A retrospective analysis of the relationship between dermatomyositis-associated interstitial lung disease and disease duration, age, arterial blood gas pH, and serum Cl− levels
Xuemei Wei and Xu Zhang contributed equally to this study and shared the first authorship.
Abstract
Background
Dermatomyositis-associated interstitial lung disease (DM-ILD) represents a severe and insidious complication of dermatomyositis (DM). The study aimed to investigate the association between DM-ILD and arterial blood gas indices, serum ion levels, and the timing of interstitial lung disease onset, with the goal of identifying potential predictors for DM-ILD.
Methods
The investigation involved the collection of basic data from 89 patients with DM hospitalized at the Chinese PLA General Hospital between January 2019 and April 2022, and 43 normal control patients hospitalized for physical examinations during the same period. Analyses were conducted to explore the relationship between DM-ILD, arterial blood gas indices, disease duration, and serum ions. A regression model to predict DM-ILD was developed using these indices, and a receiver operating characteristic curve was generated.
Results
Significant differences were observed in pH and PaO2 between the control group and the disease group (p < 0.05). The DM group exhibited higher levels of pH, actual bicarbonate, and base excess (BE) compared with the control group. In contrast, pH and BE levels were lower in the DM-ILD group than in the DM group, with these differences being statistically significant (p < 0.05). Interstitial lung disease was correlated with the duration of the disease and pH levels (p < 0.05). The cutoff values for age, disease duration, pH, and Cl− were 55.5 years, 5.5 years, 7.432, and 101.5 mmol/L, respectively. The model demonstrated a prediction sensitivity and specificity for DM-ILD of 0.809 and 0.722, respectively, with an area under the curve of 0.809.
Conclusion
Arterial blood gas analysis and serum Cl− levels may assist in predicting DM-ILD. A combined monitoring approach involving arterial blood gas pH, disease duration, age, and serum Cl− levels could enhance the accuracy of DM-ILD predictions and hold significant clinical evaluation potential.
Abbreviations
-
- AB
-
- actual bicarbonate
-
- BE
-
- base excess
-
- DM
-
- dermatomyositis
-
- DM-ILD
-
- dermatomyositis-associated interstitial lung diseases
-
- PaCO2
-
- partial pressure of carbon dioxide
-
- PaO2
-
- partial pressure of oxygen
-
- ROC
-
- receiver operating characteristic
-
- SaO2
-
- oxygen saturation
1 INTRODUCTION
Dermatomyositis (DM) is an autoimmune disease characterized by insidious or subacute onset with primary clinical manifestations of muscle weakness and skin rash. The diagnosis of DM currently depends on serological tests, imaging, electromyography, and clinical observations. As the disease progresses, it can gradually affect various organs, with interstitial lung disease (ILD) being the most common complication [1-4]. The incidence of DM ranges from 1 to 6 cases per 100 000 individual, with a female to male ratio of approximately 2:1 [5]. Notably, the overall incidence of ILD among patients with DM is 41%, and this figure rises to about 50% within the Asian population [4].
In its initial stages, dermatomyositis-associated interstitial lung disease (DM-ILD) can be highly occult and challenging to detect through conventional imaging and serological tests. When further complicated by interstitial pneumonia, reversing lung damage becomes increasingly difficult [6-8]. DM-ILD exhibits considerable heterogeneity, necessitating individualized treatment strategies based on the form of onset, rate of progression, extent of lesion involvement, and treatment response to treatment by other adverse factors. The management of DM primarily involves glucocorticoids, immunosuppressants, biological agents, and other pharmacological interventions [9, 10]. Timely intervention in DM-ILD is critical for improving patient outcomes, emphasizing the importance of early detection of ILD complications in DM patients.
Arterial blood gas analysis provides real-time insights into blood pH and oxygen levels [11]. In the presence of pulmonary interstitial lesions, alveolar gas exchange is compromised, leading to alterations in arterial oxygen partial pressure and pH levels [11]. Analysis of arterial blood gas is essential for assessing potential complications of ILD in DM patients. Additionally, serum ion levels serve as vital indicators of acid-base metabolism and overall metabolic function. Currently, hematological indicators specifically associated with DM-ILD are limited; however, serological and arterial blood testing offer the advantages of convenience and minimal invasiveness. This study aims to explore the potential roles of various biomarkers in monitoring interstitial pneumonia complications in DM by analyzing changes in blood gas indices, disease progression, complications, and other relevant factors.
2 MATERIALS AND METHODS
2.1 Human subjects
This study recruited a random sample of patients diagnosed with dermatomyositis who were hospitalized at the Chinese PLA General Hospital from January 2019 to April 2022. The cohort comprised 89 patients, including 54 females and 35 males, with ages ranging from 39 to 59 years and a median age of 49 years. Patients were categorized into two groups based on the presence or absence of ILD: the DM group and the DM-ILD group, at a ratio of 36:53. Additionally, 43 healthy controls were included, consisting of 18 males and 25 females, with a median age of 56 years (range: 39–63 years). The duration of disease was measured from the date of diagnosis until January 2023 and was expressed in whole years.
2.2 Inclusion and exclusion criteria
The inclusion criteria for the study were as follows: (1) Both the DM group and the DM-ILD group included newly diagnosed patients admitted to the Chinese PLA General Hospital, whereas the control group comprised individuals undergoing concurrent physical examinations. (2) DM diagnoses were confirmed by the rheumatology department, and DM-ILD was used to describe cases with clinically diagnosed interstitial pneumonia. The diagnostic approach adhered to the 2004 classification criteria for idiopathic inflammatory myopathy established by the European Neuromuscular Disease Center [3, 12]. (3) Comprehensive and detailed medical records were obtained for each patient. (4) Only patients in stable condition without other malignant tumors were included.
The exclusion criteria included: (1) Patients with severe chronic diseases; (2) Patients with DM who were not hospitalized specifically for DM management; (3) Patients with cachexia.
2.3 Detection methods
Prior to initial admission and any treatment, the arterial blood gas indices and venous serum K+, Na+, and Cl− levels of the patients were assessed using the hospital's laboratory management system. Arterial blood gas analysis was performed using a Roche Cobas B221 (Roche Diagnostics GmbH) instrument using the electrode method. Serological indicators were measured using a Roche Cobas-701 (Roche Diagnostics GmbH) instrument utilizing the ion electrode method. All reagents employed were genuine Roche products. Quality control results from the same day verified the accuracy of all results, which indicated no instances of out-of-control situations.
2.4 Analytical methods
This study analyzed various aspects: (1) The DM group and DM-ILD group were considered as disease groups. The general conditions, arterial blood gas indices, and venous blood ion levels were compared between these disease groups and a control group. (2) Variations in each index among different age-gender-specific disease groups were examined based on age (<50 years old vs. ≥50 years old) and gender. (3) Comparisons were made between the control group and the DM group, and between the DM group and the DM-ILD group in terms of each index's variation. (4) Correlations between blood gas pH, the presence or absence of ILD, age, gender, and the course of the disease in all patients with DM were investigated. (5) A binary logistic regression model was established utilizing arterial blood gas pH values and disease duration. (6) Receiver operating characteristic (ROC) curves were established to investigate the factors that can predict DM-ILD.
2.5 Statistical analysis
Statistical analysis was performed using SPSS software version 22.0. As the quantitative data for all groups were skewed, median (P25, P75) was employed to represent the data. The ANOVA test facilitated pairwise comparisons among the three groups. Subsequently, the Wilcoxon W test was applied to assess differences between the Control and DM groups as well as between the DM and DM-ILD groups. Findings across these comparisons were consistent and were reported using the Wilcoxon W test values. Correlation assessments were performed using Spearman rank analysis. Variables that showed significant differences between groups were included in a binary logistic regression to develop a predictive model, followed by the construction of an ROC. The Youden Index was calculated from the prediction sensitivity and specificity for each variable leading to the determination of the cutoff value corresponding to the maximum Youden Index. Data visualization and further analysis were conducted using GraphPad Prism 5 and Origin 2021 software. A significance level of p < 0.05 was established for statistical significance.
3 RESULTS
3.1 General information on the disease and control groups
The disease duration for the 89 patients ranged from 2 to 6 years (median, 5 years). Significant differences were observed in pH, PaO2, base excess (BE), and SaO2 between the control and disease groups (p < 0.05). Additionally, venous blood K+, Na+, and Cl− levels were significantly lower in the disease group compared with the control group (p < 0.001), as shown in Table 1.
| Variable | Control group | Disease group | χ2/Z | p |
|---|---|---|---|---|
| Gender (male/female) | 18/25 | 35/54 | −0.016 | 0.988 |
| Age (yrs) | 56 (39, 63) | 49 (39, 59) | −1.330 | 0.184 |
| Course of disease (yrs) | — | 5 (2, 6) | — | — |
| Complicated interstitial pneumonia (yes/no) | — | 53/36 | — | — |
| pH value | 7.384 (7.370, 7.399) | 7.407 (7.387, 7.433) | −4.418 | <0.001 |
| PaO2 (mmHg) | 89.8 (84.6, 95.9) | 82.1 (72.8, 91.8) | −2.846 | 0.004 |
| PaCO2 (mmHg) | 41.5 (39.5, 43.0) | 41.0 (37.2, 43.8) | −1.395 | 0.163 |
| AB (mmol/L) | 24.1 (23.6, 25.1) | 25.1 (23.2, 26.8) | −1.638 | 0.101 |
| BE (mmol/L) | −1.2 (−1.7, 0.0) | 0.5 (−0.9, 1.6) | −2.871 | 0.004 |
| SaO2 (%) | 97.2 (96.8, 97.6) | 96.7 (95.4, 97.2) | −2.769 | 0.006 |
| K+ (mmol/L) | 4.10 (3.96, 4.30) | 3.67 (3.44, 4.01) | −4.615 | <0.001 |
| Na+ (mmol/L) | 142.0 (139.2, 144.0) | 139.5 (136.9, 141.1) | −4.338 | <0.001 |
| Cl− (mmol/L) | 105.4 (102.2, 107.6) | 101.6 (99.5, 104.3) | −4.496 | <0.001 |
- Note: Control, normal control group; DM, Dermatomyositis patients without complications group.
- Abbreviations: AB, actual bicarbonate; BE, base excess; PaCO2, partial pressure of carbon dioxide; PaO2, partial pressure of oxygen; SaO2, oxygen saturation.
3.2 Comparison of indices between the control group, DM group, and DM-ILD group
Relative to the control group, the DM group exhibited increased levels of pH, Actual bicarbonate (AB), and BE, and decreased levels of K+, Na+, and Cl−, all with statistical significance (p < 0.05). Compared with the DM group, there was a decrease in pH and BE levels and an increase in Cl− levels in DM-ILD group, also statistically significant (p < 0.05), as shown in Figure 1.
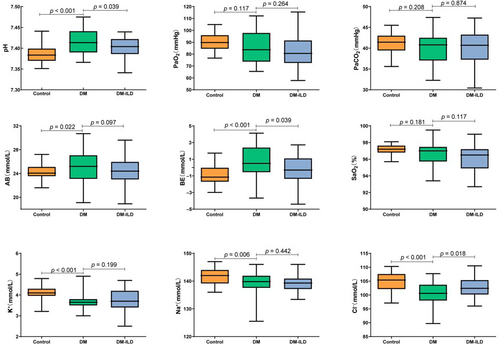
Comparative analysis of indices between the control group and DM group, and between the DM group and DM-ILD group. Control, normal control group; DM, Dermatomyositis patients without complications group; DM-ILD, Dermatomyositis-associated interstitial lung diseases group. AB, actual bicarbonate; BE, base excess; PaCO2, partial pressure of carbon dioxide; PaO2, partial pressure of oxygen; SaO2, oxygen saturation.
3.3 The relationship between ILD and disease course in DM
The course of illness in the DM-ILD subgroup was longer than in the general DM population (p = 0.013), as shown in Figure 2. The proportions of ILD among DM patients over time periods of 0–2, 3–4, 5–6, 7–8, and more than 8 years were 48.0%, 41.2%, 72.0%, 66.7%, and 88.9%, respectively, as shown in Table 2.
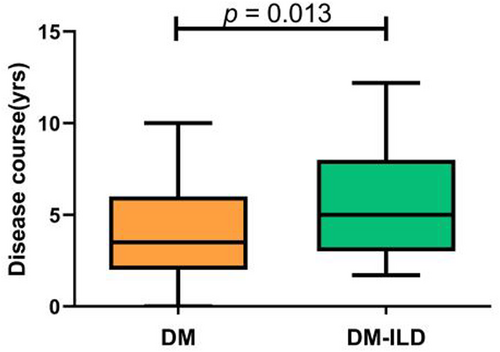
Analysis of disease course differences in patients with dermatomyositis with or without pulmonary lesions. DM, dermatomyositis patients without complications group; DM-ILD, dermatomyositis-associated interstitial lung diseases group.
| Disease course (yrs) | DM-ILD(n)/DM(n)[%] |
|---|---|
| 0–2 | 12/25 (48.0) |
| 3–4 | 7/17 (41.2) |
| 5–6 | 18/25 (72.0) |
| 7–8 | 8/12 (66.7) |
| >8 | 8/9 (88.9) |
- Note: DM, dermatomyositis patients with this disease course; DM-ILD, dermatomyositis-associated interstitial lung diseases with this disease course.
3.4 Analysis of the relationship among age, gender, and blood gas indices in patients with DM
No correlation was found between arterial blood gas indices or levels of K+, Na+, and Cl− with respect to age or gender (p > 0.05), as shown in Tables 3 and 4.
| Variable | <50 (yrs) | ≥50 (yrs) | Z | p |
|---|---|---|---|---|
| pH value | 7.403 (7.374, 7.418) | 7.417 (7.380, 7.435) | −1.603 | 0.109 |
| PaO2 (mmHg) | 83.3 (74.0, 97.7) | 81.1 (70.3, 88.8) | −1.225 | 0.220 |
| PaCO2 (mmHg) | 40.9 (37.8, 42.8) | 39.8 (35.3, 42.9) | −1.338 | 0.181 |
| AB (mmol/L) | 25.0 (23.9, 26.2) | 24.8 (22.9, 26.9) | −0.176 | 0.860 |
| BE (mmol/L) | 0.2 (−0.9, 1.3) | 0.4 (−1.5, 2.3) | −0.234 | 0.815 |
| SaO2 (%) | 96.9 (95.6, 97.6) | 96.6 (95.0, 97.2) | −1.100 | 0.271 |
| K+ (mmol/L) | 3.58 (3.28, 3.95) | 3.75 (3.54, 4.10) | −1.736 | 0.083 |
| Na+ (mmol/L) | 139.6 (137.3, 141.0) | 139.4 (137.1, 141.8) | −0.304 | 0.761 |
| Cl− (mmol/L) | 101.3 (99.2, 103.4) | 101.8 (100.3, 105.6) | −1.555 | 0.120 |
- Abbreviations: AB, actual bicarbonate; BE, base excess; PaCO2, partial pressure of carbon dioxide; PaO2, partial pressure of oxygen; SaO2, oxygen saturation.
| Variable | Male | Female | Z | p |
|---|---|---|---|---|
| pH value | 7.405 (7.382, 7.420) | 7.412 (7.390, 7.435) | −1.747 | 0.081 |
| PaO2 (mmHg) | 83.7 (75.9, 93.9) | 80.6 (71.8, 94.4) | −1.277 | 0.202 |
| PaCO2 (mmHg) | 40.9 (38.1, 45.4) | 40.6 (35.7, 42.3) | −1.483 | 0.147 |
| AB (mmol/L) | 25.1 (23.4, 27.1) | 24.8 (23.0, 26.4) | −1.571 | 0.116 |
| BE (mmol/L) | 0.4 (−1.4, 2.3) | 0.0 (−1.1, 1.4) | −0.899 | 0.369 |
| SaO2 (%) | 96.6 (95.5, 97.2) | 96.8 (95.0, 97.4) | −0.225 | 0.822 |
| K+ (mmol/L) | 3.78 (3.49, 4.20) | 3.63 (3.44, 4.00) | −1.424 | 0.154 |
| Na+ (mmol/L) | 139.1 (137.6, 140.1) | 140.0 (136.8, 141.9) | −1.348 | 0.178 |
| Cl− (mmol/L) | 100.9 (99.2, 103.8) | 101.5 (97.3, 104.6) | −0.979 | 0.328 |
- Abbreviations: AB, actual bicarbonate; BE, base excess; PaCO2, partial pressure of carbon dioxide; PaO2, partial pressure of oxygen; SaO2, oxygen saturation.
3.5 Correlation analysis among indicators in patients with DM
Significant correlations were observed for the duration of interstitial pneumonia, and between gender/age, pH/interstitial pneumonia, age/PaO2, gender/PaCO2, pH/PaCO2, PaCO2/AB, all showing statistical significance (p < 0.05), as shown in Figure 3.
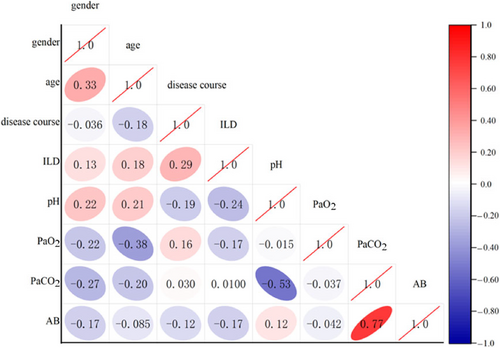
Spearman correlation analysis among indicators. Blue indicates a positive correlation and red indicates a negative correlation. The intensity of the color increases with the proximity to 1. The correlation coefficient is displayed in bold and underlined if p < 0.05. AB, actual bicarbonate; ILD, interstitial lung diseases; PaCO2, partial pressure of carbon dioxide; PaO2, partial pressure of oxygen.
3.6 The value of age, pH, Cl− and disease course in predicting DM-ILD
Differences in pH, BE, disease course and serum Cl− levels were noted between the DM and DM-ILD groups. Therefore, these four indicators were included in a binary logistic regression model. To minimize the influence of uncontrollable patient factors, age and gender were also considered. It was determined that gender and BE did not reach statistical significance and were therefore excluded from further analysis. Age, disease course, pH, and Cl− demonstrated statistical significance (p = 0.020, 0.006, 0.041, 0.016) as predictors of DM-ILD, as shown in Figure 4.
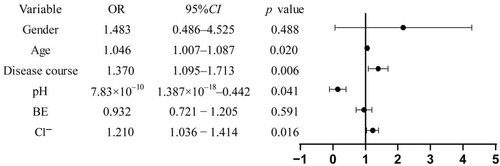
Predictive value of various indices for DM-ILD. AB, actual bicarbonate; ILD, interstitial lung diseases; PaCO2, partial pressure of carbon dioxide; PaO2, partial pressure of oxygen.
3.7 ROC curve analysis of arterial blood gas pH and disease duration for predicting DM-ILD
The ROC curve analysis identified cutoff values for age, disease duration, pH, and Cl− at 55.5 years, 5.5 years, 7.432, and 101.5 mmol/L, respectively, which provided the highest sensitivity and specificity for predicting DM-ILD. The combined assessment of these four variables showed statistical significance (p < 0.001). The sensitivity and specificity for predicting DM-ILD were 0.809 and 0.722, respectively, with an area under the curve of 0.830, as shown in Table 5 and Figure 5.
| Variable | AUC | Sensitivity | Specificity | Cutoff | p | 95% CI for AUC | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Age | 0.610 | 0.415 | 0.585 | 55.5 yrs | 0.078 | 0.488 | 0.732 |
| Disease course | 0.656 | 0.472 | 0.528 | 5.5 yrs | 0.013 | 0.543 | 0.769 |
| pH | 0.629 | 0.849 | 0.151 | 7.432 | 0.039 | 0.506 | 0.752 |
| Cl− | 0.648 | 0.642 | 0.639 | 101.5 mmol/L | 0.018 | 0.530 | 0.766 |
| Combined detection | 0.809 | 0.830 | 0.722 | — | <0.001 | 0.714 | 0.905 |
- Note: Combined detection: Age, disease course, pH, and Cl− are combined.
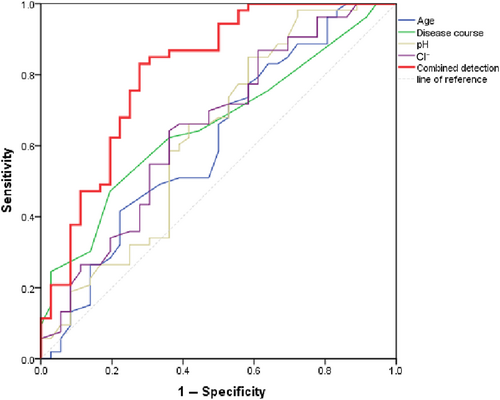
ROC curve of arterial blood gas pH and disease course and their combined predictive value for DM with interstitial pneumonia. Combined detection: Age, disease course, pH, and Cl− are combined.
4 DISCUSSION
DM is a complex disease with multiple etiological factors including immune dysfunction, genetic predispositions, environmental exposure, and infections [13, 14]. Epidemiological studies have shown a higher incidence of DM among women than men [5]. In this 3-year study with a sample size of 89 randomly selected cases, the proportion of male patients was significantly lower than that of female patients, which aligns with previous findings [5]. Patients with DM often exhibit abnormal immune functions, triggering inflammatory responses that lead to lung tissue damage through the infiltration of inflammatory cells. This process culminates in irreversible fibrosis within the pulmonary interstitium, progressing to dyspnea and other respiratory symptoms [8, 15]. The primary objective of this study was to explore the relationship between DM-ILD and both arterial blood gas indices and serum ion levels. It also aimed to analyze the timing of ILD onset and identify potential predictive indices for DM-ILD.
Our findings demonstrate that compared to the general population, individuals with DM exhibit higher pH and AB, along with reduced venous blood K+ levels. A decrease in K+ can lead to its extracellular migration, prompting an intracellular influx of H+ and Na+, which may result in a more alkaline blood pH and subsequent metabolic alkalosis. Our analysis revealed minimal differences in the PaCO2 between the two groups, suggesting that the pH alteration in DM patients is not due to respiratory factors but rather to conditions predisposing them to metabolic complications [16, 17]. Further comparisons between DM patients with and without interstitial pneumonia showed that those without the complication had elevated levels of pH, AB, and BE, which is consistent with the metabolic alkalosis discussed earlier. Conversely, the pH values in DM-ILD patients were lower than those in DM patients without interstitial complications, indicating a potential occurrence of acidosis in the presence of interstitial pneumonia.
The lungs play a pivotal role in ventilation and gas exchange within the human body. In instances where lung interstitium is compromised, pulmonary hyperventilation typically leads to alterations in PaCO2. Interestingly, our study noted that PaCO2 levels remained stable, suggesting that the observed acidosis may be predominantly driven by metabolic factors rather than respiratory changes. DM is recognized as a systemic inflammatory disorder that affects multiple organs including the lungs, kidneys, and heart. This multifaceted involvement was underscored by variations in serum ion concentrations detected in our analysis. Notably, in the absence of overt respiratory symptoms, the body often initiates a range of compensatory responses [18]. The lungs are closely related to the regulation of blood oxygen concentration; as pulmonary disease progresses, a decline in blood oxygen levels is observed [7, 19, 20]. The heart and kidneys—organs characterized by high blood flow—work synergistically. The heart ensures continuous blood flow to the kidneys, which in turn regulate this flow, creating a complementary relationship [21]. As the burden on these organs increases, fluctuations in blood flow and resultant ionic imbalances occur leading to changes in blood pH levels. Despite the presence of lung lesions, their mild nature in this study meant that PaCO2 levels were not significantly altered. The observed reduction in pH levels is likely attributable to compensatory mechanisms rather than direct respiratory alterations. This conclusion is supported by the concurrent reduction in AB, a positive shift in BE, and an increase in blood K+ levels noted during the study. In individuals with DM, the study revealed that metabolic alkalosis can develop even when the lungs are not affected. As the disease progresses and the partial pressure of oxygen declines, a reduction in metabolic bases occurs. The early onset of metabolic alkalosis before the development of interstitial pneumonitis facilitates a more effective monitoring of the lung's pathological state. Furthermore, this study identified a correlation between the duration of DM and the onset of ILD, with ILD presenting more frequently in patients who had experienced a longer duration of DM. These findings underscore the complex relationship between DM and ILD, reinforcing the interconnected nature of these conditions.
By grouping patients with DM according to age and gender, it was concluded that alterations in pH did not correlate with these factors. This observation reinforces the hypothesis of the present study that pH variations in patients with DM are predominantly linked to interstitial pneumonia rather than age or gender. Upon analyzing data—including blood gas pH, disease duration, serum Cl− levels, age, and gender—through a binary logistic model, it was determined that age, disease duration, pH, and serum Cl− were predictors of interstitial pneumonia development. The predictive odds ratio for disease duration was 1.37, indicating that with each additional year of disease duration, the likelihood of developing interstitial pneumonia was 1.37 times that of the previous year. Further analysis revealed that monitoring these four indices significantly enhances the early detection of interstitial pneumonia. ROC curves demonstrated that the combined assessment substantially outperformed individual tests in predicting interstitial pneumonia, achieving a prediction accuracy of 80.9%, with notable improvements in both sensitivity and specificity. These findings underscore the importance of regular monitoring of arterial blood gas pH and serum Cl− levels to effectively preempt interstitial pneumonia as DM progresses and patient age. Specifically, the predicted threshold for the course of the disease was found to be 5.5 years. In Table 2, ILD incidence remained below 50% for patients whose disease duration was under 5 years but increased notably between five and six years. These results suggest that ILD should be monitored closely when the disease duration reaches 5–6 years. Although arterial blood gas analysis is routinely performed and is minimally invasive, it involves slightly more trauma than venous blood collection. Based on these findings, we recommend that patients with dermatomyositis under 55 years of age undergo annual monitoring after five years from disease onset, while those over 55 years should undergo annual blood gas analyses to detect potential complications early.
The generalizability of these findings is subject to certain limitations. Notably, although this study investigated the relationship between patients with DM and arterial blood gas and ions, it is important to acknowledge that due to treatment factors, the impact of patient medication cannot be disregarded. Nonetheless, the findings of this study may serve as a reference for evaluating complications of DM during the treatment period. Given that dermatomyositis is a clinically rare disease, the sample size in this study was necessarily small. Future research will aim to enlarge the study cohort to facilitate comprehensive follow-up, including monitoring patient survival, dynamic observation of patient conditions, and enhancing service provision in clinical settings.
In conclusion, the analysis of arterial blood gas and serum Cl− seems to play a more significant indicative role in the development of DM-ILD. Furthermore, the combined monitoring of arterial blood gas pH, disease duration, patient age, and serum Cl− could prove to be an effective method for predicting DM-ILD, offering substantial potential clinical value.
AUTHOR CONTRIBUTIONS
Xu Zhang: Conceptualization (equal); data curation (equal); formal analysis (equal); resources (equal); validation (equal); writing – original draft (equal). Xuemei Wei: Conceptualization (equal); resources (equal); writing – original draft (equal). Xiaojuan Luan: Data curation (equal); resources (equal). Xiujuan Li: Data curation (equal); resources (equal). Jin Dong: Investigation (equal); validation (equal); writing – review & editing (equal). Jingzhu Nan: Formal analysis (equal); supervision (equal). Yanhong Gao: Writing – review & editing (equal).
ACKNOWLEDGMENTS
We would like to thank all those who participated in the study for their time and involvement.
CONFLICT OF INTEREST STATEMENT
All authors declare no conflicts of interest.
ETHICS STATEMENT
The studies involving human participants were reviewed and approved by the First Medical Center of Chinese PLA General Hospital, and the approved number was S2021-087-01.
INFORMED CONSENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
The datasets used or analyzed in this study are available from the corresponding author upon reasonable request.




