The variations in the natural history of high-risk human papillomavirus infections in Chinese healthy women aged 27–45 years compared with 18–26 years: A prospective cohort study
Qi Chen
State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, Fujian Province, China
National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Innovation Platform for Industry-Education Integration in Vaccine Research, NMPA Key Laboratory for Research and Evaluation of Infectious Disease Diagnostic Technology, Xiamen University, Xiamen, Fujian Province, China
Contribution: Data curation, Investigation, Visualization, Funding acquisition, Writing - original draft, Software, Formal analysis, Validation
Search for more papers by this authorXingmei Yao
State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, Fujian Province, China
National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Innovation Platform for Industry-Education Integration in Vaccine Research, NMPA Key Laboratory for Research and Evaluation of Infectious Disease Diagnostic Technology, Xiamen University, Xiamen, Fujian Province, China
Contribution: Data curation, Software, Investigation, Visualization, Writing - review & editing, Formal analysis, Validation
Search for more papers by this authorJiali Quan
State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, Fujian Province, China
National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Innovation Platform for Industry-Education Integration in Vaccine Research, NMPA Key Laboratory for Research and Evaluation of Infectious Disease Diagnostic Technology, Xiamen University, Xiamen, Fujian Province, China
Contribution: Investigation, Writing - review & editing
Search for more papers by this authorXinhua Jia
National Cancer Center, National Center for Cancer Clinical Research, The Cancer Institute, Chinese Academy of Medical Sciences/Peking Union Medical College, Beijing, China
Contribution: Investigation, Writing - review & editing
Search for more papers by this authorYufei Li
State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, Fujian Province, China
National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Innovation Platform for Industry-Education Integration in Vaccine Research, NMPA Key Laboratory for Research and Evaluation of Infectious Disease Diagnostic Technology, Xiamen University, Xiamen, Fujian Province, China
Contribution: Investigation, Writing - review & editing
Search for more papers by this authorKongxin Zhu
State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, Fujian Province, China
National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Innovation Platform for Industry-Education Integration in Vaccine Research, NMPA Key Laboratory for Research and Evaluation of Infectious Disease Diagnostic Technology, Xiamen University, Xiamen, Fujian Province, China
Contribution: Investigation, Writing - review & editing
Search for more papers by this authorXiaowen Hu
State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, Fujian Province, China
National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Innovation Platform for Industry-Education Integration in Vaccine Research, NMPA Key Laboratory for Research and Evaluation of Infectious Disease Diagnostic Technology, Xiamen University, Xiamen, Fujian Province, China
Contribution: Investigation, Writing - review & editing
Search for more papers by this authorXingcheng Huang
State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, Fujian Province, China
National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Innovation Platform for Industry-Education Integration in Vaccine Research, NMPA Key Laboratory for Research and Evaluation of Infectious Disease Diagnostic Technology, Xiamen University, Xiamen, Fujian Province, China
Contribution: Investigation, Writing - review & editing
Search for more papers by this authorGuohua Zhong
State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, Fujian Province, China
National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Innovation Platform for Industry-Education Integration in Vaccine Research, NMPA Key Laboratory for Research and Evaluation of Infectious Disease Diagnostic Technology, Xiamen University, Xiamen, Fujian Province, China
Contribution: Investigation, Writing - review & editing
Search for more papers by this authorLingxian Qiu
State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, Fujian Province, China
National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Innovation Platform for Industry-Education Integration in Vaccine Research, NMPA Key Laboratory for Research and Evaluation of Infectious Disease Diagnostic Technology, Xiamen University, Xiamen, Fujian Province, China
Contribution: Investigation, Writing - review & editing
Search for more papers by this authorZhaofeng Bi
State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, Fujian Province, China
National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Innovation Platform for Industry-Education Integration in Vaccine Research, NMPA Key Laboratory for Research and Evaluation of Infectious Disease Diagnostic Technology, Xiamen University, Xiamen, Fujian Province, China
Contribution: Investigation, Writing - review & editing, Funding acquisition
Search for more papers by this authorMengjun Liao
State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, Fujian Province, China
National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Innovation Platform for Industry-Education Integration in Vaccine Research, NMPA Key Laboratory for Research and Evaluation of Infectious Disease Diagnostic Technology, Xiamen University, Xiamen, Fujian Province, China
Contribution: Investigation, Writing - review & editing
Search for more papers by this authorLu Chen
State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, Fujian Province, China
National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Innovation Platform for Industry-Education Integration in Vaccine Research, NMPA Key Laboratory for Research and Evaluation of Infectious Disease Diagnostic Technology, Xiamen University, Xiamen, Fujian Province, China
Contribution: Investigation, Writing - review & editing
Search for more papers by this authorXuefeng Kuang
State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, Fujian Province, China
National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Innovation Platform for Industry-Education Integration in Vaccine Research, NMPA Key Laboratory for Research and Evaluation of Infectious Disease Diagnostic Technology, Xiamen University, Xiamen, Fujian Province, China
Contribution: Investigation, Writing - review & editing
Search for more papers by this authorZhe Wang
State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, Fujian Province, China
National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Innovation Platform for Industry-Education Integration in Vaccine Research, NMPA Key Laboratory for Research and Evaluation of Infectious Disease Diagnostic Technology, Xiamen University, Xiamen, Fujian Province, China
Contribution: Investigation, Writing - review & editing
Search for more papers by this authorShangying Hu
National Cancer Center, National Center for Cancer Clinical Research, The Cancer Institute, Chinese Academy of Medical Sciences/Peking Union Medical College, Beijing, China
Contribution: Investigation, Writing - review & editing
Search for more papers by this authorChunlan Zhuang
State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, Fujian Province, China
National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Innovation Platform for Industry-Education Integration in Vaccine Research, NMPA Key Laboratory for Research and Evaluation of Infectious Disease Diagnostic Technology, Xiamen University, Xiamen, Fujian Province, China
Contribution: Investigation, Writing - review & editing
Search for more papers by this authorShoujie Huang
State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, Fujian Province, China
National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Innovation Platform for Industry-Education Integration in Vaccine Research, NMPA Key Laboratory for Research and Evaluation of Infectious Disease Diagnostic Technology, Xiamen University, Xiamen, Fujian Province, China
Contribution: Investigation, Writing - review & editing, Project administration, Supervision
Search for more papers by this authorLihui Wei
Department of Obstetrics and Gynecology, Peking University People's Hospital, Beijing, China
Contribution: Investigation, Project administration, Writing - review & editing, Supervision
Search for more papers by this authorWen Chen
National Cancer Center, National Center for Cancer Clinical Research, The Cancer Institute, Chinese Academy of Medical Sciences/Peking Union Medical College, Beijing, China
Contribution: Investigation, Project administration, Writing - review & editing, Supervision
Search for more papers by this authorYingying Su
State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, Fujian Province, China
National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Innovation Platform for Industry-Education Integration in Vaccine Research, NMPA Key Laboratory for Research and Evaluation of Infectious Disease Diagnostic Technology, Xiamen University, Xiamen, Fujian Province, China
Contribution: Writing - review & editing, Validation, Methodology, Funding acquisition, Conceptualization, Supervision, Data curation, Software, Formal analysis
Search for more papers by this authorCorresponding Author
Fanghui Zhao
National Cancer Center, National Center for Cancer Clinical Research, The Cancer Institute, Chinese Academy of Medical Sciences/Peking Union Medical College, Beijing, China
Correspondence
Ting Wu, State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen 361102, Fujian Province, China.
Email: [email protected]
Fanghui Zhao, National Cancer Center, National Center for Cancer Clinical Research, The Cancer Institute, Chinese Academy of Medical Sciences/Peking Union Medical College, Beijing 100730, China.
Email: [email protected]
Contribution: Investigation, Supervision, Project administration, Writing - review & editing, Resources
Search for more papers by this authorCorresponding Author
Ting Wu
State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, Fujian Province, China
National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Innovation Platform for Industry-Education Integration in Vaccine Research, NMPA Key Laboratory for Research and Evaluation of Infectious Disease Diagnostic Technology, Xiamen University, Xiamen, Fujian Province, China
Correspondence
Ting Wu, State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen 361102, Fujian Province, China.
Email: [email protected]
Fanghui Zhao, National Cancer Center, National Center for Cancer Clinical Research, The Cancer Institute, Chinese Academy of Medical Sciences/Peking Union Medical College, Beijing 100730, China.
Email: [email protected]
Contribution: Conceptualization, Investigation, Supervision, Project administration, Writing - review & editing, Funding acquisition, Methodology, Resources, Formal analysis
Search for more papers by this authorYoulin Qiao
National Cancer Center, National Center for Cancer Clinical Research, The Cancer Institute, Chinese Academy of Medical Sciences/Peking Union Medical College, Beijing, China
Contribution: Investigation, Supervision, Project administration, Writing - review & editing
Search for more papers by this authorJun Zhang
State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, Fujian Province, China
National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Innovation Platform for Industry-Education Integration in Vaccine Research, NMPA Key Laboratory for Research and Evaluation of Infectious Disease Diagnostic Technology, Xiamen University, Xiamen, Fujian Province, China
Contribution: Conceptualization, Funding acquisition, Resources, Writing - review & editing
Search for more papers by this authorNingshao Xia
State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, Fujian Province, China
National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Innovation Platform for Industry-Education Integration in Vaccine Research, NMPA Key Laboratory for Research and Evaluation of Infectious Disease Diagnostic Technology, Xiamen University, Xiamen, Fujian Province, China
Contribution: Conceptualization, Funding acquisition, Resources, Writing - review & editing
Search for more papers by this authorQi Chen
State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, Fujian Province, China
National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Innovation Platform for Industry-Education Integration in Vaccine Research, NMPA Key Laboratory for Research and Evaluation of Infectious Disease Diagnostic Technology, Xiamen University, Xiamen, Fujian Province, China
Contribution: Data curation, Investigation, Visualization, Funding acquisition, Writing - original draft, Software, Formal analysis, Validation
Search for more papers by this authorXingmei Yao
State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, Fujian Province, China
National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Innovation Platform for Industry-Education Integration in Vaccine Research, NMPA Key Laboratory for Research and Evaluation of Infectious Disease Diagnostic Technology, Xiamen University, Xiamen, Fujian Province, China
Contribution: Data curation, Software, Investigation, Visualization, Writing - review & editing, Formal analysis, Validation
Search for more papers by this authorJiali Quan
State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, Fujian Province, China
National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Innovation Platform for Industry-Education Integration in Vaccine Research, NMPA Key Laboratory for Research and Evaluation of Infectious Disease Diagnostic Technology, Xiamen University, Xiamen, Fujian Province, China
Contribution: Investigation, Writing - review & editing
Search for more papers by this authorXinhua Jia
National Cancer Center, National Center for Cancer Clinical Research, The Cancer Institute, Chinese Academy of Medical Sciences/Peking Union Medical College, Beijing, China
Contribution: Investigation, Writing - review & editing
Search for more papers by this authorYufei Li
State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, Fujian Province, China
National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Innovation Platform for Industry-Education Integration in Vaccine Research, NMPA Key Laboratory for Research and Evaluation of Infectious Disease Diagnostic Technology, Xiamen University, Xiamen, Fujian Province, China
Contribution: Investigation, Writing - review & editing
Search for more papers by this authorKongxin Zhu
State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, Fujian Province, China
National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Innovation Platform for Industry-Education Integration in Vaccine Research, NMPA Key Laboratory for Research and Evaluation of Infectious Disease Diagnostic Technology, Xiamen University, Xiamen, Fujian Province, China
Contribution: Investigation, Writing - review & editing
Search for more papers by this authorXiaowen Hu
State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, Fujian Province, China
National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Innovation Platform for Industry-Education Integration in Vaccine Research, NMPA Key Laboratory for Research and Evaluation of Infectious Disease Diagnostic Technology, Xiamen University, Xiamen, Fujian Province, China
Contribution: Investigation, Writing - review & editing
Search for more papers by this authorXingcheng Huang
State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, Fujian Province, China
National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Innovation Platform for Industry-Education Integration in Vaccine Research, NMPA Key Laboratory for Research and Evaluation of Infectious Disease Diagnostic Technology, Xiamen University, Xiamen, Fujian Province, China
Contribution: Investigation, Writing - review & editing
Search for more papers by this authorGuohua Zhong
State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, Fujian Province, China
National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Innovation Platform for Industry-Education Integration in Vaccine Research, NMPA Key Laboratory for Research and Evaluation of Infectious Disease Diagnostic Technology, Xiamen University, Xiamen, Fujian Province, China
Contribution: Investigation, Writing - review & editing
Search for more papers by this authorLingxian Qiu
State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, Fujian Province, China
National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Innovation Platform for Industry-Education Integration in Vaccine Research, NMPA Key Laboratory for Research and Evaluation of Infectious Disease Diagnostic Technology, Xiamen University, Xiamen, Fujian Province, China
Contribution: Investigation, Writing - review & editing
Search for more papers by this authorZhaofeng Bi
State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, Fujian Province, China
National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Innovation Platform for Industry-Education Integration in Vaccine Research, NMPA Key Laboratory for Research and Evaluation of Infectious Disease Diagnostic Technology, Xiamen University, Xiamen, Fujian Province, China
Contribution: Investigation, Writing - review & editing, Funding acquisition
Search for more papers by this authorMengjun Liao
State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, Fujian Province, China
National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Innovation Platform for Industry-Education Integration in Vaccine Research, NMPA Key Laboratory for Research and Evaluation of Infectious Disease Diagnostic Technology, Xiamen University, Xiamen, Fujian Province, China
Contribution: Investigation, Writing - review & editing
Search for more papers by this authorLu Chen
State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, Fujian Province, China
National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Innovation Platform for Industry-Education Integration in Vaccine Research, NMPA Key Laboratory for Research and Evaluation of Infectious Disease Diagnostic Technology, Xiamen University, Xiamen, Fujian Province, China
Contribution: Investigation, Writing - review & editing
Search for more papers by this authorXuefeng Kuang
State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, Fujian Province, China
National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Innovation Platform for Industry-Education Integration in Vaccine Research, NMPA Key Laboratory for Research and Evaluation of Infectious Disease Diagnostic Technology, Xiamen University, Xiamen, Fujian Province, China
Contribution: Investigation, Writing - review & editing
Search for more papers by this authorZhe Wang
State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, Fujian Province, China
National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Innovation Platform for Industry-Education Integration in Vaccine Research, NMPA Key Laboratory for Research and Evaluation of Infectious Disease Diagnostic Technology, Xiamen University, Xiamen, Fujian Province, China
Contribution: Investigation, Writing - review & editing
Search for more papers by this authorShangying Hu
National Cancer Center, National Center for Cancer Clinical Research, The Cancer Institute, Chinese Academy of Medical Sciences/Peking Union Medical College, Beijing, China
Contribution: Investigation, Writing - review & editing
Search for more papers by this authorChunlan Zhuang
State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, Fujian Province, China
National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Innovation Platform for Industry-Education Integration in Vaccine Research, NMPA Key Laboratory for Research and Evaluation of Infectious Disease Diagnostic Technology, Xiamen University, Xiamen, Fujian Province, China
Contribution: Investigation, Writing - review & editing
Search for more papers by this authorShoujie Huang
State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, Fujian Province, China
National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Innovation Platform for Industry-Education Integration in Vaccine Research, NMPA Key Laboratory for Research and Evaluation of Infectious Disease Diagnostic Technology, Xiamen University, Xiamen, Fujian Province, China
Contribution: Investigation, Writing - review & editing, Project administration, Supervision
Search for more papers by this authorLihui Wei
Department of Obstetrics and Gynecology, Peking University People's Hospital, Beijing, China
Contribution: Investigation, Project administration, Writing - review & editing, Supervision
Search for more papers by this authorWen Chen
National Cancer Center, National Center for Cancer Clinical Research, The Cancer Institute, Chinese Academy of Medical Sciences/Peking Union Medical College, Beijing, China
Contribution: Investigation, Project administration, Writing - review & editing, Supervision
Search for more papers by this authorYingying Su
State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, Fujian Province, China
National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Innovation Platform for Industry-Education Integration in Vaccine Research, NMPA Key Laboratory for Research and Evaluation of Infectious Disease Diagnostic Technology, Xiamen University, Xiamen, Fujian Province, China
Contribution: Writing - review & editing, Validation, Methodology, Funding acquisition, Conceptualization, Supervision, Data curation, Software, Formal analysis
Search for more papers by this authorCorresponding Author
Fanghui Zhao
National Cancer Center, National Center for Cancer Clinical Research, The Cancer Institute, Chinese Academy of Medical Sciences/Peking Union Medical College, Beijing, China
Correspondence
Ting Wu, State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen 361102, Fujian Province, China.
Email: [email protected]
Fanghui Zhao, National Cancer Center, National Center for Cancer Clinical Research, The Cancer Institute, Chinese Academy of Medical Sciences/Peking Union Medical College, Beijing 100730, China.
Email: [email protected]
Contribution: Investigation, Supervision, Project administration, Writing - review & editing, Resources
Search for more papers by this authorCorresponding Author
Ting Wu
State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, Fujian Province, China
National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Innovation Platform for Industry-Education Integration in Vaccine Research, NMPA Key Laboratory for Research and Evaluation of Infectious Disease Diagnostic Technology, Xiamen University, Xiamen, Fujian Province, China
Correspondence
Ting Wu, State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen 361102, Fujian Province, China.
Email: [email protected]
Fanghui Zhao, National Cancer Center, National Center for Cancer Clinical Research, The Cancer Institute, Chinese Academy of Medical Sciences/Peking Union Medical College, Beijing 100730, China.
Email: [email protected]
Contribution: Conceptualization, Investigation, Supervision, Project administration, Writing - review & editing, Funding acquisition, Methodology, Resources, Formal analysis
Search for more papers by this authorYoulin Qiao
National Cancer Center, National Center for Cancer Clinical Research, The Cancer Institute, Chinese Academy of Medical Sciences/Peking Union Medical College, Beijing, China
Contribution: Investigation, Supervision, Project administration, Writing - review & editing
Search for more papers by this authorJun Zhang
State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, Fujian Province, China
National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Innovation Platform for Industry-Education Integration in Vaccine Research, NMPA Key Laboratory for Research and Evaluation of Infectious Disease Diagnostic Technology, Xiamen University, Xiamen, Fujian Province, China
Contribution: Conceptualization, Funding acquisition, Resources, Writing - review & editing
Search for more papers by this authorNingshao Xia
State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, Fujian Province, China
National Institute of Diagnostics and Vaccine Development in Infectious Diseases, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Innovation Platform for Industry-Education Integration in Vaccine Research, NMPA Key Laboratory for Research and Evaluation of Infectious Disease Diagnostic Technology, Xiamen University, Xiamen, Fujian Province, China
Contribution: Conceptualization, Funding acquisition, Resources, Writing - review & editing
Search for more papers by this authorQi Chen and Xingmei Yao contributed equally to this work.
Wen Chen, Yingying Su, Fanghui Zhao, and Ting Wu are joint senior authors who contributed equally.
Abstract
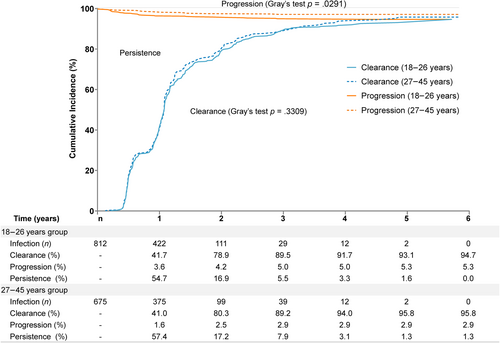
Data investigating the natural history of high-risk human papillomavirus (HR-HPV) infection in mid-adult women compared with young adult women from regions exhibiting a bimodal distribution pattern are scarce. From November 2012 to September 2019, 3681 healthy women aged 18–45 years from the control group of a bivalent HPV vaccine Phase 3 trial in China were followed over 5.5 years. At scheduled visits (Day 0, months 7, 12, 18, 24, 30, 42, 54, and 66), cervical samples were collected for ThinPrep Pap tests and HPV DNA testing, women with abnormal cytology were referred for colposcopy. Data was analyzed using Cox regression model and a competing risk model. Sensitivity analyses were performed among participants attending all scheduled visits. The incidences of HR-HPV persistent infections (over 6 months [6mPIs]) were 35.5 and 29.0 per 1000 person-years (PYs) (hazard ratio [HR] = 1.21, 95% confidence interval [CI]: 1.00, 1.46), and HR-HPV associated CIN grade 2 or greater (CIN2+) were 4.3 and 1.9 per 1000 PYs (HR = 2.31, 95% CI: 1.25, 4.26) in women aged 18–26 and 27–45 years. Competing risk models showed that the cumulative incidence of HR-HPV infections that progressed to CIN2+ was significantly higher in women aged 18–26 than in women aged 27–45 (5.3% vs. 2.9%, Gray's test p = .0291). The cumulative clearance rates of HR-HPV infections in women aged 18–26 and 27–45 were similar (94.7% vs. 95.8%, Gray's test p = .3309) during the study period. In conclusion, although mid-adult women exhibit lower incidences of HR-HPV infection and associated cervical lesions compared to young women, this population continues to face a substantial risk of acquiring causal HPV infections, which may progress to cervical lesion.
What's New?
Data on the natural history of high-risk human papillomavirus infection in middle-aged women compared to younger women in regions with a bimodal prevalence pattern are scarce. Here, the authors found that although women in China aged 27–45 years exhibit lower incidences of high-risk human papillomavirus infection and associated cervical lesions than younger women, this population continues to face a substantial risk of acquiring causal human papillomavirus infections that may progress to cervical lesions. The findings offer insights for shaping human papillomavirus vaccination policies in China and other regions with a second peak in human papillomavirus prevalence after the mid-twenties.
CONFLICT OF INTEREST STATEMENT
All authors have no conflicts of interest to declare.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
| Filename | Description |
|---|---|
| ijc35290-sup-0001-Supinfo.pdfPDF document, 1.3 MB | Data S1. Supporting Information. |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
REFERENCES
- 1de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020; 8: e180-e190.
- 2 HPV Information Center. Human papillomavirus and related diseases in the world. 2023. Accessed June 2024. https://hpvcentre.net/statistics/reports/USA.pdf
- 3Singh D, Vignat J, Lorenzoni V, et al. Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO global cervical cancer elimination initiative. Lancet Glob Health. 2023; 11(2): e197-e206.
- 4Vinodhini K, Shanmughapriya S, Das BC, Natarajaseenivasan K. Prevalence and risk factors of HPV infection among women from various provinces of the world. Arch Gynecol Obstet. 2012; 285: 771-777.
- 5Miura S, Kawana K, Schust DJ, et al. CD1d, a sentinel molecule bridging innate and adaptive immunity, is downregulated by the human papillomavirus (HPV) E5 protein: a possible mechanism for immune evasion by HPV. J Virol. 2010; 84: 11614-11623.
- 6Woodman CBJ, Collins SI, Young LS. The natural history of cervical HPV infection: unresolved issues. Nat Rev Cancer. 2007; 7: 11-22.
- 7 World Health Organization. Human papillomavirus vaccines: WHO position paper. 2022. Accessed June 2024. https://www.who.int/publications/i/item/who-wer9750-645-672
- 8Meites E, Szilagyi PG, Chesson HW, Unger ER, Romero JR, Markowitz LE. Human papillomavirus vaccination for adults: updated recommendations of the advisory committee on immunization practices. Morb Mortal Wkly Rep. 2019; 68: 698-702.
- 9Saslow D, Andrews KS, Manassaram-Baptiste D, Smith RA, Fontham ETH, American Cancer Society Guideline Development Group. Human papillomavirus vaccination 2020 guideline update: American Cancer Society guideline adaptation. CA Cancer J Clin. 2020; 70(4): 274-280.
- 10Zhao FH, Lewkowitz AK, Hu SY, et al. Prevalence of human papillomavirus and cervical intraepithelial neoplasia in China: a pooled analysis of 17 population-based studies. Int J Cancer. 2012; 131(12): 2929-2938.
- 11Demarco M, Hyun N, Carter-Pokras O, et al. A study of type-specific HPV natural history and implications for contemporary cervical cancer screening programs. EClinicalMedicine. 2020; 22:100293.
- 12Dillner J, Rebolj M, Birembaut P, et al. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ. 2008; 337:a1754.
- 13Grant LA, Dunne EF, Chesson H, Markowitz LE. Considerations for human papillomavirus (HPV) vaccination of mid-adult women in the United States. Vaccine. 2011; 29: 2365-2370.
- 14Schiffman M, Glass AG, Wentzensen N, et al. A long-term prospective study of type-specific human papillomavirus infection and risk of cervical neoplasia among 20,000 women in the Portland Kaiser cohort study. Cancer Epidemiol Biomarkers Prev. 2011; 20(7): 1398-1409.
- 15Qiao YL, Wu T, Li RC, et al. Efficacy, safety, and immunogenicity of an Escherichia coli-produced bivalent human papillomavirus vaccine: an interim analysis of a randomized clinical trial. J Natl Cancer Inst. 2020; 112(2): 145-153.
- 16Lin DY, Wei LJ. The robust inference for the cox proportional hazards model. J Am Stat Assoc. 1989; 84: 1074-1078.
- 17Zhao XL, Hu SY, Zhang Q, et al. High-risk human papillomavirus genotype distribution and attribution to cervical cancer and precancerous lesions in a rural Chinese population. J Gynecol Oncol. 2017; 28(4):e30.
- 18Zhao FH, Zhu FC, Chen W, et al. Baseline prevalence and type distribution of human papillomavirus in healthy Chinese women aged 18–25 years enrolled in a clinical trial. Int J Cancer. 2014; 135(11): 2604-2611.
- 19Rodríguez AC, Schiffman M, Herrero R, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst. 2010; 102(5): 315-324.
- 20Schiffman M, Wentzensen N. Human papillomavirus infection and the multistage carcinogenesis of cervical cancer. Cancer Epidemiol Biomarkers Prev. 2013; 22(4): 553-560.
- 21Arbyn M, Weiderpass E, Bruni L, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020; 8(2): e191-e203.
- 22Trottier H, Ferreira S, Thomann P, et al. Human papillomavirus infection and reinfection in adult women: the role of sexual activity and natural immunity. Cancer Res. 2010; 70(21): 8569-8577.
- 23Hu YM, Bi ZF, Zheng Y, et al. Immunogenicity and safety of an Escherichia coli-produced human papillomavirus (types 6/11/16/18/31/33/45/52/58) L1 virus-like-particle vaccine: a phase 2 double-blind, randomized, controlled trial. Sci Bull. 2023; 68(20): 2448-2455.
- 24Zhao FH, Wu T, Hu YM, et al. Efficacy, safety, and immunogenicity of an Escherichia coli-produced human papillomavirus (16 and 18) L1 virus-like-particle vaccine: end-of-study analysis of a phase 3, double-blind, randomised, controlled trial. Lancet Infect Dis. 2022; 22(12): 1756-1768.
- 25Wheeler CM, Skinner SR, Del Rosario-Raymundo MR, et al. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04-adjuvanted vaccine in women older than 25 years: 7-year follow-up of the phase 3, double-blind, randomised controlled VIVIANE study. Lancet Infect Dis. 2016; 16(10): 1154-1168.
- 26Arbyn M, Xu L, Simoens C, Martin-Hirsch PP. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018; 5:CD009069.
- 27Malagón T, MacCosham A, Burchell AN, et al. Proportion of incident genital human papillomavirus detections not attributable to transmission and potentially attributable to latent infections: implications for cervical cancer screening. Clin Infect Dis. 2022; 75(3): 365-371.
- 28Yao X, Chen W, Zhao C, et al. Naturally acquired HPV antibodies against subsequent homotypic infection: a large-scale prospective cohort study. Lancet Reg Health West Pac. 2021; 13:100196.
- 29Schiller JT, Markowitz LE, Kreimer AR, Lowy DR. Plotkin's Vaccines. 8th ed. Elsevier; 2023.
10.1016/B978-0-323-79058-1.00032-3 Google Scholar
- 30Herrero R, Wacholder S, Rodríguez AC, et al. Prevention of persistent human papillomavirus infection by an HPV16/18 vaccine: a community-based randomized clinical trial in Guanacaste, Costa Rica. Cancer Discov. 2011; 1(5): 408-419.
- 31Wei LH, Su YY, Hu YM, et al. Age distribution of human papillomavirus infection and neutralizing antibodies in healthy Chinese women aged 18-45 years enrolled in a clinical trial. Clin Microbiol Infect. 2020; 26(8): 1069-1075.
- 32Castle PE, Schiffman M, Herrero R, et al. A prospective study of age trends in cervical human papillomavirus acquisition and persistence in Guanacaste, Costa Rica. J Infect Dis. 2005; 191(11): 1808-1816.
- 33Goodman MT, Shvetsov YB, McDuffie K, et al. Prevalence, acquisition, and clearance of cervical human papillomavirus infection among women with normal cytology: Hawaii human papillomavirus cohort study. Cancer Res. 2008; 68(21): 8813-8824.
- 34Wei F, Guo M, Huang S, et al. Sex differences in the incidence and clearance of anogenital human papillomavirus infection in Liuzhou, China: an observational cohort study. Clin Infect Dis. 2020; 70(1): 82-89.
- 35Zhang W, Xiao J, Ma C. Clearance of high-risk HPV infection in Chinese women with normal cervical cytology. Int J Gynaecol Obstet. 2012; 118(1): 74-75.
- 36Zhang M, Zhong Y, Wang L, et al. Cervical cancer screening coverage – China, 2018–2019. China CDC Wkly. 2022; 4(48): 1077-1082.
- 37Samoff E, Koumans EH, Markowitz LE, et al. Association of chlamydia trachomatis with persistence of high-risk types of human papillomavirus in a cohort of female adolescents. Am J Epidemiol. 2005; 162(7): 668-675.
- 38Jaisamrarn U, Castellsagué X, Garland SM, et al. Natural history of progression of HPV infection to cervical lesion or clearance: analysis of the control arm of the large, randomised PATRICIA study. PLoS One. 2013; 8(11):e79260.
- 39Rowhani-Rahbar A, Hawes SE, Sow PS, et al. The impact of HIV status and type on the clearance of human papillomavirus infection among Senegalese women. J Infect Dis. 2007; 196(6): 887-894.