Etiology, clinical features, and epidemiological analysis of diarrhea patients visiting a gastrointestinal clinic in a comprehensive hospital in Beijing, China, in 2023
Abstract
Objective
To investigate the clinical features and epidemiology of diarrhea patients and analyze the current distribution of enteropathogens causing diarrhea in a comprehensive hospital in Beijing, China, in 2023.
Materials and Methods
From April to October 2023, we enrolled patients with diarrheal diseases who visited the gastrointestinal clinic in our hospital. The patients' demographic, epidemiological, and clinical features were obtained via a questionnaire. Stool samples were examined for 20 enteropathogens by multiplex polymerase chain reaction testing.
Results
We enrolled 260 patients; men and adults accounted for 55.77% and 95.77% of the patients, respectively. The median age was 37 years. Eighty-four enteropathogens, 72 bacteria and 12 viruses, were identified in 74 patients. Enteroaggregative Escherichia coli was the predominant agent. Patients with and without pathogens detected in stool samples showed no significant differences in age, sex, gastrointestinal symptoms, and stool characteristics. Possible food-related events were recorded in 57.31% of the patients. Leukocyte counts in patients with bacterial infections were higher than those of patients with viral infections and those with no detected pathogens (p < 0.05). Seasonality of bacterial distribution was observed (p < 0.05).
Conclusion
Bacteria were predominant pathogens among the diarrhea patients. The incidence of diarrhea was related to hot weather and foodborne illness. Bacterial diarrhea may cause systemic infection. The clinical symptoms of infectious diarrhea were usually non-specific and unrelated to the type of infection. Timely and comprehensive multi-pathogen surveillance might be helpful to detect suspected pathogens and promote epidemic prevention and control.
Abbreviations
-
- E. coli
-
- Escherichia coli
-
- EAEC
-
- enteroaggregative E. coli
-
- EHEC
-
- enterohaemorrhagic E. coli
-
- EPEC
-
- enteropathogenic E. coli
-
- ETEC
-
- enterotoxigenic E. coli
-
- GBD2019
-
- Global Burden of Disease Study 2019
-
- spp.
-
- species
-
- STEC
-
- Shiga toxin–producing E. coli
-
- V. cholerae
-
- Vibrio cholerae
-
- V. parahaemolyticus
-
- Vibrio parahaemolyticus
1 INTRODUCTION
Diarrheal disease is an important medical conundrum that causes significant morbidity and mortality worldwide. The Global Burden of Disease Study 2019 (GBD2019) project reported 6.58 billion cases of diarrheal disease globally, resulting in 1.53 million deaths and 80.9 million disability-adjusted life-years [1]. Diarrhea can be caused by a wide range of etiological agents, including viruses, bacteria, and parasites. Viral infections are the main cause of diarrhea in high- and upper middle-income countries, with rotavirus and norovirus being the most common pathogens [2-4]. Bacterial pathogens that cause approximately 10%–15% of diarrhea episodes in these countries were reported to be the predominant cause of diarrhea in low- and lower middle-income countries [2, 5, 6]. Many pathogens can cause bacterial diarrhea, including Vibrio cholerae (V. cholerae), Vibrio parahaemolyticus (V. parahaemolyticus), Salmonella species (spp.), Shigella spp., and different pathotypes of Escherichia coli (E. coli), such as Shiga toxin–producing E. coli (STEC), enteroinvasive E. coli, enteropathogenic E. coli, enterotoxigenic E. coli (ETEC), and enteroaggregative E. coli (EAEC) [7].
The prevalence of pathogens is usually region-specific owing to differences in economic development, local climate, and geography [8, 9]. It is necessary to study the epidemiological characteristics of enteropathogens in various regions as well as the pathogenicity data for each pathogen. In China, the majority of gastrointestinal clinics perform only routine fecal examination and bacterial culture, especially to detect V. cholerae. Most common pathogens in diarrhea cannot be accurately detected. However, accurate identification of enteropathogens could provide valuable data to determine the clinical significance of pathogens, and for surveillance and outbreak investigations.
Molecular diagnostic technology offers high sensitivity and wide coverage for the identification of diarrheal pathogens. In this study, we used multiple molecular detection to detect 20 types of common enteropathogens, 12 types of bacteria, and 8 types of viruses using stool samples from 260 diarrhea patients who visited the intestinal tract clinic in our hospital in 2023. Using the epidemiological findings, clinical manifestations, and basic laboratory test results, we studied the pathogen distribution of diarrhea patients in the local area as well as the clinical manifestations of different pathogens.
2 MATERIALS AND METHODS
2.1 Patients and settings
All patients who visited the intestinal tract clinic in our hospital with a symptoms of diarrhea from April to October 2023 were enrolled. A questionnaire was given to the patients, and they were asked to record their basic demographics, comorbidities, and associated symptoms at the time of stool specimen collection. Questions about travel history, ingestion of suspicious food, and diarrheal illness among family members, friends, or coworkers (cluster history) were also included in the questionnaire. Blood cell counts were evaluated using an automated hematology analyzer (XS-800i; Sysmex, Kobe, Japan). A mixed infection was diagnosed if more than one pathogen was identified from a stool sample.
This study was approved by the institutional ethics committees of The Seventh Medical Center of the Chinese PLA General Hospital, Beijing, China (S2024-057-01). As all data were anonymously collected and interpreted, the institutional ethics committees waived the need to obtain written informed consent from the participants.
2.2 Specimen collection and identification of enteropathogens
The stool sample was initially collected in a sterile container and submitted to the clinical laboratory within 1 day for enteropathogen analysis. The DNA/RNA extraction process of pathogen nucleic acid from stool samples was performed using a nucleic acid extraction-free reagent (NA0001000; Suzhou Zhongke Sujing Biotechnology Co., Ltd., Suzhou, China). Briefly, the procedure was as follows: 50 mg of the stool sample was placed in a centrifuge tube, and 1 ml of ribonuclease-free water was added before shaking the tube thoroughly to ensure even resuspension of the sample. The sample was then centrifuged at 845 ×g for 1 min, and 40 μL of the supernatant was transferred into a ribonuclease-free centrifuge tube. Next, 20 μL of the nucleic acid extraction-free reagent (mainly containing tris (hydroxymethyl) aminomethane and Triton X-100) was added, and the sample was incubated at 95°C–100°C for 10 min. After centrifugation at 13,523 ×g for 2 min, the supernatant was collected for further detection or storage at −80°C for future use.
A 7-nucleic acid detection kit for intestinal RNA pathogens (real-time quantitative polymerase chain reaction (RT-PCR)) and 13-nucleic acid detection kit for intestinal DNA pathogens (RT-PCR; Suzhou Zhongke Sujing Biotechnology Co., Ltd.) were used to detect 20 enteropathogens. The detailed detection procedures and result analyses were performed in accordance with the manufacturer's instructions. The 7-nucleic acid detection kit for intestinal RNA pathogens was used to detect norovirus genogroups I and II, Salmonella spp., enterovirus 71, rotavirus A, rotavirus B, and Coxsackie virus A16. The 13-nucleic acid detection kit for intestinal DNA pathogens was used to detect astrovirus, intestinal adenoviruses, Shigella spp., V. cholerae group O1, V. cholerae group O139, V. parahaemolyticus, and ETEC, EAECs, enteropathogenic E. coli, enteroinvasive E. coli, STEC, enterohaemorrhagic E. coli, and enteroaggregative shigatoxigenic E. coli.
2.3 Statistical analyses
Continuous variables were presented as the mean ± standard deviation or median (interquartile range [P25, P75]) on the basis of the distribution and homogeneity of variance. Categorical variables were reported as number and percentage. Spearman's rank correlation test, Wilcoxon rank sum test and Pearson's chi-squared test were used for intergroup comparisons. Data analyses were performed using SPSS for Windows version 27.0 (IBM Corp.). p < 0.05 was considered statistically significant for all tests.
3 RESULTS
3.1 Demographics and clinical features of the patients with diarrhea
During the study period, 260 patients were enrolled, and 260 stool specimens (one from each patient) were submitted to the clinical laboratory for microbiological analysis. Table 1 summarizes the patients' clinical features. The median age of the patients was 37 years (range: 3–88 years); 145 (55.77%) were men, and 249 (95.77%) were adults (≥18 years). The median frequency of diarrhea before visiting the clinic was five times per day (range: 1–21 times). One hundred (38.46%) patients had a fever, and 83 (31.92%) patients had vomited more than once. One hundred and eighty-nine (72.69%) and 144 (55.38%) patients experienced abdominal pain and nausea, respectively. Watery stool (74.23%) was the most common stool characteristic, followed by loose stool (23.85%). The median duration of diarrhea before visiting the clinic was 1 day (range: 0–17 days). Contact history and travel history were recorded in 27 (10.38%) and 9 (3.46%) patients, respectively. Four patients with Salmonella spp. Infection, as well as three with EAEC, two with norovirus, and one with ETEC/EAEC mixed infections, had a history of contact with other diarrhea patients before the onset of the disease. As shown in Figure 1, a possible food-related event was recorded in 149 (57.31%) patients before the onset of diarrhea and involved mainly cold drinks (18.08%), seafood (12.69%), fruits and vegetables (11.92%), deli meats (6.54%), food left out overnight (6.15%), and salads (1.92%).
| Parameter | Total (n = 260) | Viral (n = 9, 3.46%) | Bacterial (n = 63, 24.23%) | Negative detection (n = 186, 71.54%) | p |
|---|---|---|---|---|---|
| Age (years) | 37 (27, 49) | 38 (30, 72) | 37 (26, 50) | 37 (27, 48) | 0.681a |
| Sex (male) | 145 (55.77) | 7 (77.78) | 31 (49.21) | 105 (56.45) | 0.236b |
| Contact history | 27 (10.38) | 2 (22.22) | 7 (11.11) | 18 (9.68) | 0.477b |
| Symptoms and stool traits | |||||
| Fever | 100 (38.46) | 3 (33.33) | 25 (39.68) | 71 (38.17) | 0.930b |
| Abdominal pain | 189 (72.69) | 5 (55.56) | 51 (80.95) | 133 (71.51) | 0.162b |
| Nausea | 144 (55.38) | 5 (55.56) | 32 (50.79) | 106 (56.99) | 0.694b |
| Vomiting | 83 (31.92) | 4 (44.44) | 18 (28.57) | 60 (32.26) | 0.611b |
| Frequency of vomiting (times/day) | 0 (0, 1) | 0 (0, 4) | 0 (0, 1) | 0 (0, 1) | 0.570a |
| Frequency of diarrhea (times/day) | 5 (4, 8) | 5 (4, 9) | 5 (4, 7) | 5 (4, 8) | 0.908a |
| Watery stool | 193 (74.23) | 7 (77.78) | 50 (79.37) | 135 (72.58) | 0.481b |
| Loose stool | 62 (23.85) | 2 (22.22) | 13 (20.63) | 46 (24.73) | 0.779b |
| Mucous stool | 3 (1.15) | 0 (0.00) | 0 (0.00) | 1 (0.54) | — |
| Jelly like stool | 1 (0.38) | 0 (0.00) | 0 (0.00) | 3 (1.61) | — |
| Bloody stool | 1 (0.38) | 0 (0.00) | 0 (0.00) | 1 (0.54) | — |
| Laboratory test results | |||||
| Leukocyte count in blood sample | 9.42 ± 3.81 | 7.86 ± 2.40 | 9.76 ± 3.84 | 9.38 ± 3.86 | <0.05c |
| Granulocyte percentage in blood sample | 75.50 ± 12.43 | 70.92 ± 16.14 | 75.76 ± 12.54 | 75.61 ± 12.21 | 0.480c |
| Lymphocyte percentage in blood sample | 17.32 ± 10.12 | 20.52 ± 12.64 | 17.07 ± 10.24 | 17.29 ± 9.96 | 0.543c |
| Leukocyte in stool sample | 107 (41.15) | 3 (33.33) | 26 (41.27) | 77 (41.40) | 0.891b |
| Erythrocyte count in stool sample | 57 (21.92) | 1 (11.11) | 13 (20.63) | 43 (23.12) | 0.663b |
- Note: 1. Data are presented as mean ± SD, median (P25, P75) or n(%). 2. Cases of mixed bacterial infection were included in the bacterial group, while the two patients with mixed bacterial and viral infections were not included in either group.
- a Wilcoxon rank sum test.
- b Pearson's chi-squared test.
- c Spearman's rank correlation test.
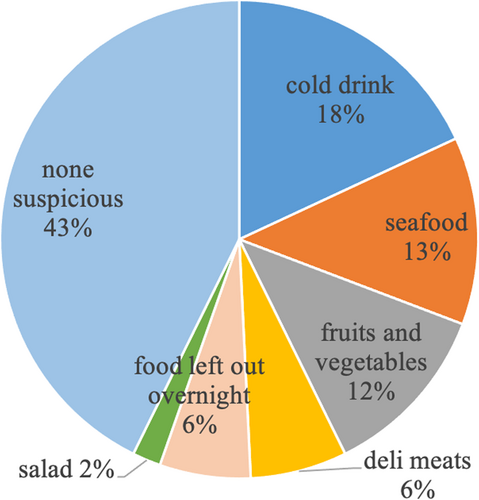
Suspicious food-related events were mentioned by the patients before the onset of diarrhea.
3.2 Comparison of the clinical features between patients with and without detected enteropathogens
Enteropathogens were detected in 74 cases, with an overall positivity rate of 28.46%. The detection results revealed that 9 patients were diagnosed with viral diarrhea, 63 with bacterial diarrhea, and two with mixed infections with both viruses and bacteria. The remaining 186 (71.54%) patients had negative detection results in their stool samples. As shown in Table 1, no significant differences were found between these three groups for sex, age, and history of contact. The frequency of fever, diarrhea, and vomiting also did not significantly differ between the groups. Regarding the stool characteristics, while there was no significant difference, a larger proportion of patients with bacterial and viral infections had watery stool (79.37% and 77.78%, respectively) compared with patients with no detected pathogens (72.58%).
The leukocyte counts of patients with bacterial infections were significantly higher than those of patients with viral infections and those without detected pathogens (p < 0.05). Other laboratory test results, including granulocyte and lymphocyte percentages in peripheral blood as well as leukocyte and erythrocyte counts in stool samples, showed no significant differences between the three groups.
3.3 Microbiological distribution of infectious gastroenteritis and the associated clinical features
A total of 84 pathogens, namely 73 bacteria and 11 viruses, were identified among the 74 patients with pathogens detected in their stool specimens (Figure 2). The viruses detected were norovirus in four patients, rotavirus in four, and intestinal adenovirus in three. EAEC (40.48%) was the most common pathogen, followed by Salmonella spp. (16.67%) and V. parahaemolyticus (7.14%). Two isolates of V. cholerae were detected in August and September (2023) respectively. Both patients had watery stools more than three times/day and sought medical attention on the day of onset. Both patients had high leukocyte counts and granulocyte percentages, and low lymphocyte percentages.
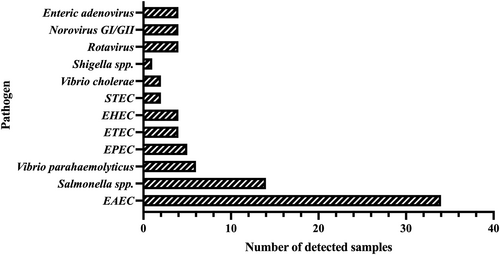
Cases of various enteropathogens detected in the stool specimens of the enrolled 260 diarrhea patients. EAEC, enteroaggregative E. coli; EHEC, enterohaemorrhagic E. coli; E. coli, Escherichia coli; EPEC, enteropathogenic E. coli; ETEC, enterotoxigenic E. coli; spp., species; STEC, Shiga-like toxin-producing E. coli.
Single bacterial and viral infections were identified in 56 (21.54%) patients and 9 (3.46%) patients, respectively. Nine patients (3.46%) had mixed infections, among whom, six had mixed infections with two different bacteria. The remaining seven patients were infected with both bacteria and virus; one patient had a triple-organism infection. EAEC and Salmonella spp. were the two most common pathogens in mixed infections. The associated enteropathogens are listed in Table 2. There was no difference in stool characteristics or symptoms between patients with single-organism infections and those with mixed infections.
| Condition of mixed pathogens | Number of patients |
|---|---|
| EAEC/ETEC | 2 |
| EAEC/Salmonella spp. | 2 |
| EHEC/Salmonella spp. | 1 |
| EAEC/Vibrio parahaemolyticus | 1 |
| STEC/Intestinal adenovirus | 1 |
| Salmonella spp./Intestinal adenovirus | 1 |
| EAEC/Vibrio parahaemolyticus/Rotavirus | 1 |
| Total | 9 |
- Abbreviations: E. coli, Escherichia coli; EAEC, enteroaggregative E. coli; EHEC, enterohaemorrhagic E. coli; ETEC, enterotoxigenic E. coli; STEC, Shiga-like toxin-producing E. coli; spp., species.
A significant difference in the seasonal distribution of bacteria was observed during the study period (p < 0.05). The numbers of bacteria detected in July and August were higher than other months (Figure 3). In August, the positivity rate was as high as 48.94%. There was no significant difference in the detection of viruses during these months. Both the diarrhea incidence rate and the bacterial detection rate lagged behind the average highest temperature.
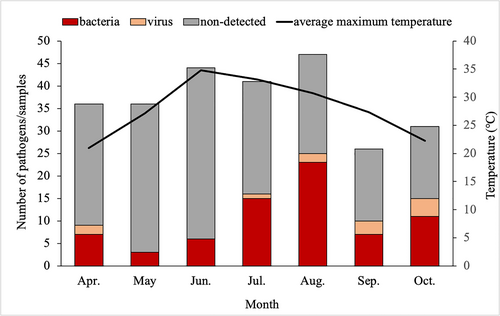
Number of diarrhea patients, detection of enteropathogens, and average highest temperature in each month of the study. The diarrhea incidence rates in June, July, and August were higher than other months. July and August had the highest bacterial detection rates. A lag phenomenon was observed for the incidence rate by months relative to the temperature curve.
4 DISCUSSION
The positivity rate in this study was 28.46%, higher than some of the historical data in Beijing but highly consistent with the national surveillance result released in 2021 [9]. Owing to the fact that the intestinal tract clinic in our hospital serves mainly adult patients, the results of this study cannot represent the infection situation of local children or adolescents. Additionally, diarrhea in adults is mainly bacterial.
Foodborne illness and high-temperature weather were the two main factors related to the positivity rate of bacterial detection in this study. Bacterial causes are commonly reported in foodborne illnesses [10]. The World Health Organization estimated the global burden of foodborne diseases, and the results showed that almost one in 10 people fall ill every year from eating contaminated food; 420 000 die as a result [11]. In our study, up to 57.31% of the diarrhea patients had concerns about eating possibly contaminated food. Cold drinks, seafood, and unwashed fruits and vegetables were the most common sources of foodborne diarrhea. The highest numbers of diarrhea patients were seen in June, July, and August. Additionally, the number of bacterial infection cases detected in July and August was higher than other months. Infectious diarrhea is related to local temperature; however, there was a lag phenomenon in our study that was similar to the results of previous studies [12, 13]. This finding suggests that weather patterns should be considered when developing surveillance programs and developing relevant public health intervention strategies [12].
EAEC was the most common pathogen detected in our study (34/260, 13.08%), similar to the results of previous studies [14]. This pathogen was associated with acute or persistent watery diarrhea in some studies, and can affect all age groups [7, 15]. The detection rate of EAECs has been increasing in Beijing since 2016. A higher positive percentage of this organism was recently reported in regions with high income levels compared with middle-income regions [15]. Further analysis is needed to determine the specific reasons for this difference.
V. cholerae infections in Beijing have recently been seen to be sporadic [16]. Common symptoms of infected individuals include diarrhea (84.09%), yellow watery stool (95.45%), abdominal pain (68.18%), nausea and vomiting (40.91%), and fever (36.36%) [16]. Currently, in China, the detection of V. cholerae relies mainly on the detection of the O1 and O139 serotypes to identify suspicious colonies on gentamicin agar medium. In this study, both V. cholerae isolates were confirmed to be non-O1/O139 serotypes through 16S rRNA sequence BLAST analysis. Both serotypes showed vigorous activity in the mobility test but were only slightly positive in the immobilization test. This method might easily lead to missed detection of non-O1/O139 V. cholerae. For suspicious colonies, further identification through biochemical or molecular diagnosis is essential.
In this study, there were no statistically significant differences in age, sex, stool characteristics, and gastrointestinal symptoms between patients with and without pathogens detected in stool samples. We consider three possible reasons for these results. First, the clinical symptoms in patients with infectious diarrhea are usually non-specific and unrelated to the type of infection. Second, the pathogen detection rate may have been lower than the actual rate owing to improper specimen collection and preservation, and delayed examination. Third, this study involved few pediatric patients, so the detection rate may be biased. In China, children with diarrhea are mainly infected with viruses, while adults aged 18–45 years are mainly infected with bacteria [9]. The majority of the patients in this study were adults; therefore, the detection rate of viruses was relatively low. Nevertheless, the leukocyte counts of patients with bacterial infection were significantly higher than those of the virus-infected patients and the undetected group, indicating that intestinal infection is prone to progress to systemic infection. This result further highlights the importance of accurate pathogen detection. It is impossible to accurately determine the cause of diarrhea and the type of infection solely on the basis of clinical manifestations.
The 71.54% of our patients whose stool samples were negative for the tested pathogens might have been infected with other reported diarrheal pathogens, such as the following viruses: Saffold cardiovirus, coronavirus, picobirnavirus, and MW (Malawi) polyomavirus [17]; bacteria: Klebsiella oxytoca [18], enterotoxigenic Bacteroides fragilis [19], and Laribacter hongkongensis [20]; and parasites. Future studies could involve testing for more pathogens compared with the current study or the use of higher coverage methods, such as targeted next-generation sequencing in enteropathogen surveillance in diarrhea patients.
This study has some limitations. First, the samples were collected from a single comprehensive hospital during part of a single year. Therefore, our results cannot be compared with a much broader epidemiological situation. Second, culture and molecular typing of pathogenic bacteria were not performed, which would have provided a better understanding of the prevalence of intestinal pathogens. Future studies will address these limitations to provide more comprehensive information.
5 CONCLUSION
In conclusion, enteropathogen surveillance in 2023 in a comprehensive hospital in China showed that foodborne sources of pathogens and hot weather are the main reasons for the increasing incidence of diarrhea and enteropathogen detection rates. Bacterial diarrhea may cause systemic infection. Therefore, timely and comprehensive multi-pathogen monitoring is helpful for accurate diagnosis and epidemiological analysis of infectious diarrhea.
AUTHOR CONTRIBUTIONS
Lihua Qi: Data curation (Lead); Funding acquisition (Lead); Writing—original draft (Lead); Writing—review & editing (Lead). Siwei Zhou: Project administration (Equal). Dongmei Gu: Methodology (Lead); Project administration (Equal).
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
This study was approved by the institutional ethics committees of The Seventh Medical Center of the Chinese PLA General Hospital, Beijing, China (S2024-057-01).
INFORMED CONSENT
As all data were anonymously collected and interpreted, the institutional ethics committees waived the need to obtain written informed consent from the participants.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




