The exploration of cell population data in clinical use: Beyond infectious diseases
Abstract
Cell population data (CPD) is regarded as the fingerprint of a blood cell at a given moment. CPD parameters harbor information associated with cell morphology and can be automatically generated using modern hematological analyzers. Various studies have revealed many unique clinical applications for CPD, especially for infectious diseases, such as sepsis. For example, one monocyte-related CPD parameter is the monocyte distribution width (MDW), which can be generated using a Beckman Coulter hematological analyzer. MDW has received FDA and CE approval for aiding in sepsis diagnosis in adult patients in the emergency department. Additionally, MDW can serve as a diagnostic biomarker in patients infected with SARS-CoV-2. CPD has also been widely explored for possible clinical applications beyond infectious diseases, such as for predicting myelodysplastic syndromes, screening for hematological malignancies, and detecting sterile inflammation. CPD parameter measurements are easily obtained and quite cost-effective, making them practical for clinical use. However, there are some potential drawbacks of CPD parameters. Some pre-analytical conditions can affect CPD values. Furthermore, CPD are specific to certain hematological analyzers and the result cannot be transferred between different analyzers. The practical usefulness of CPD reference intervals is also still questionable. In this review, wesummarize the current studies related to CPD and its clinical applications. Additional well-designed clinical studies related to CPD are still expected.
Abbreviations
-
- AHSCT
-
- autologous hematopoietic stem cell transplantation
-
- ANOVA
-
- analysis of variance
-
- ATB
-
- active tuberculosis
-
- AUC
-
- area under the curve
-
- CI
-
- confidence interval
-
- CML
-
- chronic myelogenous leukemia
-
- COPD
-
- chronic obstructive pulmonary disease
-
- COVID-19
-
- coronavirus disease 2019
-
- CPD
-
- cell population data
-
- ED
-
- emergency department
-
- HBV
-
- hepatitis B virus
-
- ICU
-
- intensive care unit
-
- IQR
-
- interquartile range
-
- LTBI
-
- latent tuberculosis
-
- LV
-
- lymphocyte volume
-
- MDS
-
- myelodysplastic syndromes
-
- MDW
-
- monocyte distribution width
-
- MLS
-
- mean lymphocyte scatter
-
- MMV
-
- mean monocyte volume
-
- MNV
-
- mean neutrophil volume
-
- NLR
-
- neutrophil-to-lymphocyte ratio
-
- RBC
-
- red blood cell
-
- SD
-
- standard deviation
-
- WBC
-
- white blood cells
1 INTRODUCTION
Cell population data (CPD) can be automatically generated by modern hematology analyzers together with the detection of single blood cells. This has recently increased interest in potential clinical applications. Multiple detectors are often equipped in hematology analyzers, which can collect a set of signals generated from blood cells. Then, specific software is used to identify and characterize the types of cells. CPD provides cell morphology-related information and the mean and standard deviation (SD) of the total signal are calculated from the defined cells following each detection. Because of this, using CPD is essentially regarded as an approach to quantitatively measure a blood cell, specifically white blood cells (WBCs; lymphocytes), at any given moment of testing. For example, the granularity of a neutrophil will increase when encountering an “invader” like a pathogen. From a CPD testing perspective, the side scatter signal of the neutrophils will change. The volume of a lymphocyte will also increase after activation following a viral infection, causing the “volume” CPD parameter of the lymphocyte to increase correspondingly.
The concept of CPD was first introduced with the LH750 hematology analyzer (Beckman Coulter). At that time, certain neutrophil CPD parameters were found to be useful in aiding the early diagnosis of bacterial infections [1]. It was then reported that lymphocyte CPD could be used to characterize the malignant cells in patients with leukemia [2]. Over time, additional brands of hematology analyzers have included technology capable of generating CPD [3, 4]. CPD is currently considered useful in many clinical applications, including sepsis [5, 6], tuberculosis [7], postoperative infection [8], myelodysplastic syndrome [9], leukemia [10] and others. Additionally, lymphocyte CPD may provide cell functional information that can be used to aid in diagnosing viral infections [11] and lymphocytosis [12]. During the coronavirus disease 2019 (COVID-19) pandemic, the potential to apply CPD was considerably explored. Many studies have revealed that certain monocyte and lymphocyte CPD parameters have diagnostic and prognostic value for SARS-CoV2 infection [13-15]. Notably, a unique monocyte volume-related CPD, called monocyte distribution width (MDW), has received FDA and CE approval for use as an early sepsis indicator in adult patients in the emergency department (ED) [16-18].
In this review, we summarize the current studies related to the clinical application of CPD. We believe that with the continuous accumulation of experimental and clinical evidence, CPD will show increased value for such applications.
2 CPD ACQUISITION
Modern automatic hematology analyzers are equipped with multiple detectors that can collect various information from the blood cells being examined. During the process, the number of cells and their categories can be determined. Cell morphology-related information can also be collected and documented using the instrument. Hematology analyzers from various brands implement these functions through different detection principles. For example, Beckman Coulter hematology analyzers, such as the DxH series, use the Coulter Principle, which involves acquiring detection information through optical and electrical signals from the cells. However, Sysmex (Kobe) hematology analyzers, such as the XN-series, use fluorescent-flow cytometry technology, where information is obtained from surfactant reagents combined with blood cell membrane and fluorescence dye staining for intracellular proteins and nucleic acids.
After a single tested blood cell, narrowly represented as a WBC, passes through the detector channel within an analyzer, a set of electronic signals is generated and collected by the instrument. Generally, in an entire anti-coagulation whole blood sample, 10–20,000 WBCs can be measured. The absolute value of each signal is documented and analyzed via the internal software; then, these signals are used to define the cell clusters with similar characteristics. Thus, the number and percentage of different cell clusters can be presented. Approximately five cell clusters are typically distinguished and recognized as lymphocyte (WBC) subsets.
Along with these defined leukocyte clusters, the mean and SD values of the cell morphology-related signals, such as cell size, granularity, and nuclear morphology, are calculated and included as CPD. In principle, each WBC subset harbors its own set of CPD that reflects the state of the blood cells at the moment of testing. CPD therefore can directly or indirectly demonstrate information associated with cell morphology [19, 20].
In this article, we mainly describe the CPD generated via the Coulter Principle in detail because it does not “destroy” the cells and can acquire the near original cell morphological information. CPD are calculated using 256 channels of each VCSn measurement, with the mean and SD of each population being determined. For VCSn, V represents volume (cell volume information), C represents conductivity (cell nucleus information), S represents scattering (intracellular granule information), and n represents the number of scatter angles (here it is five scatter angles: MALS, UMALS, LMALS, LALS, and AL2). CPD are often referred to as positional parameters that provide additional tools for investigation. For example, the volume of a neutrophil is related to the existence and percentage of immature granulocytes in the blood. Additionally, granularity-related CPD represent the granule size and extent of granulation within the cells, while lymphocyte volume CPD are associated with the activated status of lymphocytes, which can be triggered by viral infection. CPD details are normally not included in a patient's test report because nearly all CPD are labeled as “for research use only” other than MDW (launched in USA and Europe). However, the patient's CPD results can be downloaded from the instrument for data analysis or scientific study purposes.
3 CPD CLINICAL APPLICATIONS
3.1 Reference ranges
In one study, the specific age and gender reference ranges for blood routine and CPD parameters were established for the XN-2000 hematology analyzer (Sysmex). In total, 280 healthy adults were enrolled, with their peripheral blood samples being tested. Finally, 36 routine blood test results and 57 CPD parameters were collected for each sample. The patients were categorized into three age groups: 20–40, 41–60, and >60 years old. Each age group was then separated into males and females, resulting in six total groups. Among the 36 routine test results, 22 showed significant differences related to gender or age. For the 57 CPD parameters examined, 44 of them were significantly different. Additionally, certain red blood cell (RBC)-, lymphocyte-, and platelet-related CPD parameters showed decreasing trends in women and older patients [21]. Another study with a larger sample size was conducted in the Dutch Lifelines cohort to establish the reference intervals for the Sysmex XN hematological parameters. In this study, 15 803 healthy individuals aged 20–92 years were enrolled, and then 105 parameters (including CPD) were analyzed. The results showed that many RBC-related parameters could be separated by gender, including the RBC count, hemoglobin levels, hematocrit levels, mean corpuscular hemoglobin concentration, and reticulocyte production index. A minority of CPD parameters were found to be moderately influenced by body mass index and smoking, while no significant differences between age groups were observed [22].
An additional study enrolled individuals from a multi-ethnic population in Malaysia. The CPD reference intervals were established for the Unicel DxH 800 automated hematology analyzer (Beckman Coulter). The data were obtained from a previously published work in Malaysia, where 2725 voluntary adult participants were enrolled, comprising both genders and three principal races (Malay, Chinese, and Indian)[23]. In this reference interval study, 1077 apparently healthy Malaysian adults (both genders, ≥19 years old) were enrolled. Finally, analysis of variance (ANOVA) tests suggested that there were no significant differences among the CPD parameters with respect to age, gender, or racial group [24].
As previously mentioned, MDW is a unique monocyte volume-related CPD parameter derived from the Beckman Coulter DxH 900 automated hematology analyzer that has received FDA and CE approval as a biomarker for aiding the diagnosis of sepsis in adult patients in the ED. Therefore, the MDW reference range is of particular interest. A study established the reference interval of MDW in healthy participants using three different statistical approaches. By the non-parametric method, the lower and upper reference limits were 16.22 (90% confidence interval [CI]: 15.78–16.47) and 23.15 (90% CI: 22.80–24.10), respectively, without outlier removal. After outlier removal, they were 16.44 (90% CI: 16.21–16.67) and 22.99 (90% CI: 22.33–23.22), respectively. By the robust method, the lower and upper reference limits were 16.29 and 22.98, respectively, without outlier removal, and 16.50 and 22.67, respectively, after outlier removal. Using the Harrell–Davis bootstrap method, the respective lower and upper reference limits were 16.19 and 23.24 without outlier removal. With outlier removal, they were 16.43 and 22.93. Additionally, no significant differences were found between the gender or age groups [25].
The results of these studies raise further questions and point to unmet needs. More investigation is required to establish the reference intervals for different populations, such as children and older individuals.
3.2 CPD in infectious diseases
3.2.1 Sepsis
Sepsis is an extreme immune response to an infection. In 2016, a new definition of sepsis (sepsis 3.0) was developed [26], which stated that sepsis is a life-threatening organ dysfunction caused by the unregulated immune response of a host. Urgent treatment is needed when sepsis occurs because it can rapidly lead to organ failure and even death. Early recognition of sepsis is crucial for improving the treatment and survival of patients. Therefore, the development of biomarkers for rapid diagnosis and severity assessment is greatly needed. With the establishment of new technologies, an increasing number of new biomarkers have been introduced into clinical use. One of the CPD parameters, MDW, reflects the variability of monocyte size in peripheral blood and thus has been widely investigated for applications in sepsis.
Monocytes are known to play key roles in the sepsis initiation and progression processes. They are a heterogeneous cell population in peripheral blood, with some subsets being activated and others not following a microorganism invasion. Therefore, the monocyte volume varies after activation, resulting in an increased volume SD. MDW reflects the volume SD of the tested monocytes, which is the theoretical basis for the application of MDW in sepsis. A demonstration of MDW in a normal population and sepsis patients is shown in Figure 1.
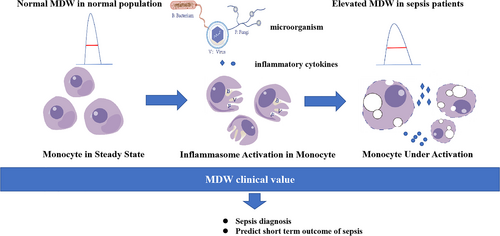
Demonstration of monocyte distribution width in normal population and sepsis patients.
Crouser et al. conducted a blind, prospective cohort study from two different EDs, which has been defined as a landmark study of MDW application in such patients [16]. In this work, 1320 subjects were consecutively enrolled from the ED, with patients being divided into three groups, SIRS, infection, and sepsis, according to final clinical assessment. MDW was measured using a UniCel DxH 800 hematology analyzer. The results showed that MDW could identify sepsis and distinguish these patients from the other groups (area under the curve [AUC]: 0.79; 95% CI: 0.73–0.84). Moreover, the MDW level was associated with the sepsis severity. Additionally, another study showed that MDW was a potential biomarker for the early screening of ED patients with the risk of developing sepsis (AUC: 0.96; 95% CI: 0.95–0.98) [27].
Many previous studies have demonstrated further applications of MDW for sepsis in the ED. A combination of the sepsis index, mean monocyte volume (MMV), and MDW significantly improved the early diagnosis of sepsis [28, 29]. Similarly, after combining MDW with other parameters, such as the WBC count [30], neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio [31], the AUC values were significantly improved. Additionally, the diagnostic performance of MDW in sepsis has been demonstrated to be comparable to that of PCT or CRP, which are commonly used clinically. However, using a combination of MDW with PCT or/and CRP did not show any significantly enhanced diagnostic performance compared with using a single biomarker. This indicated the need for selecting appropriate biomarkers based on clinical practice [32]. Overall, a meta-analysis [18] suggested that the performance of MDW in sepsis screening is reflected regardless of Sepsis 2.0 and Sepsis 3.0 criteria. Furthermore, because MDW has a clinically acceptable performance in sepsis diagnosis, it can also be used as a complement to the SIRS score or qSOFA score. Notably, it is simple to obtain and is quite cost-effective.
Other work involving the application of MDW in intensive care unit (ICU) or neonatal patients has also been reported. In a pilot study from the ICU [33], the MDW values were significantly higher in sepsis patients than in those without sepsis at admission. A study by Polilli et al. [34] compared the MDW values among patients with sepsis (sepsis and septic shock) and those without sepsis, with the results showing that the MDW values were significantly higher with sepsis. The median MDW value was 26.23 (interquartile range [IQR]: 23.48–29.83) in the sepsis group and 28.97 (IQR: 21.27–37.21) in the septic shock group. Interestingly, the pathogen type (bacterium, virus, or fugus) had no impact on the observed elevation of MDW. This indicated that MDW could be used as a general diagnostic biomarker of sepsis, but not as a tool to differentiate the pathogen type. In a prospective study that enrolled ICU patients [17], the AUC value of MDW for diagnosing sepsis was comparable to that of PCT, which was 0.79 (95% CI: 0.77–0.80) and 0.76 (95% CI: 0.74–0.78), respectively. However, MDW was superior to CRP and WBC for this purpose. Moreover, MDW was found to serve as a prognostic predictor of sepsis in ICU patients [35, 36].
There is very little literature on the application of CPD in neonatal sepsis. Nam et al. conducted a study in pediatric septic patients [37], finding that the MDW values increased relative to the control. The AUC value of MDW in sepsis diagnosis was (0.77, 95% CI: 0.72–0.83), better than that of CRP (0.74, 95% CI: 0.68–0.80). In addition, MDW could be used to predict mortality. Celik et al. [38] performed a study in 227 newborns, with the enrolled patients divided into a sepsis group (40 proven sepsis cases, 76 clinically diagnosed sepsis cases) and control group (111 excluding sepsis). The results showed that the MDW values were higher in the septic patients than in the control individuals. Furthermore, the MDW values were significantly higher in late-onset septic patients compared with those in early onset patients. No significant difference in MDW values was found between the patients with gram-positive blood culture and those with gram-negative blood cultures.
It is very important to differentiate sepsis from the inflammatory status because the consequences and treatment methods for the patient are quite different. Unfortunately, there is still no gold standard biomarker for this purpose, although several potential biomarkers have been explored [39]. From a CPD perspective, a prospective study enrolled four patient groups: 66 with sepsis, 59 with pneumonia (indicated for local infection), 92 with rheumatoid arthritis (indicated for chronic inflammation), and 56 with coronary artery bypass graft operation (indicated for noninfectious inflammation). The CPD parameters were obtained using a Unicel DxH800 [40]. The results showed that the mean neutrophil volume (MN-V-NE) value was highest in the sepsis group compared with the values in the other groups. The SD of neutrophil volume (SD-V-NE) value was higher in the sepsis group and could be used to differentiate sepsis from the other conditions examined. The combination of several CPD parameters, SD-V-NE + SD-V-LY + SD-V-MO, SD-V-NE + SD-V-MO, and MN-V-NE + SD-V-NE + SD-C-LY + SD-V-MO, had higher AUC values than PCT.
3.2.2 COVID-19
COVID-19 results from an infection with the SARS-CoV-2 virus. Most infected individuals experience mild to moderate respiratory illness and can recover without special treatment. However, some patients will become critically ill, with the development of severe pneumonia, acute respiratory distress syndrome, or multiple organ dysfunction syndrome. Therefore, recognizing infected patients at risk of severe disease or having poor prognosis deserves more attention.
Generally, older patients and those with other comorbidities, such as diabetes, cardiovascular disease, chronic respiratory disease, or various malignancies, are more likely to develop serious symptoms or complications with COVID-19. Additionally, lymphocytopenia and inflammatory storms are quite common critically ill patients, which prompts us to search for relevant predictive biomarkers that may be clinically applicable. Circulating monocytes play key roles in the initiation and progression of inflammatory storms by producing inflammatory mediators when aberrantly activated [41, 42]. Significant differences in monocyte morphology and function were found between COVID-19 patients and healthy individuals. Interestingly, it was also demonstrated that the MDW value was significantly higher in COVID-19 patients (mean: 27.3 ± 4.9) compared with that in non-COVID-19 patients (mean: 20.3 ± 3.3), with the value increasing with the severity of disease. These results indicate the potential for using MDW as an indicator of COVID-19 prognosis [43].
In addition to MDW, other CPD parameters have been studied for COVID-19-related applications. Naoum et al. [44] found that lymphopenia and eosinopenia were typical in all COVID-19 patients, with increased WBC and neutrophil counts also being observed. CPD analysis indicated that increased volumes of neutrophils, lymphocytes, and monocytes were associated with COVID-19, whereas the conductivity increased for lymphocytes but decreased for neutrophils. Some parameters showed good diagnostic performance, such as the lymphocyte count (AUC = 0.858) and monocyte volume (AUC = 0.837). Vasse et al. [45] analyzed CPD of the different leukocyte subpopulations in 322 confirmed COVID-19 patients, 285 SARS-CoV-2-negative patients, and 137 healthy controls. Using a random forest classifier, an algorithm was then developed based on the combination of four monocyte-related CPD parameters to discriminate COVID-19 patients from others. This algorithm was tested in an external validation cohort, which prospectively enrolled 222 patients from the ED. The results showed that 60.5% of these enrolled patients were correctly identified, while 10.3% of the 136 enrolled SARS-CoV-2-negative patients were misclassified using the protocol (specificity of 89.7% and sensitivity of 60.5%). False negatives mainly came from patients with low inflammatory state and false positives mainly appeared in patients who already had sepsis. Overall, CPD parameters could potentially be used as a screening tool and aid in the early diagnosis of COVID-19.
Recent studies have also shown a significantly increased diagnostic performance when using a combination of CPD parameters. For example, the Lymph Index is calculated as follows: (mean lymphocyte volume [LV] x LV-SD)/(mean lymphocyte conductivity [LC]). When it was combined with MDW, the AUC value was 0.89 [46]. Additionally, NLR >5 plus MDW ≥20 could distinguish a SARS-CoV-2 infection from upper respiratory tract infections or influenza [47]. Riva et al. [48] reported that MDW was strongly correlated with other biomarkers, such as CRP, fibrinogen, and ferritin, in COVID-19 patients. The MDW value was also correlated with clinical outcome (survival or death).
3.2.3 Other infectious diseases
Viral infections
Lymphocytes are a part of anti-virus immune responses, making lymphocyte-related CPD parameters relevant in viral infections. One study investigated the usefulness of detecting morphological changes in lymphocytes in patients with hepatitis B virus (HBV) infection [49]. CPD parameters were collected from 37 HBV active patients, 140 HBV carriers, and 655 controls. The results showed significant increases in lymphocyte volume-related CPD (LV and LV-SD) and a significant decrease in lymphocyte conductivity-related CPD (LC) in patients with HBV infection (both HV active and carriers) compared with the findings in the patients in the control group. CPD parameters related to monocyte volume and conductivity showed similar results. Using a Lymph Index cut-off value of 18.2 achieved 91.7% sensitivity and 95.7% specificity values for identifying active HBV in these patients. These investigators aimed to further evaluate the performance of CPD in differentiating various viral infections and acute bacterial infections. Another study was performed which enrolled 72 patients with viral infections, 46 with acute bacterial infections, and 204 controls. The results showed that the Lymph Index significantly increased in the viral infection group, whereas there was only a slight increase in the acute bacterial infection group. With a Lymph Index cut-off value of ≥12.92, the sensitivity for diagnosing viral infections reached 91.7% and the specificity reached 97.2% [11].
The application of CPD parameters for identifying bacterial and viral infections was conducted in a retrospective observational study [50]. In total, 602 children who came to the hospital for treatment were enrolled, with their CPD data obtained using a UniCel DxH800. A diagnostic decision rule was established using statistical analysis to screen and differentiate the viral infections. This rule included a total of 21 CBC and CPD parameters. Using this rule, 74/77 (96.1%) viral infections, 2/54 (3.7%) normal samples, 1/17 (5.9%) tuberculosis cases, and 6/71 (8.5%) bacterial infections were identified. Compared with the findings in the normal control group, the sensitivity for distinguishing viral infections was 96.1% and the specificity was 96.3%. This study suggests that the ability to distinguish viral infections in children can be improved by combining CBC and CPD parameters.
Tropical fever
Tropical fevers are defined as infections that are unique to tropical or subtropical regions, including dengue fever, rickettsial infections, and malaria. However, with economic development and population shifts, patients are not restricted to these regions and some sporadic cases are reported. Because patients with different infections have overlapping clinical symptoms, quick and accurate diagnosis at arrival can sometimes be difficult, especially in non-tropical regions. A CBC test will often be ordered by a physician for a patient with a fever, and CPD parameters can be obtained simultaneously. This demonstrates the possibility and availability of using CPD for aiding the diagnosis of tropical fever.
A study from India reported the value of CPD parameters in patients with tropical infections [51]. It was a cross-sectional study, with 324 cases enrolled and analyzed, including malaria (50.0%), dengue (30.9%), leptospirosis (13.9%), typhoid (4.0%), and rickettsial infections (1.2%), along with 324 healthy controls. CBC and CPD parameters were generated using the UniCel DxH800. The results showed that some CBC parameters had significantly different mean values between control and cases, including hemoglobin, WBC count, differential leukocyte count, RBC count, hematocrit, red cell distribution width, platelet count, and plateletcrit. There were also differences among various infections. For example, the mean lymphocyte, monocyte, and neutrophil volumes were much higher in malaria and dengue fever infections than in leptospirosis, typhoid, and rickettsial infections. The CPD parameters were least altered in typhoid fever, but the conductivity and light scatter of eosinophils increased. This study showed the potential use of CPD in different types of tropical fevers, potentially supporting the development of a specific diagnostic algorithm.
A study in Thailand developed a predictive score for dengue infections, which included CBC and MDW [52]. This showed that the median MDW value was higher in dengue patients than in non-dengue patients (29.7% [95% Cl: 26.5–34.7] versus 24.2% [95% Cl: 21.1–27.8]). Additionally, when the disease severity increased in confirmed dengue patients, the MDW value also increased. Some independent factors for prediction of dengue infection were WBC count <4 × 109/L (score 1), platelet count <100 × 109/L (score 1), and MDW >24 (score 1). With the cut-off score as ≥ 1, the respective sensitivity and specificity values for dengue infection prediction were 92.2% and 40.8% in the training set and 88.9% and 44.1% in the validation set. The AUC values were 0.839 (95% Cl: 0.779–0.899) and 0.742 (95% Cl: 0.674–0.811) in the training set and validation set, respectively. A previous study aimed to identify malaria and dengue infections, as well as to distinguish them from other febrile illnesses, using blood parameters (including CPD). The CPD were generated using an LH750 hematology analyzer (Beckman Coulter) [53]. The results showed that malaria and dengue cases had significant leukocyte abnormalities compared with cases of other febrile illnesses. Furthermore, such discriminant functions could be demonstrated via internal software calculations, which would then trigger the alarm to order specific tests for additional confirmation. Another study involved leukocyte CPD with an XN1000 hematology analyzer (Sysmex) [54]. This work had patients with acute febrile illnesses enrolled, including 97 dengue cases, 202 dengue-negative controls, and 100 healthy individuals as normal controls. In the dengue group, a significant increase in HFLC was observed. The HFLC% could discriminate dengue from dengue-negative patients using a cut-off value of 1.75, with 52% sensitivity and 90% specificity. LY-X, LY-Z, LY-WX, LY-WZ, and MO-X were identified as independent predictors for dengue fever through a regression analysis. Therefore, CPD parameters may be used as a tool to discriminate dengue infections from other febrile illnesses in a rapid and economic manner.
Other infections
Additional publications have reported the application of CPD in other infections, indicating its extended clinical potential. Sun et al. evaluated the diagnostic efficacy of monocyte-related CPD parameters and monocyte chemotactic protein (MCP)-1 for distinguishing active tuberculosis (ATB) from latent tuberculosis (LTBI) [55]. Ninety-seven patients with ATB, 113 patients with LTBI, and 101 healthy controls were enrolled, and then the CPD parameters were measured using a Unicel DxH800. Compared with the LTBI patients and healthy controls, the ATB patients had significantly increased values for the average volume SD of monocytes, average conductivity of monocytes, and MCP-1 levels. The sensitivity and specificity values reached 93.8% and 93.1% respectively using these three indicators. These findings suggest that such monocyte volume parameters and MCP-1 levels could potentially be used as preliminary clinical assessment indicators to differentiate ATB from LTBI.
For chronic obstructive pulmonary disease (COPD) patients, effectively managing exacerbations is the most important. In such conditions, however, discriminating infection-related from non-infection-related acute exacerbations of COPD (AECOPD) is difficult, resulting in the overuse of antibiotics, bacterial resistance, and even false treatments. In a retrospective study, 45 AECOPD patients were enrolled (22 bacterial and 23 nonbacterial), and then CBC, CPD, blood cell differentiation type, and certain serum biomarkers were tested and analyzed [56]. The results showed that the basophil percentage had a better AUC value (0.80). With sensitivity values ≥90%, the NLR and CD4+ T cell percentage demonstrated the highest specificity (57%), with AUC values of 0.755 and 0.747, respectively. Both the mean volume and SD of neutrophils had good performance (AUC values of 0.846 and 0.804, respectively). Regression models suggested that the specificity increased with the addition of leukocyte populations, PCT, and CRP, compared with CRP alone. Although no single test showed sufficient accuracy for predicting bacterial AECOPD, combining several parameters may improve this.
3.3 CPD beyond infectious disease
3.3.1 Hematological disorders
As described earlier, CPD parameters reflect the morphological characteristics of blood cells. Therefore, they can also be classified as hematologic tests, which can help diagnose diseases derived from blood and bone marrow cells. Bone marrow biopsies and aspirates are more specialized tests for diagnosing hematologic diseases. However, these tests are invasive, so surrogate blood tests that are more “convenient and friendly” are urgently needed.
For example, myelodysplastic syndromes (MDS) are clonal hematological diseases that result from dysfunctional blood cells. Patients display chronic cytopenia and dysplasia, with some quickly progressing into acute myeloid leukemia. Assessing peripheral blood cell morphology can be used as a tool for diagnosing MDS. Previous studies have attempted to add a “flag” on the automatic hematology analyzer for each test with an “abnormal” blood cell finding to trigger the manual examination of morphology to further confirm the dysgranulopoiesis or to initiate a bone marrow biopsy [57]. However, the sensitivity, specificity, and cut-off values of these assays vary [58, 59]. One study aimed to establish a screening method for the detection of dysplastic cell morphology in MDS patients using CPD parameters measured automatically with the DxH800 automatic hematology analyzer (Beckman Coulter) [58]. Peripheral blood was collected from 43 patients with low-grade or high-grade MDS and 21 controls. The results indicated that all the mean light scatter values from neutrophils were significantly lower in high-grade MDS compared with those in the controls. The SD values of each neutrophil CPD parameter differed significantly among the three groups. The mean median angle light scatter (MN-MALS-NE) and mean upper median angle light scatter (MN-UMALS-NE) were different between the low-grade MDS and control groups. In addition, MN-MALS-NE could be used as an individual predictive factor for MDS, achieving a sensitivity of 63% and specificity of 67% with a cut-off value of ≤133. Additionally, the SD of neutrophil upper median angle light scatter (SD-UMALS-NE) could also predict MDS, with a cut-off value of ≥11.16 having 77% sensitivity and 82% specificity. Thus, this study showed that the CPD parameter heterogeneity could contribute to predicting MDS.
Hematological malignancies often originate from the bone marrow, where hematopoietic stem cells develop into WBCs, RBCs, or platelets. Various diagnostic techniques have been developed and clinically applied, such as biopsy, flow cytometry, radiology exams, blood cell tests, and genetics technology. CBC is one of the fundamental tests and CPD generated using hematology analyzers can provide various useful information for hematological disorder screening. One study enrolled newly diagnosed chronic myelogenous leukemia (CML) patients and related controls, with CPD parameters being collected and compared [10]. The results showed that certain CPD parameters, such as the mean neutrophil volume (MNV), mean monocyte volume (MMV), mean lymphocyte scatter (MLS), SD of the mean neutrophil volume (MNV-SD), and SD of the mean neutrophil conductivity (MNC-SD) were higher in the untreated CML group compared with those in the other groups. Notably, the combination of MNV >163.0 and MNC-SD >12.69 can be used for CML diagnosis, with 89.5% sensitivity and 100% specificity.
Recently, machine learning algorithms have become quite popular in diagnostic studies. Research from KonKuk University Medical Center presented a novel idea of utilizing machine learning algorithms to screen hematological malignancies using CPD parameters [60]. Here, 882 cases (457 patients with a hematologic malignancy and 425 controls without a hematologic malignancy) were enrolled, then the data were analyzed using seven machine learning models. The results showed outstanding diagnostic performance using the ANN model, which achieved the highest accuracy (82.8%) and AUC ± SD (93.5% ± 2.6) values. The ANN model (based on CPD parameters) appeared to be an efficient method for screening hematological malignancies in a clinical laboratory.
To treat many hematological malignancies, especially leukemia, autologous hematopoietic stem cell transplantation (AHSCT) is frequently adopted. After AHSCT, a sufficient quantity of hematopoietic stem cells surviving within the recipient's body is a foundation for a successful outcome. It has been suggested that the morphological changes of myeloid cells in peripheral blood can reflect the bone marrow stimulation process, which may be used to predict the AHSCT (CD34+ stem cells) outcome. As has been emphasized, CPD parameters can reflect the morphological changes in leukocytes and may therefore be used to demonstrate the changes of myeloid cells from peripheral blood and indirectly reflect the transplantation efficacy. A study found that 2–4/μL before the increase of CD34+ cell count in peripheral blood, neutrophil CPD parameter changes could be detected. Additionally, the MN-V-NE correlated well with the CD34+ cell count, suggesting that such neutrophil CPD could be used as a surrogate for the CD34+ cell count in peripheral blood. In certain circumstances, CPD could be used to improve the management of patients undergoing AHSCT [61].
3.3.2 Inflammation
Inflammation is triggered by pathogen infections and can also occur in sterile conditions. Identifying sterile inflammation is also important because it can cause tissue damage and lead to more critical illnesses.
CPD may be used in the early detection of sterile inflammation because inflammatory responses involve the activation of immune cells. For example, cardiac surgery could trigger sterile inflammation that is responsible for post-operative morbidity. In a cohort study that included 1453 patients undergoing cardiopulmonary bypass, CPD parameters were collected at three time points (pre-operation, post-operation, and on day 5 after operation). The data were compared between patients with and without post-operation complications (supra-ventricular arrhythmia, stroke, acute renal failure, and/or death) [62]. The results showed that the values of some neutrophil parameters, including CPD parameters, increased with operation. These included the neutrophil count, NE-SCC (reflects granularity), NE-SFL (reflects intensity), and NE-FSC (reflects size), while the heterogeneity of neutrophil granularity (NE-WX) decreased. Furthermore, the lymphocyte count and LY-Y (reflects intensity) decreased with operation, but the LY-X (reflects granularity) and LY-Z (reflects size) increased. Less lymphocyte heterogeneity was observed. Patients who developed composite complications had a higher neutrophil count, as well as at the period of pre-operation, with more heterogeneous neutrophils. After adjustment with the EuroSCORE2, some CPD parameters (NE-WX, NE-WY, and LY-X) were found to be associated with the occurrence of post-operation complications.
Acute pancreatitis is a relatively common but quite serious inflammatory disease. Early recognition and evaluation of disease severity is of great clinical importance. CPD can also be used as a predictive marker for acute pancreatitis severity. In a prospective observational study [63], which enrolled patients with acute pancreatitis from the ED, 42 CPD parameters were compared between 103 acute pancreatitis patients and 62 healthy controls. Acute pancreatitis patients were further divided into mild acute pancreatitis (n = 30), moderate acute pancreatitis (n = 42), and severe acute pancreatitis (n = 31). Among the 42 CPD parameters examined, four were the highest in patients with severe acute pancreatitis at admission, with significant differences observed among the three groups over the three days of patient admission. Subsequently, a multiple factor logistic regression model was established, forming a scoring system based on these four CPD parameters (SD_LALS NE, MN_LALS LY, SD_LMALS MO, and SD_AL2 MO) for diagnosing severe acute pancreatitis. The sensitivity, specificity, and AUC values of this model were 96.8%, 65.3%, and 0.87, respectively. In addition, this scoring system can be used to predict acute pancreatitis severity, ICU transport, and mortality. This was comparable to other commonly used clinical indicators, such as SOFA, APACHE II, PCT, CRP, and WBC count.
4 DISCUSSION
This review summarizes the current major clinical studies on CPD applications in infectious diseases, as well as in other diseases. CPD parameters serve as morphological characteristic indicators and can be used as leukocyte action and functional change markers. For example, MDW can serve as a biomarker for peripheral monocyte activation, an early diagnostic biomarker of adult sepsis in the ED, and a prognostic indicator of sepsis patients in the ICU, as well as in neonates and children. Additionally, MDW is valuable for supporting the diagnostic and prognostic evaluation of COVID-19 patients.
CPD can be easily measured via automatic hematology analyzers, such as the Beckman Coulter Unicel DxH series and Sysmex XN series, which are widely used and studied. CPD can be measured simultaneously with routine blood tests (also called CBC) without additional technical operation or reagents, empowering the excellent feasibility of potential clinical application. Using CPD parameters can also have health-related economic effects. In a corresponding study, approximately 67% of sepsis patients benefited from MDW results because of early recognition, resulting in the antibiotic use duration being reduced from 3.98 to 2.07 h. It was proposed that the use of MDW for the early diagnosis of sepsis could lower the total cost by $712,783 per hospital per year [64]. Because MDW is the only CPD parameter that has received FDA and CE approval, other clinical studies and explorations of CPD applications are being conducted. Laboratory developed tests for CPD parameters may potentially be launched, which would allow them to benefit more patients.
Compared with other host response-related biomarkers, CPD parameters have comparable efficacy for the diagnosis of infectious diseases. As mentioned earlier, the performance of MDW for diagnosing sepsis was equal to that of PCT, while it even had good sensitivity, NPV 86.4% (95% CI: 65.1–97.1) for ruling out sepsis [34]. When combined with the WBC count, the AUC value reached 0.89 [16]. The combination of MDW and other biomarkers (PCT, CRP) could enhance the diagnostic performance, but the changes were not statistically significant [32, 65]. In COVID-19 patients, CPD showed favorable applications for diagnosis and prognosis evaluations. Combinations with other indicators led to better performance. MDW has also received CE approval for aiding the diagnosis of COVID-19. In this review, we mainly discuss the leukocyte CPD parameters. However, in a broad sense, RBC and platelet CPD should also be involved. Various publications have already described the unique application of these CPD parameters. For instance, a novel algorithm with RBC-related parameters was developed, named the αβ-algorithm, which was calculated as ([MN-LMALS-RET × RDW]—MCH). This could discriminate the α from the β-thalassemia trait. The respective sensitivity and specificity values were 92% and 90%, with the AUC value reaching 0.97 with a cut-off value of 1742.5 [66]. CPD deserves further exploration for potential clinical applications. The main published clinical applications of CPD are summarized in Table 1.
| Clinical application/Site | Population | CPD parameter | Cutoff (if available) | Sensitivity/Specificity/AUC | Reference |
|---|---|---|---|---|---|
| Sepsis diagnosis/ED | Adult | MDW | 20.5 | 0.77/0.73/0.79; 95% CI, 0.73–0.84 | Crouser et al. CHEST 2017; 152(3):518–526. [16] |
| Prediction of short-term outcome of sepsis/ED and ICU | Adult | MDW (on day 3 since admission) | 26.20 | 0.78/0.68/0.731; 95% CI, 0.650–0.812 | Liu et al. Clin Chem Lab Med 2023; 62(3):562–571. [36] |
| Sepsis diagnosis | Newborns | MNV, NDW, MMV, MDW, MNS | MNV, NDW, MMV and, MDW were higher in infants with sepsis than in controls. MNS was lower in the patients with sepsis. | – | Celik, H.T et al. Pediatr Int 2016; 58(2):119–125. [38] |
| Prediction of sepsis in critically ill patient/ICU | Adult | MDW | >23.0 | 0.75/0.89/0.82; 95% CI, 0.75–0.89 | Polilli, E et al. BMC Emerg Med 2021; 21(1):147. [34] |
| Sepsis diagnosis/ICU | Adult | MDW | >24.63 | 0.67/0.78/0.79; 95% CI: 0.77–0.80 | Piva, E et al. Clin Chem Lab Med 2021; 59(7):1307–1314. [17] |
| Differentiation sepsis from inflammatory status | Adult | CPD combinations: Type 1 (SD-V NE + SD-V LY + SD-V MO); Type 2 (MN-V NE + SD-V NE + SD-C LY + SD-V MO); Type 3 (SDV NE + SDV MO) | – | Type 1: 0.85/0.75/0.87; 95% CI, 0.82–0.92 | Cevlik, T et al. J Intensive Care Med; 2023. 38(4):382–390. [40] |
| Type 2: 0.83/0.76/0.86; 95% CI, 0.82–0.92 | |||||
| Type 3: 0.83/0.73/0.85; 95% CI, 0.81–0.91 | |||||
| Diagnosis COVID-19 infection/ED | Adult | MDW | 20 | 0.98/0.65/0.91; 95% CI, 0.86–0.96 | Ognibene, A et al. Clin Chim Acta 2020; 509: 22–24. [43] |
| Predict outcomes in COVID-19 patients | Adult | MDW | 26.4 | 0.75/0.7/0.76; 95% CI, 0.66–0.87 | Riva, G et al. Sci Rep 2021; 11(1): 12716. [48] |
| Discrimination COVID 19+ from COVID 19- patients/ED | Adult | (1) SD-V-Mo; | (1) 21.71 | (1) 0.92/0.63/0.82; 95% Cl, 0.786–0.849 | Vasse, M et al. Int J Lab Hematol 2021; 43(1):116–122. [45] |
| (2) 4 CPD parameters to form a discriminating protocol (SD-V-MO, MN-C-MO, SD-LMALS-MO, SD-UMALS-MO) | (2) SD-V-MO >23, MN-C-MO >121.3, SD-LMALS-MO >25.7, SD-UMALS-MO >11.4 | (2) 0.90/0.61 | |||
| Diagnosis COVID-19 infection/hospitalized patients | Adult | (1) MDW | (1) 20.11 | (1) 0.83/0.73/0.86; 95% Cl, 0.80–0.92 | Zeng, X et al. Int J Lab Hematol 2020; 42(6):e266-e269. [46] |
| (2) MDW + lymph index | (2) 0.615 | (2) 0.81/0.79/0.89; 95% Cl, 0.85–0.94 | |||
| Distinguishing COVID-19 from upper respiratory tract infections or influenza/outdoor patients | Adult | MDW, NLR | MDW ≥20 + NLR <3.2 | AUC 0.84; 95% Cl, 0.74–0.94 | Lin, H.A et al. PLoS One 2020; 15(11):e0241262. [47] |
| Diagnosis of HBV infection | Adult | Lymph-Index | 18.2 | 91.7/95.7/0.98 | Zhu, Y et al. Lab Hematol 2011; 17(3):22–26.[49] |
| Diagnosis viral infection | Adult | Lymph-Index | ≥12.92 | 0.92/0.97/0.99 | Zhu, Y et al. Int J Infect Dis 2013; 17(7):e490-e493 [11] |
| Identification of bacterial and viral infections | Children | A diagnosis decision rule composed of 21 CBC and CPD parameters | – | 0.96/0.96 | Jung, YJ et al. Int J Lab Hematol 2012; 34(3):283–289 [50] |
| Prediction of dengue infection | Adult | WBC <4 × 109/L (score 1), platelet <100 × 109/L (score 1) and MDW >24 | Score ≥1 | 0.92/0.41/0.84; 95% Cl, 0.78–0.90 | Poottasane, N et al. Am J Trop Med Hyg 2023; 109(4):926–932. [52] |
| Discrimination of dengue-positive and dengue-negative patients | Adult | HFLC%; (LY-X, LY-Z, Ly-WX, LY-WZ, and MO-X are independent predictors for dengue fever) | 1.75 | 0.52/0.90/72% positive predictive value, and 80% negative predictive value | Chhabra, G et al. Int J Lab Hematol 2022; 44(3):477–482. [54] |
| Prediction of MDS | Adult | SD-UMALS-NE | ≥12.46 | 0.77/0.81/0.82; 95% Cl, 0.71–0.93 | Shestakova, A et al. Cytometry B Clin Cytom 2021; 100(3): 299–311. [58] |
| Prediction the severity of acute pancreatitis severity/ED | Adult | SD LALS NE < 36.17 (score 1), MN LALS LY > 34.50 (score 1), SD LMALS MO < 16.62 (score 1), SD AL2 MO < 17.2 (score 1) | Median scores of SAP and non-SAP were 1 and 3, respectively. | 0.97/0.65/0.87 | Wang, Y et al. Clin Lab Anal 2021; 35(7):e23863. [63] |
| Distinguishing untreated CML from reactive neutrophilia | Adult | MNV; MNC-SD; MLS | MNV >163.046; | 0.89/0.91/0.96; | Gaspar, BL et al. Biomed J 2019; 42(2):93–98. [10] |
| MNC-SD >12.69; | 0.95/0.96/0.99; | ||||
| MLS> 81.827 | 0.95/0.94/0.98 | ||||
| Screening hematologic malignancies | Adult | 41 parameters involving CPD | >0.05 | Accuracy 82.8%, AUC±SD (93.5% ± 2.6%) | Syed-Abdul, S et al. Sci Rep 2020; 10(1):4583. [60] |
| Detection of sterile inflammation/patients underwent cardiac surgery | Adult | NE-WX, NE-WY and LY-X | Pre-operative NE-WX, NE-WY and LY-X biomarkers levels were associated with post-operative complications | – | Nguyen, M et al. Front Immunol 2022; 13: 1101937. [62] |
| Prediction the expected outcome of transplantation of CD34+ stem cells/patients underwent hematopoietic stem cell transplantation | Adult | MN-V-NE, ImmNeIndex (MN-V-NE × SD-V-NE)/100) | There was a good correlation between MN-V-NE and ImmNeIndex with CD34+ count (r = 0.67 and 0.65 respectively) | – | Golubeva, V et al. Transfus Apher Sci 2014; 50(1):39–45. [61] |
However, some potential drawbacks of CPD applications cannot be ignored. Firstly, pre-analytical conditions and anticoagulants can affect CPD values [67]. The FDA approved MDW detection from whole blood vein samples with K2EDTA. However, sample tubes with K3EDTA are used in many laboratories, which will demonstrate higher MDW values than those using K2EDTA [25]. Generally, there is a 1.5-fold offset of the MDW value between K2EDTA and K3EDTA (as reported by the manufacturer, Beckman Coulter) [68]. Potential differences for other CPD parameters should also be carefully tested and analyzed. Secondly, CPD are specific to a specific hematology analyzer, with the same type of analyzer sometimes showing variations [69]. The analyzers within a lab (same types of analyzers) should be carefully adjusted before use for CPD parameter acquisition. A key point of difficultly is that labs often use different brands of hematology analyzers (e.g., Mindary, Sysmex, and Beckman Coulter). Although different hematology analyzers can measure the morphological related-parameters through their own technologies, these parameters are somewhat similar. However, only Beckman Coulter hematological analyzers measure this parameter using the Coulter Principle (through current, voltage, and light scattering) without any cell staining or disruption. Thus, CPD measured using Beckman Coulter hematological analyzers is closest to the primitive morphological state of measured cells. The parameter measurements obtained using different instruments are therefore not comparable. No publication has described the operation of harmonizing the CPD results between different hematological analyzers. We especially recommend Beckman Coulter hematological analyzers because more than half of the publications on CPD clinical applications have used this manufacturer's instruments. Because the CPD results cannot be transferred between different analyzers, each lab establishes its own CPD test and analysis rules. If needed, optical adjustments may be attempted to harmonize the CPD results between different hematological analyzers. Thirdly, the long-term stability of CPD is not well understood. One study validated the time-dependent analytical stability of MDW, suggesting that these measurements were stable within 4 h and that testing MDW more than 6 hours after blood collection should be avoided [70]. Studies on other CPD parameters and different situations may be performed by the lab if certain CPD parameters are used for clinical needs. Even if the local CPD reference intervals have been established, the CPD results should still be carefully reviewed for each clinical scenario [21, 22].
5 CONCLUSION
CPD parameters have been widely studied in infectious diseases. CPD is also expected to have clinical value in non-infectious diseases, especially those related to systemic inflammation. In this review, we describe how CPD parameters have been found to serve as important indicators of activated leukocytes in circulation. For example, MDW plays key roles in sepsis diagnosis and prognosis evaluation. CPD parameters are morphological indicators of blood cells, including of the volume and nuclear and intracellular granules. We speculate that CPD contains significant and important information that can be used in additional disease niches related to blood cell dysregulation. Potential clinical applications include the prediction of cytokine storms, infection differentiation in patients with immune abnormalities (autoimmune diseases, immunodeficiency), and early identification of infections in post-organ transplantation patients.
AUTHOR CONTRIBUTIONS
Shayuanzi Huang, Yin Liu, Liu Qian analyzed the data and prepared the first draft of the manuscript. Dong Wang and Juan Zhou participated in the conception and design of the study, Dong Wang, Liu Qian and Yin Liu constructively revised the manuscript, and they share corresponding authorship. All authors commented on previous versions of the manuscript and approved the final version.
ACKNOWLEDGMENTS
This work was supported by the basic and applied basic research projects of Guangdong Province (2021A1515220040).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
ETHICS STATEMENT
Not applicable.
INFORMED CONSENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
There are no data for this review.




