Quantitative in vivo detection of adipose tissue browning using diffuse reflectance spectroscopy in near-infrared II window
Kapil Dev
Laboratory of Bio Optical Imaging, Singapore Bioimaging Consortium, Agency for Science, Technology and Research (A*STAR), Singapore
Search for more papers by this authorU. S Dinish
Laboratory of Bio Optical Imaging, Singapore Bioimaging Consortium, Agency for Science, Technology and Research (A*STAR), Singapore
Search for more papers by this authorSmarajit Chakraborty
Fat Metabolism and Stem Cell Group, Singapore Bioimaging Consortium, Agency for Science, Technology and Research (A*STAR), Singapore
Search for more papers by this authorRenzhe Bi
Laboratory of Bio Optical Imaging, Singapore Bioimaging Consortium, Agency for Science, Technology and Research (A*STAR), Singapore
Search for more papers by this authorStefan Andersson-Engels
Irish Photonic Integration Centre (IPIC), Tyndall National Institute, Cork, Ireland
Department of Physics, University College Cork, Cork, Ireland
Search for more papers by this authorShigeki Sugii
Fat Metabolism and Stem Cell Group, Singapore Bioimaging Consortium, Agency for Science, Technology and Research (A*STAR), Singapore
Cardiovascular and Metabolic Disorders Program, Duke-NUS Medical School, Singapore
Search for more papers by this authorCorresponding Author
Malini Olivo
Laboratory of Bio Optical Imaging, Singapore Bioimaging Consortium, Agency for Science, Technology and Research (A*STAR), Singapore
Correspondence
Malini Olivo, Laboratory of Bio Optical Imaging, Singapore Bioimaging Consortium, Agency for Science, Technology and Research (A*STAR), Singapore.
Email: [email protected]
Search for more papers by this authorKapil Dev
Laboratory of Bio Optical Imaging, Singapore Bioimaging Consortium, Agency for Science, Technology and Research (A*STAR), Singapore
Search for more papers by this authorU. S Dinish
Laboratory of Bio Optical Imaging, Singapore Bioimaging Consortium, Agency for Science, Technology and Research (A*STAR), Singapore
Search for more papers by this authorSmarajit Chakraborty
Fat Metabolism and Stem Cell Group, Singapore Bioimaging Consortium, Agency for Science, Technology and Research (A*STAR), Singapore
Search for more papers by this authorRenzhe Bi
Laboratory of Bio Optical Imaging, Singapore Bioimaging Consortium, Agency for Science, Technology and Research (A*STAR), Singapore
Search for more papers by this authorStefan Andersson-Engels
Irish Photonic Integration Centre (IPIC), Tyndall National Institute, Cork, Ireland
Department of Physics, University College Cork, Cork, Ireland
Search for more papers by this authorShigeki Sugii
Fat Metabolism and Stem Cell Group, Singapore Bioimaging Consortium, Agency for Science, Technology and Research (A*STAR), Singapore
Cardiovascular and Metabolic Disorders Program, Duke-NUS Medical School, Singapore
Search for more papers by this authorCorresponding Author
Malini Olivo
Laboratory of Bio Optical Imaging, Singapore Bioimaging Consortium, Agency for Science, Technology and Research (A*STAR), Singapore
Correspondence
Malini Olivo, Laboratory of Bio Optical Imaging, Singapore Bioimaging Consortium, Agency for Science, Technology and Research (A*STAR), Singapore.
Email: [email protected]
Search for more papers by this authorAbstract
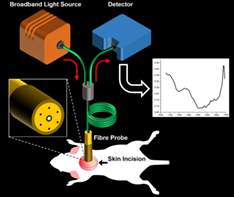
White adipose tissue (WAT) and brown adipose tissue (BAT) biologically function in an opposite way in energy metabolism. BAT induces energy consumption by heat production while WAT mainly stores energy in the form of triglycerides. Recent progress in the conversion of WAT cells to “beige” or “brown-like” adipocytes in animals, having functional similarity to BAT, spurred a great interest in developing the next-generation therapeutics in the field of metabolic disorders. Though magnetic resonance imaging and positron emission tomography could detect classical BAT and WAT in animals and humans, it is of a great challenge in detecting the “browning” process in vivo. Here, to the best of our knowledge, for the first time, we present a simple, cost-effective, label-free fiber optic-based diffuse reflectance spectroscopy measurement in the near infrared II window (~1050-1400 nm) for the quantitative detection of browning in a mouse model in vivo. We could successfully quantify the browning of WAT in a mouse model by estimating the lipid fraction, which serves as an endogenous marker. Lipid fraction exhibited a gradual decrease from WAT to BAT with beige exhibiting an intermediate value. in vivo browning process was also confirmed with standard molecular and biochemical assays.
Supporting Information
| Filename | Description |
|---|---|
| jbio201800135-sup001-author-biographies.docxWord 2007 document , 1.3 MB | Author Biographies |
| jbio201800135-sup002-AppendixS1.docxWord 2007 document , 2.7 MB |
FIGURE S1 Representative intensity normalized diffuse reflectance spectrum from BAT, control WAT and beige (treated WAT) adipose tissue FIGURE S2 CL treatment induced UCP1 expression in CL treated WAT (Beige). (A) UCP1 IHC images of WAT either treated with saline (WAT) or CL (Beige) and (B) BAT upon saline BAT(C) and CL (BAT(CL) treatments are shown. Beige showed increased UCP1 staining as pointed by arrows as compared to WAT. Scale bar represents 100 μm (representative images from n = 4 in each group). |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
REFERENCES
- 1S. Gesta, C. R. Kahn, in Adipose Tissue Biology (Ed: M. E. Symonds), Springer International Publishing, New York, 2017 Ch. 5.
10.1007/978-3-319-52031-5_5 Google Scholar
- 2M. Klingenspor, A. Bast, F. Bolze, Y. Li, S. Maurer, S. Schweizer, M. Willershäuser, T. Fromme, in Adipose Tissue Biology (Ed: M. E. Symonds), Springer International Publishing, New York, 2017 Ch. 4.
10.1007/978-3-319-52031-5_4 Google Scholar
- 3J. Wu, P. Boström, L. M. Sparks, L. Ye, J. H. Choi, A. H. Giang, M. Khandekar, K. A. Virtanen, P. Nuutila, G. Schaart, K. Huang, H. Tu, W. D. van Marken Lichtenbelt, J. Hoeks, S. Enerbäck, P. Schrauwen, B. M. Spiegelman, Cell 2012, 150, 366.
- 4W. Wang, P. Seale, Nat. Rev. Mol. Cell Biol. 2016, 17, 691.
- 5L. Z. Sharp, K. Shinoda, H. Ohno, D. W. Scheel, E. Tomoda, L. Ruiz, H. Hu, L. Wang, Z. Pavlova, V. Gilsanz, S. Kajimura, PLoS One 2012, 7, e49452.
- 6A. M. Cypess, L. S. Weiner, C. Roberts-Toler, E. Franquet Elia, S. H. Kessler, P. A. Kahn, J. English, K. Chatman, S. A. Trauger, A. Doria, G. M. Kolodny, Cell Metab. 2015, 21, 33.
- 7K. L. Townsend, Y. H. Tseng, Int J Obes Suppl. 2015, 5, S15.
- 8H. H. Hu, Crit. Rev. Biomed. Eng. 2015, 43, 161.
- 9L. Sun, J. Yan, L. Sun, S. S. Velan, M. K. S. Leow, Diabetes Metab. 2017, 43, 401.
- 10R. Hao, L. Yuan, N. Zhang, C. Li, J. Yang, J. Pediatr. Endocrinol. Metab. 2012, 25, 233.
- 11X. Wang, L. J. Minze, Z. Z. Shi, J. Vis. Exp. 2012, 23, 4060.
- 12H. H. Hu, D. L. Smith, K. S. Nayak, M. I. Goran, T. R. Nagy, J. Magn. Reson. Imaging 2010, 31, 1195.
- 13H. H. Hu, C. D. G. Hines, D. L. Smith, S. B. Reeder, Magn. Reson. Imaging 2012, 30, 323.
- 14K. N. Bhanu Prakash, S. K. Verma, J. Yaligar, J. Goggi, V. Gopalan, S. S. Lee, X. Tian, S. Sugii, M. K. Leow, K. Bhakoo, S. S. Velan, MAGMA 2016, 29, 277.
- 15N. L. Reddy, T. A. Jones, S. C. Wayte, O. Adesanya, S. Sankar, Y. C. Yeo, G. Tripathi, P. G. McTernan, H. S. Randeva, S. Kumar, C. E. Hutchinson, T. M. Barber, J. Clin. Endocrinol. Metab. 2014, 99, E117.
- 16H. H. Hu, T. G. Perkins, J. M. Chia, V. Gilsanz, AJR Am. J. Roentgenol. 2013, 200, 177.
- 17H. H. Hu, L. Yin, P. C. Aggabao, T. G. Perkins, J. M. Chia, V. Gilsanz, J. Magn. Reson. Imaging 2013, 38, 885.
- 18J. Reber, M. Willershäuser, A. Karlas, K. Paul-Yuan, G. Diot, D. Franz, T. Fromme, S. V. Ovsepian, N. Bézière, E. Dubikovskaya, D. C. Karampinos, C. Holzapfel, H. Hauner, M. Klingenspor, V. Ntziachristos, Cell Metab. 2018, 27, 689.
- 19S. He, Y. An, X. Li, X. Wei, Q. Sun, Z. Wu, J. Y. Qu, J. Biophotonics 2018, e201800019. https://doi.org/10.1002/jbio.201800019.
- 20R. A. Schwarz, W. Gao, D. Daye, M. D. Williams, R. Richards-Kortum, A. M. Gillenwater, Appl. Optics 2008, 47, 825.
- 21B. Yu, A. Shah, V. K. Nagarajan, D. G. Ferris, Biomed. Opt. Express 2014, 5, 675.
- 22W. C. Lin, D. I. Sandberg, S. Bhatia, M. Johnson, S. Oh and J. Ragheb, J. Biomed. Opt. 2010, 15, 061709.
- 23J. Antonsson, O. Eriksson, P. Blomstedt, A. T. Bergenheim, M. I. Hariz, J. Richter, P. Zsigmond, K. Wårdell, J. Neural Eng. 2008, 5, 185.
- 24P. Rejmstad, J. D. Johansson, N. Haj-Hosseini, K. Wårdell, J. Biophotonics 2017, 10, 446.
- 25Z. Volynskaya, A. S. Haka, K. L. Bechtel, M. Fitzmaurice, R. Shenk, N. Wamg, J. Nazemi, R. R. Dasari, M. S. Feld, J. Biomed. Opt. 2008, 13, 024012.
- 26M. D. Keller, S. K. Majumder, M. C. Kelley, I. M. Meszoely, F. I. Boulos, G. M. Olivares, A. Mahadevan-Jansen, Lasers Surg. Med. 2010, 42, 15.
- 27G. Dupuis, M. Elias, L. Simonot, Appl. Spectrosc. 2002, 56, 1329.
- 28G. N. Stamatas, B. Z. Zmudzka, N. Kollias, J. Z. Beer, Br. J. Dermatol. 2008, 159, 683.
- 29R. Nachabé, B. H. W. Hendriks, M. van der Voort, A. E. Desjardins, H. J. C. M. Sterenborg, Biomed. Opt. Express 2010, 1, 1432.
- 30R. Nachabé, B. H. W. Hendriks, M. van der Voort, A. E. Desjardins, H. J. C. M. Sterenborg, J. Biomed. Opt. 2010, 15, 037015.
- 31L. L. de Boer, B. G. Molenkamp, T. M. Bydlon, B. H. W. Hendriks, J. Wesseling, H. J. C. M. Sterenborg, T. J. M. Ruers, Breast Cancer Res. Treat. 2015, 152, 509.
- 32G. Ganesan, R. V. Warren, A. Leproux, M. Compton, K. Cutler, S. Wittkopp, G. Tran, T. O'Sullivan, S. Malik, P. R. Galassetti, B. J. Tromberg, Int. J. Obes. (Lond) 2016, 40, 1292.
- 33S. Nirengi, T. Yoneshiro, H. Sugie, M. Saito, T. Hamaoka, Obesity 2015, 23, 973.
- 34U. S. Dinish, C. L. Wong, S. Sriram, W. K. Ong, G. Balasundaram, S. Sugii, M. Olivo, Sci. Rep. 2017, 7, 41357.
- 35A. M. Smith, M. C. Mancini, S. Nie, Nat. Nanotechnol. 2009, 4, 710.
- 36R. Hennessy, S. L. Lim, M. K. Markey and J. W. Tunnell, J. Biomed. Opt. 2013, 18, 037003.
- 37N. Reistad, J. Nilsson, O. V. Timmermand, C. Sturesson, S. Andersson-Engels, Proc. SPIE 2015, 9531, 9314E.
- 38J. G. Granneman, H. P. Moore, Trends Endocrinol. Metab. 2008, 19, 3.
- 39D. Langin, D. Ekholm, M. Ridderstråle, M. Lafontan, P. Belfrage, Biochim. Biophys. Acta 1992, 1135, 349.
- 40K. Mössenböck, A. Vegiopoulos, A. J. Rose, T. P. Sijmonsma, S. Herzig, T. Schafmeier, PloS One 2014, 9, e110428.
- 41J. Lee, K. A. Keuter, J. Kim, A. Tran, A. Uppal, D. Mukai, S. B. Mahon, L. C. Cancio, A. Batchinsky, B. J. Tromberg, M. Brenner, Mil. Med. 2009, 174, 615.




