Control of optical transparency and infrared laser heating of costal cartilage via injection of iohexol
Corresponding Author
Yulia M. Alexandrovskaya
Institute of Photon Technologies, Federal Scientific Research Centre “Crystallography and Photonics” of the Russian Academy of Sciences, Moscow, Russia
Correspondence
Yulia M. Alexandrovskaya, Institute of Photon Technologies, Federal Scientific Research Centre "Crystallography and Photonics" of the Russian Academy of Sciences, Pionerskaya St. 2, 142190 Troitsk, Moscow, Russia.
Email: [email protected]
Search for more papers by this authorEvgeniy G. Evtushenko
M.V. Lomonosov Moscow State University, Moscow, Russia
Search for more papers by this authorMariya M. Obrezkova
M.V. Lomonosov Moscow State University, Moscow, Russia
Search for more papers by this authorValery V. Tuchin
Research-Educational Institute of Optics and Biophotonics, Saratov State University, Saratov, Russia
Laboratory of Laser Diagnostics of Technical and Living Systems, Institute of Precision Mechanics and Control RAS, Saratov, Russia
Interdisciplinary Laboratory of Biophotonics, Tomsk State University, Tomsk, Russia
Search for more papers by this authorEmil N. Sobol
Institute of Photon Technologies, Federal Scientific Research Centre “Crystallography and Photonics” of the Russian Academy of Sciences, Moscow, Russia
IPG Medical Corporation, Marlborough, Massachusetts
Search for more papers by this authorCorresponding Author
Yulia M. Alexandrovskaya
Institute of Photon Technologies, Federal Scientific Research Centre “Crystallography and Photonics” of the Russian Academy of Sciences, Moscow, Russia
Correspondence
Yulia M. Alexandrovskaya, Institute of Photon Technologies, Federal Scientific Research Centre "Crystallography and Photonics" of the Russian Academy of Sciences, Pionerskaya St. 2, 142190 Troitsk, Moscow, Russia.
Email: [email protected]
Search for more papers by this authorEvgeniy G. Evtushenko
M.V. Lomonosov Moscow State University, Moscow, Russia
Search for more papers by this authorMariya M. Obrezkova
M.V. Lomonosov Moscow State University, Moscow, Russia
Search for more papers by this authorValery V. Tuchin
Research-Educational Institute of Optics and Biophotonics, Saratov State University, Saratov, Russia
Laboratory of Laser Diagnostics of Technical and Living Systems, Institute of Precision Mechanics and Control RAS, Saratov, Russia
Interdisciplinary Laboratory of Biophotonics, Tomsk State University, Tomsk, Russia
Search for more papers by this authorEmil N. Sobol
Institute of Photon Technologies, Federal Scientific Research Centre “Crystallography and Photonics” of the Russian Academy of Sciences, Moscow, Russia
IPG Medical Corporation, Marlborough, Massachusetts
Search for more papers by this authorAbstract
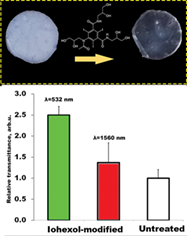
Infrared (IR) laser impact has no analogues for rapid and safe cartilage reshaping. For better penetration of radiation optical clearing agents (OCAs) can be applied. In present work, the effect of low-osmolality agent iohexol on costal cartilage is studied. Specifically, it is shown that ½ of total increase of optical transparency occurs in 20 minutes of immersion. Maximally, cartilage transparency on 1560 nm can be increased in 1.5 times. Injection of iohexol results in increased tissue hygroscopicity, lower drying rate and higher percentage of bound water. Effective diffusion coefficients of water liberation at 21°C are (5.3 ± 0.4) × 10−7 and (3.3 ± 0.1) × 10−7 cm2/s for untreated and iohexol-modified tissue, respectively. Raman spectroscopy of irradiated iohexol solution reveals its photo and thermo-stability under clinically used IR laser energies up to 350 W/cm2 for exposure times of several seconds. At energies higher than 500 W/cm2 [Correction added on 5 September 2018, after first online publication: This unit has been changed] decomposition of iohexol occurs rapidly through formation of molecular iodine and fluorescent residue.
Supporting Information
| Filename | Description |
|---|---|
| jbio201800090-sup-0001-author-biographies.docxapplication/docx, 966.4 KB | Author Biographies |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
REFERENCES
- 1E. Genina, A. Bashkatov, K. Larin, V. Tuchin, in Laser Imaging and Manipulation in Cell Biology (Ed: F. S. Pavone), Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim 2010, p. 115.
- 2L. Guo, R. Shi, C. Zhang, D. Zhu, Z. Ding, P. Li, J. Biomed. Opt. 2016, 21(8), 081202.
- 3E. Costa, A. Moreira, D. de Melo-Diogo, I. Correia, Opt. Laser Technol. 2018, 106, 94.
- 4L. Chen, G. Li, Y. Li, Y. Li, H. Zhu, L. Tang, P. French, J. McGinty, S. Ruan, Sci. Rep. 2017, 7, 12218.
- 5T. Lagerweij, S. Dusoswa, A. Negrean, E. Hendrikx, H. de Vries, J. Kole, J. Garcia-Vallejo, H. Mansvelder, W. Vandertop, D. Noske, B. Tannous, R. Musters, Y. van Kooyk, P. Wesseling, X. Zhao, T. Wurdinger, Angiogenesis 2017, 20, 533.
- 6D. Chen, N. Zeng, Q. Xie, H. He, V. Tuchin, H. Ma, Biomed. Opt. Express 2017, 8(8), 3559.
- 7O. Baum, Y. Soshnikova, E. Sobol, A. Korneychuk, M. Obrezkova, V. Svistushkin, O. Timofeeva, V. Lunin, Lasers Surg. Med. 2011, 43(6), 511.
- 8E. Sobol, A. Sviridov, A. Omelchenko, V. Bagratashvili, M. Kitai, S. E. Harding, N. Jones, K. Jumel, N. Mertig, W. Pompe, Y. Ovchinnikov, A. Shekhter, V. Svistushkin, Biotechnol. Genet. Eng. Rev. 2000, 17, 553.
- 9Y. Alexandrovskaya, K. Sadovnikov, A. Sharov, A. Sherstneva, E. Evtushenko, A. Omelchenko, M. Obrezkova, V. Tuchin, V. Lunin, E. Sobol, J. Biophotonics 2017, 11(2), e201700105.
- 10N. Sudheendran, M. Mohamed, M. G. Ghosn, V. V. Tuchin, K. V. Larin, J. Innov. Opt. Health Sci. 2010, 3(3), 169.
- 11Omnipaque package insert. GE Healthcare Inc., 2007, https://www3.gehealthcare.com/~/media/documents/us-global/products/contrast-media_non-gatekeeper/clinical-product-information/omnipaque/gehealthcare_omnipaque-prescribing-information.pdf (accessed: July 2018).
- 12H. S. Thomsen, S. K. Morcos, BJU Int. 2000, 86(1), 1.
- 13F. Hallouard, N. Anton, P. Choquet, A. Constantinesco, T. Vandamme, Biomaterials 2010, 31, 6249.
- 14R. E. Latchaw, S. L. Albers, Prog. Cardiovasc. Dis. 2017, 59, 555.
- 15A. Tsigginou, C. Gkali, A. Chalazonitis, E. Feida, D. E. Vlachos, F. Zagouri, I. Rellias, C. Dimitrakakis, Br. J. Radiol. 2016, 89(1067), 20160397.
- 16A. W. Palmer, R. E. Guldberg, M. E. Levenston, Proc. Natl. Acad. Sci. U.S.A. 2006, 103(51), 19255.
- 17L. N. M. Hayward, C. M. J. de Bakker, L. C. Gerstenfeld, M. W. Grinstaff, E. F. Morgan, J. Orthop. Res. 2013, 31(4), 567.
- 18A. V. Baskov, I. A. Borshchenko, A. B. Shekhter, V. A. Baskov, A. E. Guller, E. N. Sobol, J. Spine 2015, 4, 210.
10.4172/2165-7939.1000210 Google Scholar
- 19E. Sobol, A. Shekhter, A. Baskov, in Lasers for Medical Applications: Diagnostics, Therapy and Surgery (Ed: H. Jelinkova), Woodhead Publishing Limited, Oxford, UK 2013, p. 628.
10.1533/9780857097545.4.628 Google Scholar
- 20E. Sobol, A. Shekhter, A. Guller, O. Baum, A. Baskov, J. Biomed. Opt. 2011, 16, 080902.
- 21O. I. Baum, A. I. Omelchenko, I. O. Ryzhkov, M. V. Obrezkova, V. V. Lunin, E. N. Sobol, Doklady Biochem. Biophys. 2009, 428(1), 261.
- 22A. Bykov, T. Hautala, M. Kinnunen, A. Popov, S. Karhula, S. Saarakkala, M. T. Nieminen, V. Tuchin, I. Meglinski, J. Biophotonics 2016, 9(3), 270.
- 23A. Bykov, T. Hautala, M. Kinnunen, A. Popov, S. Karhula, S. Saarakkala, M. T. Nieminen, V. Tuchin, Proc. SPIE 2015, 9540, 95400A.
- 24E. Sobol, Phase Transformations and Ablation in Laser-Treated Solids, John Wiley & Sons, New York 1995, p. 4.
- 25M. Chaplin, Water Structure and Science, http://www1.lsbu.ac.uk/water/water_vibrational_spectrum.html#k (accessed: September 2017).
- 26V. N. Bagratashvili, E. N. Sobol, A. P. Sviridov, V. K. Popov, A. I. Omelchenko, S. M. Howdle, J. Biomech. 1997, 30(8), 813.
- 27W. J. Coumans, Chem. Eng. Proc. 2000, 39, 53.
- 28J. Jeong, J. Jung, W. J. Cooper, W. Song, Water Res. 2010, 44(15), 4391.
- 29S. Allard, J. Criquet, A. Prunier, C. Falantin, A. Le Person, J. Yat-Man Tang, J. P. Croué, Water Res. 2016, 15(103), 453.
- 30S. Giannakis, M. Jovic, N. Gasilova, M. Pastor Gelabert, S. Schindelholz, J. M. Furbringer, H. Girault, C. Pulgarin, J. Environ. Manage. 2017, 15(195), 174.
- 31C.-H. Liu, M. Singh, J. Li, Z. Han, C. Wu, S. Wang, R. Idugboe, R. Raghunathan, E. N. Sobol, V. V. Tuchin, M. Twa, K. V. Larin, Modern Technol Med 2015, 7(1), 44.
- 32K. A. Kulmala, R. K. Korhonen, P. Julkunen, J. S. Jurvelin, T. M. Quinn, H. Kröger, J. Töyräs, Med. Eng. Phys. 2010, 32(8), 878.
- 33M. Padalkar, R. Spencer, N. Pleshko, Ann. Biomed. Eng. 2013, 41(11), 1. https://doi.org/10.1007/s10439-013-0844-0.
- 34G. Varsanyi, S. Szoke, Vibrational Spectra of Benzene Derivatives, Academic Press, New York and London 1969, p. 143.
- 35D. W. Mayo, F. A. Miller, R. W. Hannah, Course Notes on the Interpretation of Infrared and Raman Spectra, John Wiley & Sons Inc., Hoboken 2003.
- 36B. A. Kolesov, Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 179, 216.
- 37B. M. Auer, J. L. Skinner, J. Chem. Phys. 2008, 128(22), 224511.
- 38S. Salkic, L. H. Eckler, M. J. Nee, J. Raman Spectrosc. 2013, 44, 1746.
- 39P. Deplano, F. A. Devillanova, J. R. Ferraro, F. Isaia, V. Lippolis, M. L. Mercuri, Appl. Spectrosc. 1992, 46, 1625.
- 40E. N. Sobol, O. I. Baum, A. I. Omelchenko, Y. M. Soshnikova, A. V. Yuzhakov, E. M. Kas'yanenko, A. V. Tokareva, A. V. Baskov, V. M. Svistushkin, L. V. Selezneva, A. B. Shekhter, Quantum Electron. 2017, 47(10), 935.
- 41E. Sobol, A. Shekhter, A. Baskov, V. Baskov, O. Baum, I. Borchshenko, V. Golubev, A. Guller, I. Kolyshev, A. Omeltchenko, A. Sviridov, O. Zakharkina, Proc. SPIE 2009, 7179, 71790B.
10.1117/12.822302 Google Scholar
- 42H. Estrada, E. Sobol, O. Baum, D. Razansky, Laser Phys. Lett. 2014, 11, 125601.
- 43E. A. Genina, A. N. Bashkatov, V. V. Tuchin, Expert Rev. Med. Devices 2010, 7(6), 825.
- 44A. M. Smith, M. C. Mancini, S. Nie, Nat. Nanotech. 2009, 4, 710.
- 45A. Y. Sdobnov, V. V. Tuchin, J. Lademann, M. E. Darvin, J. Phys. D Appl. Phys. 2017, 50, 285401.
- 46E. Sobol, O. Baum, A. Shekhter, S. Wachsmann-Hogiu, A. Shnirelman, Y. Alexandrovskaya, I. Sadovskyy, V. Vinokur, J. Biomed. Opt. 2017, 22(9), 091515.
- 47S. L. Jacques, Phys. Med. Biol. 2013, 58, R37.
- 48B. Hiller, R. K. Hanson, Exp. Fluids 1990, 10(1), 1.
- 49S. V. Kireev, S. L. Shnyrev, Laser Phys. 2011, 21, 1775.
- 50C. J. Williamson, J. Chem. Educ. 2011, 88(6), 816.
- 51T. Masiello, N. Vulpanovici, J. W. Nibler, J. Chem. Educ. 2003, 80(8), 914.




