp.) following the manufacturer's instruction. To visualize immunoblots, we used a luminescent image analyzer (Chemidoc XSR + System Bio-Rad) and the Image LabTM Software (Bio-Rad Laboratories). Only bands below the saturation limit were analyzed. The chemiluminescence was expressed in terms of volume of specific immunoreactive bands and, in each lane, the protein level was normalized to the α-tubulin level. Three technical replicates were performed for each Western blot.
2.9 Statistical analysis
Unpaired Student's t tests (α = 0.05) and two-way ANOVA (α = 0.05) were applied to evaluate statistical significance, respectively using the software GraphPad Prism 7.00 and Free Statistics Software v1.2.1 (https://www.wessa.net/rwasp_Two%20Factor%20ANOVA.wasp/).
3 RESULTS
3.1 Cerium oxide nanoparticle characterization
Figure 3 shows a representative TEM image of CONPs (a), the diffraction pattern of the powder (b), as well as the XPS wide scan (c), with high-resolution spectra collected on the binding energy regions typical for Ce 3d and O 1 s peaks (d).
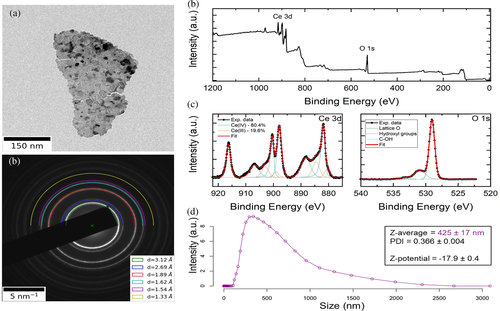
Powder electron diffraction pattern show sharp diffraction rings indicating that the nanoparticles have a crystalline nature compatible with that one of cubic ceria.54
Concerning XPS, From the wide scan we could confirm the high purity of the nanomaterial, as the observed peaks could all be assigned to either cerium or oxygen, a part from a low intensity carbon peak at ~285 eV, partially overlapping with Ce 4 s ones. The high-resolution spectra are shown after background subtraction, together with the outcome of the fitting procedure. The best fit on the Ce 3d spectrum was obtained with five sets of spin-orbit split doublets, three of which representative of Ce(IV) (light blue profiles in the figure) and two of Ce(III) (orange profiles) oxides, as described in.55 The splitting between the two components of each doublet has been set to 18.6 eV, as reported in,56 while the intensity ratio between the two components is set to 3:2, due to spin-orbit coupling. The relative content of Ce3+ and Ce4+ species in the sample was calculated from the ratio of the sum of the integrated areas of the XPS 3d peaks related to the two oxidation states to the total integral area for the whole Ce 3d region. The acquired XPS spectrum is consistent with a Ce3+ percent concentration of 19.6 ± 2.0% (Ce4+ 80.4 ± 2.0%), corresponding to a Ce3+/Ce4+ ratio of about 0.24. The O 1 s spectrum was fitted with three peaks. The main one, centered at 529.0 ± 0.2 eV, corresponds to lattice oxygen in CeO2, in agreement with the literature.57 To obtain the best fit, two low intensity peaks were centered at 530.9 ± 0.2 eV and 533. 3 ± 0.2 eV, and are assigned to hydroxides and C–OH groups at the surface of the CONPs.57 DLS measurements (Figure 3(e)) are in line with our previous reports,43 namely an hydrodynamic size of 425 ± 17 nm, a PDI of 0.366 ± 0.004, and a Z-potential of −17.9 ± 0.4 mV.
3.2 Altered gravity and tissue regeneration
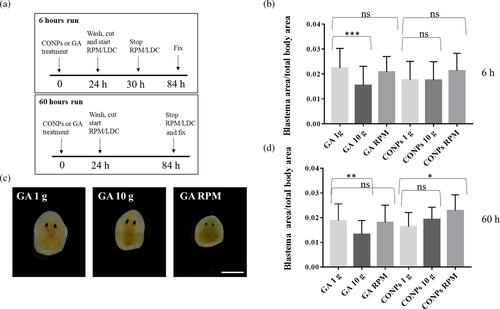
With the aim to understand the effect of gravity on tissue regeneration, we performed a morphometric analysis of 3-day-old blastema area of planarians exposed to simulated altered gravity, with or without previous treatment with CONPs and according to the experimental design depicted in Figure 4(a). In order to normalize differences in blastema area that might depend on the use of animals of different sizes, we initially investigated the dependence of these two variables. Regression analysis of blastema area with respect to body area, performed throughout the dataset, shows a linear correlation (R = 0.7, significance F close to 0). For this reason, we chose the ratio between blastema area and total body area as the most appropriate parameter to evaluate effects on tissue regeneration. Our data indicate that 6 or 60 h exposition to simulated hypergravity produced a significant reduction in the head fragment regeneration, and this reduction was rescued by CONP treatment (Figure 4(b)-(d) and Figure S1). RPM did not affect tissue regeneration at both 6 and 60 h, and CONP supplementation slightly increases the process at 60 h (Figure 4(c)-(d), and Figure S1). No statistically significant differences were detected between groups for tail fragments (Figure S2a-b).
3.3 Altered gravity and cell proliferation rate
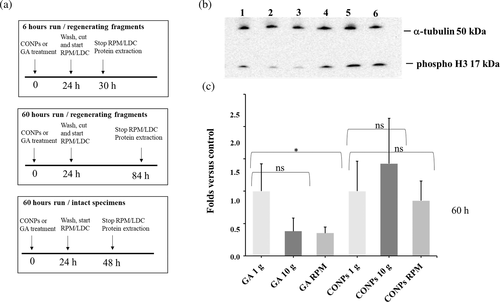
To assess the effect of altered gravity on cell proliferation, we analyzed the expression of phospho-histone H3, a marker of mitosis, in regenerating and intact animals treated with CONPs or GA according to the experimental design indicated in Figure 5(a). We did not detect significant changes in the expression of the mitotic marker in both intact animals (exposed to simulated altered gravity for 60 h; Figure S2c) and in regenerating fragments exposed to artificially altered gravity for only 6 h (Figure S2d). On the contrary, a significant reduction in the number of mitotic cells in regenerating fragments exposed to RPM for 60 h was detectable. A similar trend, although not statistically significant, was also detectable in regenerating fragments exposed for 60 h to artificial hypergravity (p value from 0.05 to 0.1 for the different technical replicates; Figure 5(b)-(c) and Figure S1). Pre-treatment of animals with CONPs induced a rescue in animals exposed to simulated altered gravity with respect to the control (Figure 5(b)-(c) and Figure S1).
3.4 Altered gravity and cell differentiation
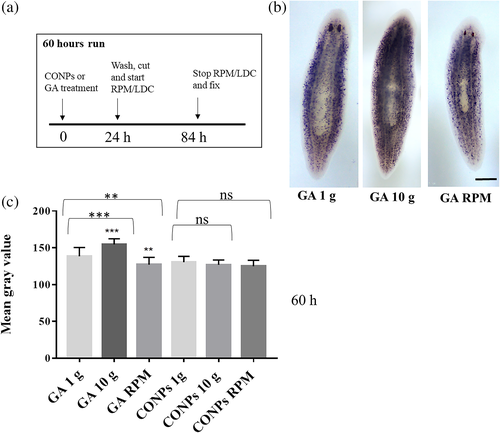
To understand the effect of simulated altered gravity on cell commitment, we analyzed the expression of NB.21.11.e, a marker of early neoblast progeny towards epidermal cell lineage, by WISH experiments performed on intact planarians exposed to artificially altered gravity for 60 h with or without previous treatment with CONPs (Figure 6(a) and Figure S1). In particular, we exposed animals to altered gravity for 60 h and sacrificed them, a time point at which the effect can be evaluated on early epidermal progeny.50 We found that NB.21.11.e expression is increased upon simulated hypergravity and reduced upon simulated microgravity (Figure 6(b)-(c) and Figure S1). These differences were not detectable in animals treated with CONPs (Figure 6(b)-(c) and Figure S1).
3.5 Altered gravity and cell death
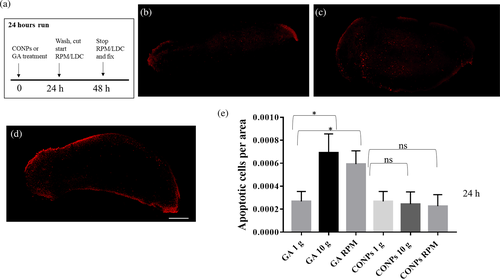
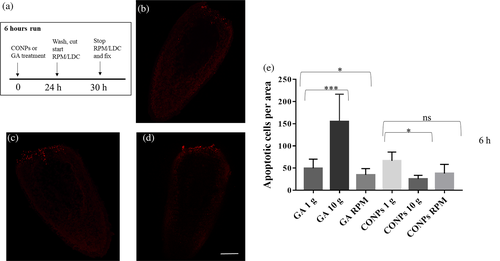
The effect of altered gravity on cell death was analyzed by whole-mount TUNEL assay in intact planarians and regenerating fragments at 6 and 60 h after amputation. We detected a significant increase in the number of apoptotic cells in intact animal treated with GA and exposed to simulated altered gravity for 24 h (Figure 7 and Figure S1). The number of apoptotic cells clearly increased 6 h after amputation in animals exposed to artificial hypergravity with respect to controls, while it also resulted in a significant reduction in animals in the RPM (Figure 8 and Figure S1). No difference was observed in animals exposed to altered gravity after 60 h of regeneration (Figure S2e). The treatment of both intact and regenerating animals with CONPs counteracted the alteration in the number of apoptotic cells (Figure 7, 8 and Figure S1).
4 DISCUSSION
Planarians are extremely resilient to the perturbation of cell homeostasis processes, including traumatic events such as extensive tissue damage. Their sturdiness resides in the adaptive response of a plastic body where homeostatic processes are continuously adjusted by cross-talk between stem cells and differentiated body parts, which have been suggested to represent a global stem cell niche.31 For these reasons, unbalancing regenerative and homeostatic performances of planarians is challenging. Accordingly, artificially altered gravity did not permanently perturb planarian regeneration even in extreme conditions. Indeed, animals were able to complete a functional regeneration, although we observed a reduced regenerative capability in head fragments following exposure to simulated hypergravity. Higher susceptibility of head fragments – with respect to tail fragments – to factors perturbing stem cell activity has been commonly demonstrated for these organisms.58 This phenomenon is probably due to the lower number of stem cells spread in this part of the body, so that minimal variations in the number of neoblasts induce a more readily observable phenotype. In general terms, a reduction in the regeneration ability might owe to reduced cell proliferation and/or an increased cell death. These two biological phenomena are indeed finely regulated during planarian regeneration, both showing two waves of activity, that is, one early after cut and the other later. While apoptosis and proliferation share this biphasic pattern, their distribution in the body is opposite. For apoptosis, the early peak occurs near the wound surface, and the second one is dispersed throughout the body59; the exact contrary occurs for proliferation, being mitotic cells accumulated under the growing blastema only in the second proliferation burst.60 A maximal impact upon blastema growth is therefore expected in case of an increase in the first peak of apoptosis and of a decrease in the second peak of proliferation. Slight changes in the number of apoptotic or mitotic cells spread far from the regeneration site are unlikely to directly perturb blastema growth.
In all altered gravity conditions tested in this study, we monitored a reduction of the proliferative activity in the second peak, which is consistent with a reduced blastema growth. On the contrary, we monitored an increase in apoptotic cells under the wound surface in early regeneration only in artificial hypergravity conditions. In the RPM, the number of apoptotic cells localized below the wound is slightly reduced. This difference might be at the basis for the different effect upon blastema growth observed in microgravity versus hypergravity simulations. Indeed, in this latter case the combination of two adverse effects, that is a reduced proliferation at the blastema site and an increased apoptosis under the wound surface, might concur to the slight reduction of the blastema growth rate. We currently do not know which cells underwent apoptosis, but, as at the same time (6 h) at which we observed an increase in apoptosis, we also monitored a number of mitosis comparable to controls; so, it is possible to hypothesize that this process preferentially affects post-mitotic cells. Accordingly, it has been demonstrated that the two regenerative apoptotic waves predominantly, if not exclusively, occurred in differentiated cells.59 We can thus imagine that a first early consequence of hypergravity is the induction of cell-death processes: this is also in line with what we observed in intact animals, where an increment in TUNEL-positive cells was monitored after 24 h of 10 g exposure. Altered gravity is also known to influence cell determination and differentiation in different types of cells (22, 61-64), and it also has an effect on cell differentiation in planarians, a process that can be easily followed by studying differentiation towards the epidermal lineage, for which early and late molecular markers are available.50 Obtained data show again an opposite effect of hypergravity and microgravity experiments. Indeed, a significant increase of NB-21.11.e-positive cells (marker for enhanced differentiation) was detectable after simulated hypergravity exposure, while its significant reduction was produced by artificial microgravity exposure indicating a possible demonstration of the gravity continuum paradigm.65
Pre-treatment with CONPs rescued the negative impact of simulated altered gravity on the blastema growth, cell proliferation, apoptosis, and cell differentiation. We can assume that protective effect is due to internalized nanoparticles, as we extensively washed planarian before exposition to altered gravity conditions. Owing to concomitance on CONP crystalline surface of both Ce4+ and Ce3+ states,40 CONPs show self-regenerating antioxidant properties and ROS-scavenger functions, resulting into anti-inflammatory activity.66 Hence, CONPs have had several biomedical applications, and showed a protective role in disorders characterized by ROS overproduction.67-69 Since several literature sources report on overproduction of ROS induced by altered gravity,70-73 we hypothesize that oxidative unbalance might be the principal cellular stress at which planarian cells are exposed following altered gravity, despite additional concurrent causes cannot be ruled out. Thus, we speculate that upon hypergravity exposure cells experience an increase in ROS production, to which some respond activating a cell-death program. In turn, increases in cell death may influence the cellular homeostasis of the animal, inducing premature cell differentiation and perturbing activation of neoblasts and their subsequent accumulation to form a blastema. Further experiments will be necessary to validate our hypothesis.
5 CONCLUSION
Altered gravity represents one of the main challenges for space exploration. Understanding the molecular bases of the noxious effects of hyper- and microgravity is, therefore, vital. However, it is hard to perform altered-gravity research on whole organisms, and this becomes especially true for space experimental campaigns. Simpler biological models are needed.
We utilized planarians as a straightforward platform for artificially altered gravity experiments, characterizing their molecular responses to different gravity regimens. Planarians are evolutionary distant from humans, but molecular mechanisms and fundamental signaling pathways are well conserved between the two taxa, so that our data can be useful for translational research.
Upon exposure to altered gravity, regenerating planarians experience transient growth delays, likely due to aberrations in tissue turnover dynamics; these, in turn, depend on a combination of increased cell death and an overall reduced proliferation capacity. When CONPs are administered, such adverse phenomena become largely unapparent; given the strongly supported involvement of oxidative stress in mediating the damages of altered gravity, and considering the recognized role of CONPs as an antioxidant, we hypothesize that such rescue effects are mostly due to CONP protecting planarians from the ROS imbalance caused by the gravitational insult.
Our findings strengthen the validity of planarian worms as a suitable model for space biology, and contribute to the understanding of the effects produced by altered gravity, as well as to the future application of emerging nanomaterials in biomedicine.
ACKNOWLEDGMENTS
We thank Dr. Maria Grazia D'Elia for assistance on Western blotting and TUNEL assay experiments. We would also like to thank the ESA-ESTEC TEC-MMG LIS Lab, especially Mr. Alan Dowson for his support in preparation and during this study.
This work has received funding from the European Space Agency (ESA) through the grant number CORA-GBF-2017-001.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




