In vivo sun protection factor and UVA protection factor determination using (hybrid) diffuse reflectance spectroscopy and a multi-lambda-LED light source
Funding information: Bundesministerium für Bildung und Forschung, Grant/Award Number: FKZ 03ZZ0131D; Courage + Khazaka electronic GmbH
Abstract
The sun protection factor (SPF) values are currently determined using an invasive procedure, in which the volunteers are irradiated with ultraviolet (UV) light. Non-invasive approaches based on hybrid diffuse reflectance spectroscopy (HDRS) have shown a good correlation with conventional SPF testing. Here, we present a novel compact and adjustable DRS test system. The in vivo measurements were performed using a multi-lambda-LED light source and an 84-channel imaging spectrograph with a fiber optic probe for detection. A transmission spectrum was calculated based on the reflectance measured with sunscreen and the reflectance measured without sunscreen. The preexposure in vitro spectrum was fitted to the in vivo spectrum. Each of the 11 test products was investigated on 10 volunteers. The SPF and UVA-PF values obtained by this new approach were compared with in vivo SPF results determined by certified test institutes. A correlation coefficient R2 = 0.86 for SPF, and R2 = 0.92 for UVA-PF were calculated. Having examined various approaches to apply the HDRS principle, the method we present was found to produce valid and reproducible results, suggesting that the multi-lambda-LED device is suitable for in-vivo SPF testing based on the HDRS principle as well as for in-vivo UVA-PF measurements.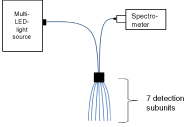
Abbreviations
-
- DRS
-
- diffuse reflectance spectroscopy
-
- HDRS
-
- hybrid diffuse reflectance spectroscopy
-
- SPF
-
- sun protection factor
-
- UVAPF
-
- UVA protection factor
1 INTRODUCTION
Skin cancer is the most common cancer worldwide and incidences have been increasing steadily over the last decades [1, 2]. Beside seeking shade and wearing protective clothing, applying sunscreen products is an important strategy of photoprotection [3]. The efficacy of a sunscreen product is dependent on the type and amount of UV-filters [4], the vehicle of the product [5, 6], as well as its quality to create a continuous film over the surface of the skin [7]. Commercial sunscreen formulations are constantly being adapted to the state of research and customized to target susceptible groups like children or dermatological patients.
The sun protection factor (SPF) value quantifies the degree of protection of an individual sun protection product. It is a relative measure of how much longer a person can stay in the sun before developing an erythema response to the solar ultraviolet (UV) rays. For each new sunscreen product, the SPF value must be tested. Guidelines, such as those from the International Organization for Standardization (ISO) define an in vivo procedure, in which the ratio between the UV dose leading to a minimal erythemal response (MED) on protected skin and on unprotected skin, is calculated with a minimum of 10 voluntary test subjects [8].
Consequently, the current procedure requires that the tested volunteers are being intentionally exposed to UV irradiation, raising ethical concerns. Sufficient evidence shows that UV radiation is the main environmental cause of non-melanoma skin cancer [9]. It is considered to be the main environmental cause of cutaneous melanoma, too [10]. Therefore, the European Commission for safety of non-food products has advised the development of a non-invasive method for the determination of SPFs [11].
In the past decades, efforts have been made to develop an in vitro procedure: However, no approach measuring the transmission of the sunscreen on roughened polymethylmethacrylate (PMMA) plates has been broadly accepted, yet [12, 13]. While the shape of the absorption curve represents the effectiveness of the sunscreen filters reliably, the magnitude of the absorbance is often either over- or underestimated, because artificial substrates cannot imitate the interaction between sunscreen formulations and skin [5, 14, 15].
Therefore, a method is required, which allows measurements directly on the human skin without inducing damage due to irradiation.
SPF determination via hybrid diffuse reflectance spectroscopy (HDRS) fulfills these criteria.
By comparing the skin's remitted light before and after the application of a sunscreen, the absolute UVA absorption spectrum can be evaluated. Due to high light extinction in the UVB range, the shape of the UVB absorbance spectrum can be measured using an in vitro transmission spectrum and then adjusted to the in vivo diffuse reflectance spectroscopy (DRS) measurements. Different approaches using two monochromators or a polychromatic light source showed a good correlation with conventional SPF testing [16-18].
In the following, a new DRS device for SPF and UVA-PF measurements is presented that uses an LED-based light source, thus providing an adjustable and compact test system, since LEDs can be pulsed, and the UVA and the UVB range can be individually adapted.
2 MATERIALS AND METHOD
2.1 Diffuse reflectance spectroscopy setup
The in vivo measurements were performed with a DRS device, using a customized multi-lambda-LED light source with 8 LEDs at wavelengths from 290 to 400 nm. A customized 84-channel imaging spectrograph was used for detection. A customized fiber optic probe captured 7 measuring positions and two detection distances simultaneously (= 14 channels). At every probe position, the optimal exposure time (max. 4 seconds) was evaluated by a sample measurement with short exposure time. Thereby, overexposure at positions with high reflectance was avoided, while the total erythema effective dose was limited to below 30 J/m2. This corresponds to about 20% of the MED of skin type I and a lower fraction for skin types II and III (e.g. ≈10% for skin type II) [19, 20]. The LED settings (and thus the overall UV illumination spectrum) chosen ensured an optimal signal to noise ratio of the skin reflectance.
Reflectance values for each of the 84 detection fibers were derived from the reflectance image. On this level, a square root was applied in order to achieve a “transmission-like” weighting of values before robust averaging was used for calculation of values on the level of individual channels, measurement position, and probe position, respectively. Reflectance values without sunscreen were averaged over all measurement positions. The transmission was obtained dividing the reflectance measured with sunscreen by the reflectance measured without sunscreen. The result of the measurement was a robust average transmission spectrum for each volunteer and each sunscreen.
2.2 In vivo measurement
The test subjects met the criteria defined in ISO 24444 [8], having skin phototypes I, II and III and no disqualifying skin conditions, such as, sun damage or pigmentation marks. A positive vote for the study design had been obtained from the ethics committee of the Charité-Universitätsmedizin Berlin, and all test subjects had provided their written informed consent. 10 subjects were tested for each test sample. Before starting a new measurement series, the device was calibrated for the specific volunteer to check the constancy of the light emittance. Five measurements per test area (10 × 10 cm) were performed before test product application. A total of 20 spectra of all 4-test areas was used for further calculation. An amount of 2.0 mg/cm2 was applied to the back of the test subjects. 25 to 30 minutes after the application 20 measurements were carried out in the test area.
2.3 Hybrid diffuse reflectance spectroscopy procedure
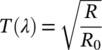 ()
()The in vitro data were provided by the sunscreen manufacturers. The transmission values of the in vivo DRS measurement TDRS(λ) are divided by the preexposure in vitro value at each wavelength between 320 and 330 nm, to determine the scaling factor CHDRS'λ at each wavelength. The scaling value CHDRS'0 is the arithmetic mean of these 11 CHDRS'λ values. The values of TPMMA(λ) from 290 to 320 nm are multiplied by the scaling value CHDRS'0 and combined with TDRS(λ) values from 321 to 400 nm to form the transmission spectrum THDRS(λ) from 290 to 400 nm (THDRS(λ)290-400nm). Figure 1 shows representative spectra of different stages of the HDRS procedure.
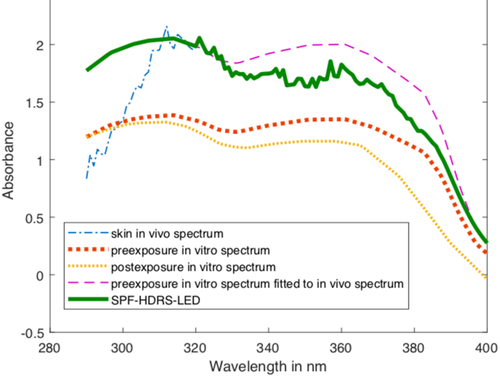
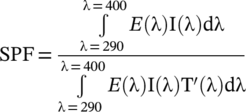 ()
()THDRS(λ)290–400 nm is multiplied by CPD'(λ) before calculating SPFHDRS according to Equation (2), where E(λ) = erythemal action spectrum, I(λ) = spectral irradiance, T'(λ) = postexposure transmission THDRS(λ)290–400 nm, and d(λ) = wavelength interval.
We decided to apply the HDRS principle to the in vivo and the preexposure in vitro spectra. Alternatively, one could first correct the in vivo spectra for photodegradation and then use the postexposure in vitro spectra to obtain the HDRS spectra, see Table 2.
2.4 Test products
Eleven commercially available test products with an SPF ranging from 12 to 73 and a UVA-PF ranging from 5.8 to 36.6 were investigated. Included were lotions and sprays, products without any physical filters as well as one product with titanium dioxide (nano) and zinc oxide. The samples also differed in their photostability. The test products and test protocols from certified test institutes were provided by the manufacturer.
3 RESULTS
The SPF and UVA protection factor (UVA-PF) values obtained by this new approach were compared with in vivo SPF results determined by certified test institutes, as illustrated in Figures 2 and 3. A linear regression through zero with a slope of 1.43 and a correlation coefficient R2 = 0.86 for SPF and a slope of 1.29 and R2 = 0.92 for UVA-PF was obtained. The error bars of the reference values correspond to the 17% maximum standard error, which is allowed according to ISO 24444 [8].
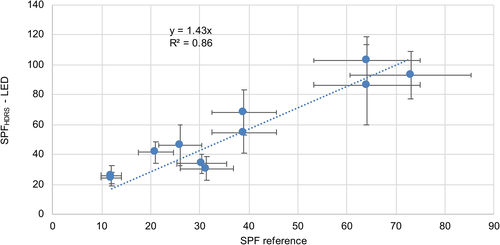
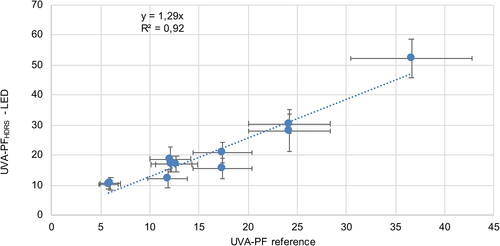
The sunscreens included in the evaluation with their reference, SPFHDRS-LED and UVA-PFHDRS-LED values are shown in Table 1.
| Sunscreen nr. | Type of formulation | UV filter | Reference SPF of manufacturer measured by test institute (n, number of volunteers) | SPF +/-SDa measured by multi-lambda-LED device, corrected for photodegradation | Reference UVA-PF of manufacturer measured by test institute | UVA-PF +/-SDa measured by multi-lambda-LED device, corrected for photodegradation |
|---|---|---|---|---|---|---|
| 1 | O/W lotion | Organic UV filters | 12 (n = 10) | 24.6+/−3.8 | 5.8 | 10.3 +/−1.3 |
| 2 | O/W lotion | Organic UV filters | 12 (n = 10) | 26.2+/−6.7 | 6 | 10.6+/−2.1 |
| 3 | O/W spray | Organic UV filters | 21 (n = 11) | 41.6+/−7.2 | 12.2 | 17.0+/−2.6 |
| 4 | O/W lotion | Organic UV filters | 26 (n = 10) | 46.4+/−13.8 | 12.1 | 18.8+/−4.2 |
| 5 | Oil spray | Organic UV filters | 30.4 (n ≥ 10) | 34.0+/−6.4 | 17.4 | 15.6+/−3.2 |
| 6 | O/W lotion | titanium dioxide (nano) and zinc oxide | 31.4 (n = 11) | 30.8+/−7.8 | 11.8 | 12.3+/−3.1 |
| 7 | O/W lotion | Organic UV filters | 39 (n = 10) | 54.9+/−14.0 | 12.7 | 17.0+/−2.7 |
| 8 | O/W spray | Organic UV filters | 39 (n = 10) | 68.1+/−15.4 | 17.4 | 20.9+/−3.4 |
| 9 | O/W lotion | Organic UV filter | 64 (n = 10) | 102.9+/−16.2 | 24.2 | 30.5+/−3.4 |
| 10 | O/W spray | Organic UV filter | 64 (n = 10) | 86.7 +/−26.9 | 24.2 | 28.1+/−7.0 |
| 11 | O/W lotion | Organic UV filter | 73 (n = 10) | 93.0+/−15.7 | 36.6 | 52.2+/−6.5 |
- a Standard deviation.
In addition to the SPFHDRS values, in vivo DRS data obtained by the multi-lambda device were compared with results determined by test institutes. Taking the photostability into account, an R2 = 0.78 was calculated for sunscreen products up to SPF 26 (n = 4), as shown in Figure 4. When considering only test subjects with skin photo type I (n = 2–4), an R2 = 0.9 was calculated for sunscreen products with SPF 26 and lower.
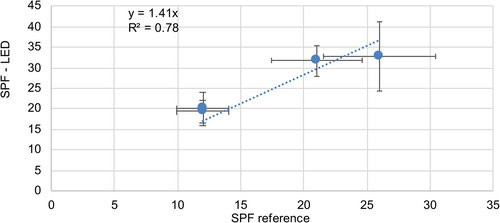
In addition to the SPFHDRS values, in vivo DRS data obtained by the multi-lambda device were compared with results determined by test institutes. Taking the photostability into account, an R2 = 0.78 was calculated for sunscreen products up to SPF 26 (n = 4), as shown in Figure 4. When considering only test subjects with skin photo type I (n = 2–4), an R2 = 0.9 was calculated for sunscreen products with SPF 26 and lower.
4 DISCUSSION
During the project implementation, various approaches to apply the HDRS principle have been examined. Each of these approaches used a scaling factor C'λ of each wavelength either between 320 and 330 nm or between 340 and 350 nm to adjust the in vitro spectrum to the in vivo DRS measurements. Different approaches for aging (correction for photodegradation) were investigated. They included no aging at all, calculating the ratio of photodegradation (RPD) based on Rohr et al. [17] and determining a wavelength dependent scaling factor. The correction for photodegradation was done after application of the HDRS principle and alternatively before calculating a HRDS spectrum. In addition, both versions were carried out using either transmission or absorption values for all the calculation steps. The correlation coefficients for all of these options are shown in Table 2.
| Type of in vitro spectra used for HDRS spectrum | No aging | Aging with RPD | Aging with a wavelength dependent factor | |||||
|---|---|---|---|---|---|---|---|---|
| Pre-exposure in vitro data | Post-exposure in vitro data | Pre-exposure in vitro data | Post-exposure in vitro data | Preexposure in vitro data | Postexposure in vitro data | |||
| Type of spectra used for calculation of HDRS spectra and aging | Based on absorption values | Based on transmission values | Based on absorption values | Based on transmission values | ||||
| 320–330 nm | 0.81 | 0.76 | 0.76 | 0.81 | 0.88 | 0.86 | 0.85 | 0.83 |
| 340–350 nm | 0.8 | 0.78 | 0.55 | 0.79 | 0.78 | 0.79 | 0.84 | 0.84 |
The method presented above was found to produce the most valid and best reproducible results, which indicates that the multi-lambda device is suitable for SPF testing based on the HDRS principle as well as for UVA-PF measurements. An external device for testing photostability is required, yet.
Compared to waiting 24 hours for erythema formation using conventional SPF determination, this approach is much faster: Measuring one SPF takes about 15 minutes per volunteer plus the time of application and incubation (independent of the formulation).
With an effective dose of less than 20% of the MED of skin type I the technology is non-invasive. No erythemal response is being induced. This makes MED reading, a soft skill often discussed as an inter-grader variable factor of SPF testing, obsolete [22, 23].
The spectroscopic measurements are independent of a biological response and thus not influenced by anti-inflammatory components of certain commercial sunscreen formulations, which can otherwise lead to an overestimation of SPF values [24].
The compact LED-based setup offers a high flexibility in terms of spectrum, light intensity and time. The duration of illumination can be adapted to measurement purposes. Each LED can be individually adjusted in order to tune and to omit specific wavelength ranges. This could be beneficial, for example, to investigate particular wavelength-dependent properties of sunscreen products.
Apart from SPF values based on the HDRS principle, the multi-lambda-LED device produced DRS in vivo values that showed a good accuracy in sunscreen products with SPF 26 and lower, especially when testing subjects with skin type I. This is in accordance with previous experiments using an older Xenon lamp-based spectroscopic DRS setup, where the comparably high reflectance of pig ear skin was mostly in good agreement with SPF values [25]. Pure in vivo measurements were also shown for a setup using one UVB LED and a photodiode for detection [26]. Now the multi-lambda-LED light source with 8 LEDs covering the whole UV range allows more spectrally resolved and thus accurate measurements. These could be beneficial for testing the reproducibility of SPF values, for example, to determine the sweat-resistance or homogeneity of a sunscreen product [7, 27]. Furthermore, the in vivo spectra may be useful for research purposes, since skin filter interactions are measured in the UVA and in the UVB range. For sunscreen products with SPF values higher than 26 the remitted light signal becomes insufficient for the determination of SPFs. In order to pursue a non-invasive, pure in vivo method to determine high SPF values, too, a modified, more powerful multi-lambda-LED device with a cooling system will be developed.
5 CONCLUSION
Overall, we present a compact and adjustable measurement system using a multi-lambda-LED light source that is suitable for non-invasive and fast SPF testing based on the HDRS principle. In addition, DRS in vivo measurements can be performed and used for research purposes.
ACKNOWLEDGMENTS
We thank Jürgen Helfmann for his contribution in the early phase of the program. We are thankful to Mathias Rohr for providing photostability measurements. Open access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST
G.W., C.R., and G.K. are employed by Courage + Khazaka electronic GmbH, which is the industrial partner of the joint research project with an interest in commercialization.
AUTHOR CONTRIBUTIONS
Georg Wiora, Carina Reble, Carolin Maria Throm, Martina C. Meinke and Jürgen Lademann were involved in conceptualization. Carina Reble, Georg Wiora and Georg Khazaka, construction and building up the system. Georg Wiora, Carolin Maria Throm and Johannes Schleusener were involved analyzing the data. Carolin Maria Throm, Sabine Schanzer, Georg Wiora, Carina Reble, Johannes Schleusener, Ludger Kolbe and Hans Karrer were involved in investigation. Carolin Maria Throm writing-original draft; Carina Reble, Johannes Schleusener, Martina C. Meinke and Jürgen Lademann writing-review and editing. Jürgen Lademann, Martina C. Meinke, Georg Khazaka and Georg Wiora, project management.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




