Enhanced phototoxicity of photodynamic treatment by Cx26-composed GJIC via ROS-, calcium- and lipid peroxide-mediated pathways
Abstract
In spite of the promising initial treatment responses presented by photodynamic therapy (PDT), 5-year recurrence rates remain high level. Therefore, improvement in the efficacy of PDT is needed. There are reports showing that connexin(Cx) 26-composed gap junctional intercellular communication (GJIC) enhances the intercellular propagation of “death signal”, thereby increasing chemotherapeutic cytotoxicity. However, it is unclear whether Cx26-formed GJIC has an effect on PDT phototoxicity. The results in the present study showed that Cx26-composed GJ formation at high density enhances the phototoxicity of Photofrin-PDT. When the Cx26 is not expressed or Cx26 channels are blocked, the phototoxicity in high-density cultures substantially reduces, indicating that the enhanced PDT phototoxicity at high density is mediated by Cx26-composed GJIC. The GJIC-mediated increase in PDT phototoxicity was associated with ROS, calcium and lipid peroxide-mediated stress signaling pathways. The work presents the ability of Cx26-composed GJIC to enhance the sensitivity of malignant cells to PDT, and indicates that maintenance or increase of Cx26-formed GJIC may be a profitable strategy towards the enhancement of PDT therapeutic efficiency. Picture: The survival response of Photofrin-PDT in Dox-treated (Cx26 expressing, GJ-formed) and Dox-untreated cells (Cx26 non-expressing, GJ-unformed) at high-cell density condition.
1 Introduction
Photodynamic therapy (PDT) is a novel oncotherapy exerting a selective cytotoxic activity toward malignant cells via reactive oxygen species (ROS) generated by energy transduction from photosensitizers after excitation by visible light. Photofrin was the first photosensitizer approved by FDA in 1995 and Photofrin-mediated PDT is the most widely used for reducing obstruction and relieving symptoms in patients with obstructing esophageal cancer 1. In spite of promising initial treatment responses after PDT, 5-year recurrence rate remains at high level 2. Consequently, improving the therapeutic efficacy of PDT is needed.
Gap junction (GJ) channel, which is constituted by two hemichannels composed by six connexin (Cx) subunits, connects the cytoplasm of adjacent cells and mediates direct intercellular movement of cytoplasmic signaling molecules 3. Gap junctional intercellular communication (GJIC) is driven by the propagation of the molecules with the molecule weight less than 1.5 kDa. This communication pathway plays a vital role in cellular proliferation, differentiation and tumor suppression 4, 5. It has been reported that GJIC holds the potential to regulate the sensitivity of chemotherapeutic agents or ionizing radiation 6, 7. Reports showed that increased cisplatin toxicity was observed when GJIC between the target cells was present 8. Such an effect has also been noticed when malignant cells were treated with ionizing radiation, in which GJ increases the sensitivity of human skin fibroblasts to ionizing radiation-induced cell death 6.
An inference from the above results is that the sensitivity of cytotoxic substance modulated by GJIC depends on the “death signal” which can diffuse through GJ channels to surrounding cells and cause cell death. This is called a “bystander effect”. It is noteworthy that Cx26, which is one of the connexins (Cxs) family members that form GJ channels, has a capability to mediate a “bystander effect” that enables toxic substances to be transmitted to neighboring cells and cause cell damage 9. There are reports showing that enhancement of Cx26-composed GJIC by baicalein increases the cytotoxicity of cisplatin 10, in contrast, inhibition of Cx26-formed GJIC by propofol depresses the cisplatin cytotoxicity 11. The reports of Cx26-composed GJIC dependent component of cisplatin cytotoxicity suggests that the enhancement of Cx26-formed GJIC could increase the intercellular diffusion of “death signal”, resulting in the augment of cytotoxic action of chemotherapeutics. However, it is unclear whether Cx26-formed GJIC has an effect on the phototoxicity of PDT.
Studies reported that the enhanced amounts of intracellular ROS, Ca2+ and lipid peroxides such as ceramide and 4-hydroxynonenal (4-HNE) are responsible for cellular damage induced by PDT [12–14]. Studies have documented that mitochondrial ROS can diffuse through GJ channels, thereby presenting themselves as an oxidative stress signal 15 and intracellular Ca2+ can propagate via GJ for the regulation of cellular function 3. In addition, ceramide and 4-HNE may have the possibility to propagate through GJ channels due to their small molecular weights. In view of these reports, it is reasonable to hypothesize that Cx26-composed GJIC might have an effect on the PDT phototoxicity.
Therefore, this study was aimed to investigate the effect of Cx26-composed GJIC on the phototoxicity of Photofrin-PDT in HeLa cells transfected with Cx26 and the underlying mechanisms. The results illustrated that Cx26-composed GJIC could enhance PDT phototoxicity in transfected HeLa cells. This effect was associated with the increased amounts of intracellular ROS, Ca2+, ceramide and 4-HNE by GJ. The work presents the ability of Cx26-composed GJIC to enhance the sensitivity of malignant cells to PDT, and indicates that maintenance or increase of Cx26-formed GJIC may be a beneficial strategy towards the enhancement of PDT therapeutic efficacy.
2 Materials and methods
2.1 Materials
Photofrin® (75 mg/vail) was supplied by Union Med. Group Limited Company, Hong Kong, China. G418 was from Amrseco, America. Doxycycline (Dox) was purchased from Abcam. The reagents for cell culture were purchased from Life Technology. Ceramide and 4-HNE ELISA kits were purchased from Shanghai Enzyme-linked Biotechnology Co., Ltd, China. Fluo-3-Am was from Beyotime, China. Other reagents were all provided from Sigma-Aldrich unless otherwise noted.
2.2 Cell line and cell culture
Stably transfected HeLa cell line was a generous gift from Prof. Tao Liang (Sun Yat-Sen University at Guangzhou city, China). The cells were stably transfected to express Cx26 channels under the control of a bidirectional tetracycline-inducible promoter, described by Koreen et al. 16 Cells were grown in DMEM maintained with 10 % fetal bovine serum (FBS), 200 μg/mL hygromycin B, and 100 μg/mL G418 sulfate. Cx26 expression was induced with 1 μg/mL Dox for 48 h.
2.3 Photofrin incubation and 18α-GA treatment
Photofrin was dissolved in 5 % dextrose solution without exposure to light to prepare stock solution (10 mg/mL), and then diluted to a series of concentrations by DMEM before use. After incubation with different concentrations of Photofrin for 4 h at 37 °C in the dark, the cells were cultured in Photofrin-free complete medium before irradiation. After PDT, the cells were then kept culturing in drug-free complete medium for another 24 h at 37 °C without exposure to light until CCK-8 assay was used to assess phototoxicity of Photofrin. 18α-GA was dissolved in DMSO at 10 mM, diluted to final concentration of 10 μM in culture medium, and added to the cells 2 h before Photofrin incubation.
2.4 In vitro photosensitivity
Phototoxicity was assessed by a CCK-8 (Dojindo Molecular Technologies, Japan) assay under the conditions of high and low cell density, where the formation of GJ is allowed or not, respectively. For the condition of high-cell density, cells in the logarithmic phase were seeded into 96-well plates at 3×104 cells/cm2 in order that the cells were at 70 %-100 % confluent when exposure to Photofrin. At this density, a single cell could contact with 3 to 5 surrounding cells on average, promoting gap junctions (GJs) formation. As for the condition of low-cell density, cells were seeded into 96-well plates at 3×103 cells/cm2. At this density, there was no probability for the formation of GJs as they did not contact each other. After incubation with Photofrin (1-10 μg/mL), the cells were irradiated by an argon-pumped dye laser at 630 nm (20 mW/cm2 and 2 J/cm2). After PDT, 10 μL CCK-8 solution was added and incubated with cells for 2 h. The OD values were detected at 450 nm to assess cellular survival using Enzyme-labeling instrument (Elx808, Bio Tek, America).
2.5 “Parachute” dye-coupling assay
The “Parachute” dye-coupling assay was performed as described in our previous study to assess GJ function 17. Briefly, donor and receiver cells were seeded in 24-well plates until they were grown to 70 %–100 % confluence. Donor cells were loaded with 5 μM calcein-acetoxymethyl ester, which can diffuse through GJ channel after conversion into gap junction–permeable dye calcein, and then seeded onto the receiver cells at 1:150 donor/receiver ratio after trypsinization. The donor cells were permitted to contact with the surrounding receiver cells for 4 h at 37 °C for GJ formation. The average amount of the receiver cells receiving dyes transmitted by each donor cell was determined using fluorescence microscope and normalized to the controls.
2.6 Western blot assay
The extracted protein samples were separated in 10 % Tris-glycine gels by SDS-polyacrylamide gel electrophoresis and then transferred to a nitrocellulose transfer membrane (Excell Bio, China). Cx26 antibody (dilution 1:5000) was incubated at 4 °C overnight. Secondary antibody was IRDye 800CW purified immunoglobulin-conjugated anti-rabbit (dilution 1:10000). Immunopositive bands were visualized at Ex/Em=778 nm/795 nm.
2.7 Intracellular ROS and Ca2+ measurement using flow cytometer
Flow cytometer was performed to measure intracellular ROS and Ca2+ concentration according to previous descriptions 17, 18. Briefly, cells, which were 70 % - 100 % confluent, were exposed to 1 μg/mL Photofrin for 4 h or incubated with Photofrin-free medium for 4 h (control group). With respect to intracellular ROS detection, cells were then dyed with 20 μM dichlorodihydrofluorescein diacetate (DCFH-DA) for 1 h. 30 min after irradiated by an argon-pumped dye laser at 630 nm at fluence rate of 20 mW/cm2 for 2 J/cm2, the cells were washed and harvested with PBS. As for intracellular Ca2+ measurement, after incubated with or without Photofrin for 4 h, cells dyed with 5 μM Fluo-3-Am for another 1 h. After irradiation at 630 nm (20 mW/cm2 and 2 J/cm2), the cells were then gently washed and harvested with fresh Ca2+ balanced salt solution (BBS) (10 mM HEPES, 2 mM MgCl2, 140 mM NaCl, 2 mM CaCl2 and 2.8 mM KCl, pH 7.2) or Ca2+ free BBS (10 mM HEPES, 140 mM NaCl, and 2.8 mM KCl, pH 7.2). The fluorescence intensities of ROS and Ca2+ were measured using a flow cytometer (MACS Quantify, Germany).
2.8 Ceramide and 4-HNE detection using ELISA assay
After cells (5×105 cells/mL) were seeded into 6-well plates and grown to 70 % - 100 % confluence, they were exposed to 1 μg/mL Photofrin for 4 h, or incubated with Photofrin-free medium for 4 h (control group), and then illuminated at 630 nm with the light intensity of 20 mW/cm2 and 2 J/cm2. 1 h after PDT, the harvested cells (1×106 cells/mL) were sonicated and the supernatant was collected. The concentration of ceramide and 4-HNE in the supernatant was measured by ELISA assay using a specific quantitation kit, according to the protocols. Three replications of each sample were averaged, and a standard curve was prepared for each ELISA plate.
2.9 Statistical analysis
The data were expressed as mean±SD. The variance was analyzed using the SPSS software for Windows 20.0 using one-way analysis of variance (ANOVA) or t-test. P<0.05 was considered to be statistically significant.
3 Results
3.1 Phototoxicity of Photofrin-mediated PDT is cell density dependent
The effect of Photofrin-mediated PDT on the survival of Cx26-expressing HeLa cells at low density (3×103 cells/cm2, GJ-unformed) and at high density (3×104 cells/cm2, GJ-formed) was demonstrated in Figure 1. Photofrin could remarkably decrease the survival of cells in a concentration-dependent manner at both high density and low cell density. The phototoxic effect of Photofrin-PDT was significantly increased at high-density condition, when the cells contact with one another, than at low density. In specific, at 1, 2.5 and 5 μg/mL Photofrin, the survival fraction of cells at low-density significantly increased than that at high-density condition. However, at 10 μg/mL of Photofrin, there was no significant difference in survival fraction between low- and high-density cultures. The results suggest that at low Photofrin concentration, the photocytotoxicity increases when there are chances for intercellular gap junctional contacts.
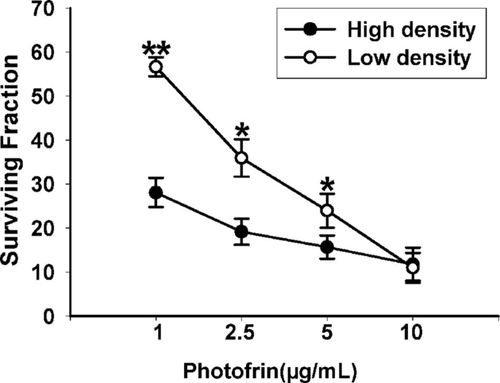
Phototoxicity of HeLa cells expressing Cx26 by Photofrin-mediated PDT is cell-density dependent. The survival of cells exposure to different concentrations of Photofrin at high cell density (3×104 cells/cm2) or at low cell density (3×103 cells/cm2) was assessed by CCK-8 assay. The data in different groups were expressed as the mean ± SD from 3 experiments. Statistical difference between groups was assessed by t-test using SPSS 20.0. *P<0.05, **P<0.01, versus high-density group.
3.2 Cx26–composed GJIC mediates cell- density dependence of phototoxicity of PDT
The cell-density dependent phototoxicity of Photofrin-PDT in Cx26-expressing cells indicates that Cx26-composed GJIC may play a role in phototoxicity of PDT. In order to investigate the effect of Cx26-composed GJIC on the sensitivity of Photofrin-PDT, we performed two methods to manipulate Cx26 expression and GJ function at high-density condition: void of Dox induction of Cx26 expression and inhibition of GJ channels using GJIC inhibitor. As shown in Figure 2 A, Dox induced expression of Cx26 whereas Cx expression was not detected in cells untreated with Dox. In addition, parachute assay, as depicted in Materials and Methods section, was utilized to evaluate GJIC between cultured cells. Donor cells labeled with calcein, a GJ-permeable dye, were seeded onto receiver cells and incubated with them for 4 h. The experiments were implemented in the presence of Dox and/or 18α-glycyrrhetinic acid (18α-GA), a non-selective GJIC inhibitor 19. The amount of the receiver cells labeled with calcein per donor cell was counted. The function of GJIC was determined by the amount of receiver cells receiving dyes from the seeded donor cells. As shown in Figure 2B, Dox induced the emergence of GJIC and no GJIC was detected in Dox-untreated cells. Pretreatment of Dox-treated cells with 10 μM 18α-GA leaded to substantially reduced GJIC.
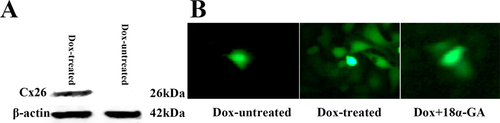
Cx26 expression induced by Dox and inhibition of GJ channels by GJIC inhibitor. A, Cx26 expression was induced by Dox, as illustrated by Western blot. B, 10 μM 18-α-GA inhibits dye coupling through Cx26-composed gap junctional channels in HeLa cells, as illustrated by “parachute” dye-coupling assay.
Figure 3 A shows the survival response of Photofrin-PDT in Dox-treated (Cx26 expressing, GJ-formed) and Dox-untreated cells (Cx26 non-expressing, GJ-unformed) at high-cell density condition. After treatment with Dox, the cells exhibited much more sensitivity to Photofrin-PDT with approximately 3-fold (1 μg/mL Photofrin) and 2-fold (2.5 μg/mL Photofrin) survival respectively less than Dox-untreated cells. Pretreating Cx26-expressing cells with 10 μM 18α-GA, a GJIC inhibitor shown to remarkably suppress Cx26-composed GJ channels at this concentration (Figure 2B), decreased the phototoxicity of Photofrin-PDT, thereby leading to substantially increased survival at high-density cultures (Figure 3B). Specifically, at 1 and 2.5 μg/mL Photofrin, the survival of 18α-GA-treated cells increased by a factor of approximately 3 and 2 respectively than that of 18α-GA-untreated cells at high-density condition. However, at 5 and 10 μg/mL of Photofrin, the surviving fractions between Dox-treated and –untreated cells, or 18α-GA-treated and -untreated cells exhibited no significant difference.
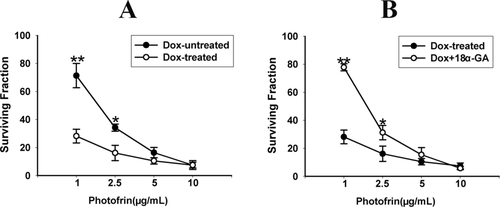
Roles of Dox and 18α-GA in the survival of HeLa cells after PDT. The data in different groups were expressed as the mean ± SD from 3 to 4 experiments. Statistical difference between groups was assessed by t-test using SPSS 20.0. *P<0.05, **P<0.01, versus Dox-treated group or Dox +18α-GA group.
Together, these data show that at low Photofrin concentration, Cx26-composed GJ formation at high density enhances the phototoxicity of Photofrin-PDT since the phototoxicity at high-cell density condition substantially reduces when Cx26 is not expressed or Cx26 channels are inhibited. In conclusion, the results illustrate that the enhancement of photocytotoxicity of Photofrin-PDT at high density is mediated by Cx26-composed GJIC.
3.3 Cx26-composed GJIC enhances intracellular ROS generation after PDT
It has been established that ROS is allowed to diffuse through GJ in spite of its short lifetime 20. Thus, ROS might have a role in the GJIC-mediated enhancement of photocytotoxicity induced by Photofrin-PDT. The results illustrated that Photofrin- PDT led to a significant increase in the intracellular ROS generation. To be specific, the GJ-formed cells (Dox-treated) generated significant higher quantities of ROS than GJ-unformed cells (Dox-untreated) when the cells were treated with 1μg/mL Photofrin (Figure 4). In addition, pretreatment of Dox-treated cells with GJIC inhibitor resulted in a remarkable decrease in the amount of intracellular ROS (Figure 4). These findings suggested that the formation of Cx26-composed GJ had an ability to promote the generation of ROS, which may be related to the increased PDT phototoxicity by Cx26-composed GJIC.
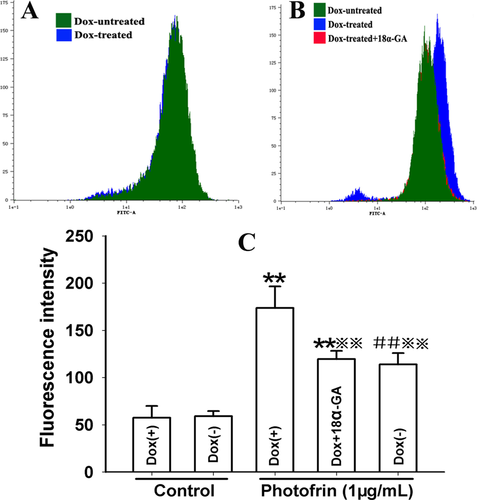
Cx26-composed GJIC enhances intracellular ROS generation after PDT. The cells were treated with Dox or GJIC inhibitor, 18α-GA, incubated with DCFH-DA and irradiated with or without 1 μg/mL Photofrin. 30 min after irradiation, the fluorescence intensity of ROS was detected using flow cytometry. A: control; B: 1 μg/mL Photofrin; C: The fluorescence intensity of ROS. The data in different groups were expressed as the mean ± SD from 3 experiments. Statistical difference between groups was assessed by one way ANOVA using SPSS 20.0. **: P<0.01, significantly different from control group (Dox-treated); ##: P<0.01, significantly different from control group (Dox-untreated);
 : P<0.01, significantly different from Photofrin group (Dox-treated).
: P<0.01, significantly different from Photofrin group (Dox-treated).
3.4 Cx26-composed GJIC increases intracellular Ca2+ release after PDT
ROS produced after PDT may lead to the damage to intracellular Ca2+ store, causing an increase in intracellular Ca2+ concentration ([Ca2+]i). The above finding that GJIC-mediated increased intracellular ROS production after PDT suggests the possibility that GJ formation has an effect on intracellular Ca2+ release after PDT. For confirming the possibility, cells were irradiated in Ca2+-free balanced salt solution (BBS) after incubation with 1 μg/mL Photofrin. Figure 5 illustrated that PDT caused a remarkable rise in [Ca2+]i. Notably, GJ-formed cells produced significantly higher [Ca2+]i compared to cells without GJ. Moreover, after Dox-treated cells were exposure to GJIC inhibitor, [Ca2+]i was significantly decreased. These results suggest that the presence of Cx26-composed GJIC may result in the enhanced release of intracellular Ca2+ after PDT.
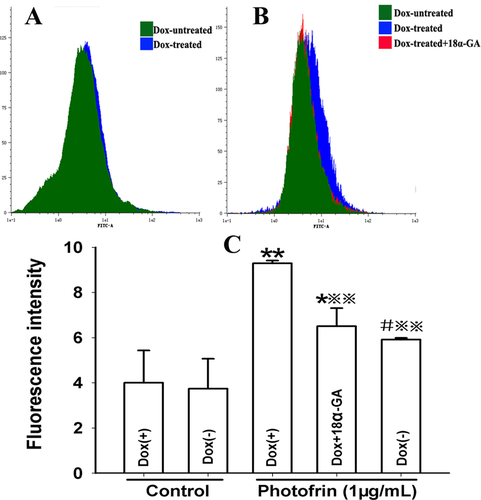
Cx26-composed GJIC increases intracellular Ca2+ release after PDT. The cells were treated with Dox or GJIC inhibitor, 18 α-GA, incubated with Fluo-3-Am and irradiated with or without 1 μg/mL Photofrin in Ca2+- free BBS. After irradiation, the fluorescence intensity of Ca2+ was detected using flow cytometry. A: control; B: 1 μg/mL Photofrin; C: The fluorescence intensity of Ca2+. The data in different groups were expressed as the mean ± SD from 3 experiments. Statistical difference between groups was assessed by one way ANOVA using SPSS 20.0. *: P<0.05, **: P<0.01, significantly different from control group (Dox-treated); #: P<0.05, significantly different from control group (Dox-untreated); : P<0.01, significantly different from Photofrin group (Dox-treated).
3.5 Cx26-composed GJIC stimulates the extracellular Ca2+ influx after PDT
It has been reported that an increase in [Ca2+]i, which greatly results from Ca2+ influx after PDT is responsible for PDT-induced cell death 17, 21. Since Ca2+ can diffuse GJ channels and hemichannels 3, the formation of GJ channels might facilitate PDT-triggered Ca2+ influx. For investigating the effects of Cx26-composed GJIC on the extracellular Ca2+ influx, cells in Ca2+ BBS were treated and irradiated with 1 μg/mL Photofrin. As illustrated in Figure 6, there was a significantly increase in [Ca2+]i in cells treated with Dox (GJ-formed) compared to those without GJ. Additionally, pretreatment of Dox-treated cells with GJIC inhibitor, [Ca2+]i markedly decreased. These results suggest that Cx26-composed GJIC may stimulate the influx of extracellular Ca2+ after PDT.
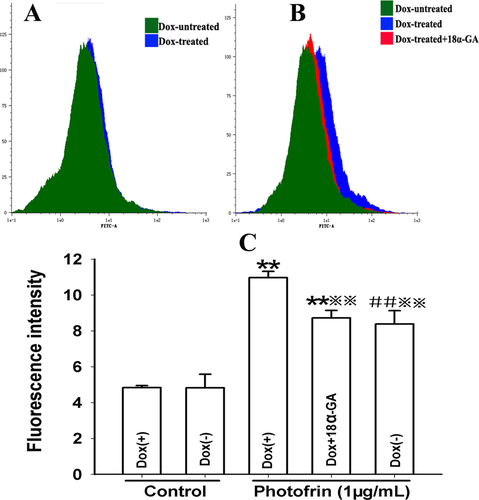
Cx26-composed GJIC stimulates the extracellular Ca2+ influx after PDT. The cells were treated with Dox or GJIC inhibitor, 18 α-GA, incubated with Fluo-3-Am and irradiated with or without 1 μg/mL Photofrin in fresh Ca2+ BBS. After irradiation, the fluorescence intensity of Ca2+ was measured using flow cytometry. A: control; B: 1 μg/mL Photofrin; C: The fluorescence intensity of Ca2+. The data in different groups were expressed as the mean ± SD from 3 experiments. Statistical difference between groups was assessed by one way ANOVA using SPSS 20.0. **: P<0.01, significantly different from control group (Dox-treated);##: P<0.01, significantly different from control group (Dox-untreated); : P<0.01, significantly different from Photofrin group (Dox-treated).
3.6 Cx26-composed GJIC increases ceramide and 4-HNE production after PDT
The accumulation of intracellular ceramide and 4-HNE after PDT might have a possibility to pass through GJ channels because of their small molecular weights. Therefore, these lipid peroxides might have a role in the increased phototoxicity of Photofrin-based PDT by Cx26-composed GJIC. As shown in Figure 7, Photofrin-based PDT results in a rise in the amount of intracellular ceramide and 4-HNE generation either in Dox-treated/-untreated cells. Specifically, the quantities of ceramide and 4-HNE in the Dox-treated cells (GJ-formed) were markedly higher than that in Dox-untreated (GJ-unformed) cells. Pretreatment of Dox-treated cells with GJIC inhibitor led to a significantly reduction in the generation of ceramide and 4-HNE. These results indicate that the presence of Cx26-composed GJIC may increase the generation of intracellular ceramide and 4-HNE after PDT, which may be involved in a Cx26-composed GJ-dependent enhancement of phototoxicity induced by Photofrin-mediated PDT.
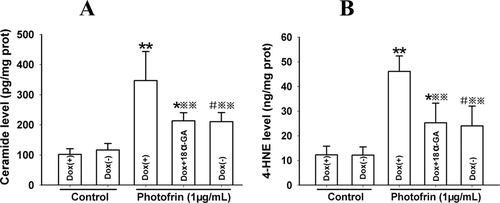
Cx26-composed GJIC augments ceramide and 4-HNE production after PDT. The cells were treated with Dox or GJIC inhibitor, 18α-GA and irradiated with or without 1 μg/mL Photofrin. 1 h after irradiation, the quantities of ceramide and 4-HNE were measured using ELISA assay. A: ceramide; B: 4-HNE. The data in different groups were expressed as the mean ± SD from 3 experiments. Statistical difference between groups was assessed by one way ANOVA using SPSS 20.0. *: P<0.05, **: P<0.01, significantly different from control group (Dox-treated); #: P<0.05, significantly different from control group (Dox-untreated); : P<0.01, significantly different from Photofrin group (Dox-treated).
4 Discussion
It is generally believed that lack of GJIC is widely associated with tumorigenic phenotype; however, GJIC can be reduced, maintained or even increased in some cases. For example, Cx40 and Cx26 expressions are up-regulated in testicular malignancy compared to testis 22, and Cx43 expression can be up-regulated or down-regulated according to the tumor type 23. Additionally, the expressions of Cx43, Cx40, Cx26 and Cx32 are maintained in small lung cancers 24, 25. In these cases, one would expect GJIC to play a role in the efficacy of antineoplastic therapy.
Our previous studies showed that a substantial enhancement of Photofrin- and 2-[1-Hexyloxyethyl]-2-Devinylpyropheophorbide-A (HPPH)-mediated PDT phototoxicity depended on Cx32-composed gap junctional coupling among the target cells [17, 26]. This study shows that there is a significant Cx26-composed GJIC-dependent effect of PDT cytotoxicity after cells were treated with low concentration of Photofrin. This effect is deficient in low-cell density condition due to void of GJ formation (Figure 1). Notably, we show that in high-cell density condition, in which there is opportunity for GJ contacts between the cells, attenuation of GJIC either by pretreatment with GJ channel inhibitor 18α-GA or inhibiting Cx26 expression, decreases PDT-induced cytotoxicity (Figure 2). This work demonstrates that increasing Cx26-composed GJIC can enhance the phototoxicity of Photofrin-mediated PDT, and indicates that therapeutic strategies designed to up-regulate Cx26 expression or to maintain Cx26-composed GJ function could enhance therapeutic efficacy of PDT.
The diffusion of cytotoxic substances via GJ complexes has broadly investigated. These substances are generally considered as the molecules involved in cellular death pathways 3. It has been established that intracellular ROS production induced by PDT causes cellular damage by attacking DNA, proteins and lipids 27, and mitochondrial ROS is considered as a molecule spreading via GJ channels as an oxidative stress signal 3, 15. Therefore, intracellular ROS may play a role in Cx26-composed GJIC-dependent component of PDT phototoxicity. Our results showed that the amount of ROS produced by PDT in GJ-formed cells was remarkably increased than that in GJ-unformed cells (Figure 4). Additionally, inhibiting Cx26-composed GJ by 18α-GA could reduce ROS generation after PDT (Figure 4). These results indicate that the increased Photofrin phototoxicity caused by Cx26-composed GJIC may arise from the intercellular ROS transfer.
Reports have indicated that PDT can stimulate Ca2+ influx and the release of Ca2+ from intracellular calcium stores, resulting in an enhancement in [Ca2+]i, which may ultimately lead to cell apoptosis and death 28, 29. It has been reported that Ca2+ can diffuse through GJ complexes to surrounding neighbors 3. Accordingly, Cx26-composed GJ complexes may impact on PDT cytotoxicity by regulating extracellular Ca2+ influx and/or intracellular Ca2+ release. It should be noted, however, that Ca2+ influx and increased [Ca2+]i may cause the closure of GJ channels 30. It is generally believed that the effective [Ca2+]i on GJ channels relies on the procedure used to enhance [Ca2+]i the type of cell and the species of expressed Cx 31. The results that a Cx26-composed GJIC-mediated increase in [Ca2+]i in the presence or absence of extracellular Ca2+ demonstrated that the enhancement of [Ca2+]i after PDT exhibit no effect on Cx26-composed junctional coupling (Figure 5 and 6). More importantly, the data suggest that Cx26-composed GJIC can make contribution to the enhanced influx of Ca2+ and intracellular Ca2+ release. Together, it can be concluded that the increased Photofrin-mediated phototoxicity due to Cx26-composed GJIC may be related to the intracellular calcium pathways.
There are reports showing that PDT results in intracellular increases in ceramide and 4-HNE generation 13, 14. These lipid peroxides have been reported to play a role in triggering apoptosis under PDT-mediated stress condition 32, 33. Since the molecular weights of ceramide and 4-HNE are all less than 1.5kDa, the upper limit of penetrable molecules through GJ channels, they are likely to propagate via GJ complexes. Therefore, in theory, the Cx26-composed GJIC-mediated increase in Photofrin-mediated phototoxicity could be mediated via this mechanism. The findings that GJIC mediated by Cx26 could enhance the intracellular levels of ceramide and 4-HNE after PDT suggest that the elevated phototoxicity of Photofrin-mediated PDT by Cx26-composed GJIC may be implicated in the lipid peroxide-mediated stress signaling pathways (Figure 7).
The work reported here represents the demonstration of Cx26-composed GJIC-dependent cytotoxicity in response to PDT and suggests that the functionality of Cx26-composed GJIC in malignancies may be a significant determiner of the response to tumor therapy mediated by PDT. This brings about a few therapeutic thoughts. First of all, designing strategies to elevate the expression of Cx26 or increase Cx26-formed GJIC may improve the sensitivity of cancer cells to PDT. In contrast, factors that attenuate the functionality of Cx26-formed GJ channels may result in insensitivity of malignant cells to PDT, leading to remarkable reduction in therapeutic efficacy.
Acknowledgements
This project was supported by the Natural Science Foundation of China (No. 81402946), Initializing Fund of Xuzhou Medical University, China (No. D2014017 and D2014010) and the Natural Science Research grant of Higher Education of Jiangsu province, China (No. 14KJD310002).
Author biographies
Please see Supporting Information online.




