Efficacy and safety of everolimus plus exemestane in patients with hormone receptor-positive, HER-2-negative advanced breast cancer: Results from the open-label, multicentre, non-interventional BRAWO study
Abstract
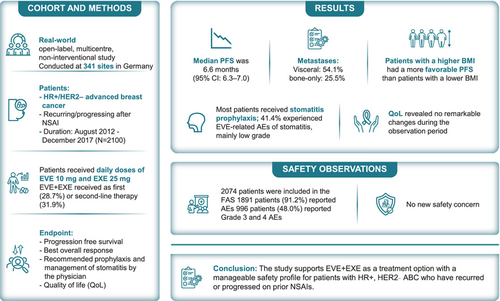
BRAWO, a real-world study, assessed the efficacy, quality of life (QoL) and safety of EVE + EXE in postmenopausal women with HR+/HER2– advanced breast cancer (ABC) in routine clinical practice. Postmenopausal women with HR+/HER2-ABC with recurrence or progression after a NSAI were included. Primary Observation parameters included the evaluation of the effectiveness of EVE + EXE. A multivariate-analysis using Cox proportional hazard model was built to identify predictors of progression. Overall, 2100 patients were enrolled (August 2012–December 2017); 2074 were evaluable for efficacy and safety analyses. Majority of patients (60.6%) received EVE + EXE as first (28.7%) or second-line (31.9%) therapy. Visceral metastases were present in 54.1% patients. Median progression-free survival (mPFS) reported as 6.6 months (95%CI: 6.3–7.0). Multivariate-analysis in a subset of patients (n = 1837) found higher body mass index (BMI) and non-visceral metastases to be independent predictors of favorable PFS. Patients with a BMI of 20 to <25 had a mPFS of 6.0 (95%CI: 5.4–6.4) and those with a BMI ≥30 had mPFS of 8.5 (95%CI: 6.9–9.9). 41.2% patients achieved stable disease and 7.3% partial response. No major changes were observed QoL; 86.4% patients received stomatitis prophylaxis and 41.4% experienced EVE related AEs of stomatitis, mainly low grade. AEs occurred in 91.2% of patients, of which stomatitis (42.6%) and fatigue (19.8%) were most frequent. The BRAWO study provides real-world evidence of efficacy and safety of EVE + EXE in patients with HR+, HER2− ABC. A high BMI and the absence of visceral metastases were independent predictors of PFS in this cohort of patients.
Graphical Abstract
What's new?
The non-interventional BRAWO study assessed efficacy and safety of everolimus and exemestane (EVE + EXE) for patients with HR+, HER2-advanced breast cancer. The study also assessed patients' quality of life during the treatment. BRAWO assessed the combination treatment under real-world conditions using evidence from a clinical practice in Germany. They found a median progression-free survival of 6.6 months, which broadly agreed with the findings from earlier clinical trials. EVE + EXE did not significantly affects quality of life. Most common adverse events were stomatitis and fatigue; most patients did receive prophylactic treatment against stomatitis. Higher BMI and non-visceral metastases predicted longer progression-free survival.
CONFLICT OF INTEREST STATEMENT
DL has received honoraria for Advisory Board and oral presentations from Amgen, AstraZeneca, Daiichi-Sankyo, Loreal, Onkowissen, Pfizer, Novartis, Roche, Teva, Pierre Fabre, and High5md. TD has received advisory board honoraria from Novartis, Daiichi Sankyo, Lilly, and IOMEDICO. MS reports personal fees, advisory board and lectures from AstraZeneca, BioNTech, Daiichi-Sankyo, Gilead, Lilly, MSD, Novartis, Pantarhei, Pfizer, Pierre Fabre, Roche, and SeaGen. AH reports speaker bureau from Roche, Novartis, Lilly, MSD, AstraZeneca, Daiichi-Sankyo, GSK, Gilead, EXACT Sciences, Menarini stemline, Pfizer; Honoria from Roche, Novartis, Lilly, MSD, AstraZeneca, SeaGen, GSK, EXACT Sciences, Rimeses, Teva, Onkowissen, Gilead, Menarini stemline, Pfizer, Amgen, Pierre Fabre, Daiichi-Sankyo, Asai; Consulting/advisory role, Roche, Novartis, MSD, AstraZeneca, GSK, EXACT Sciences, Rimeses, Teva, Onkowissen, Gilead, Menarini stemline, Pfizer, Amgen, Pierre Fabre, Daiichi-Sankyo; Travel Grant from Roche, Novartis, Lilly, AstraZeneca, GSK, EXACT Sciences, Gilead, Menarini stemline, Pfizer, Daiichi-Sankyo. CJ reports lecturer fee from AstraZeneca, Roche, Novartis, Pfizer, Gilead, EXACT Sciences. PAF reports personal fees from Novartis, grants from Biontech, grants and personal fees from Pfizer, personal fees from Daiichi-Sankyo, Astra Zeneca, Eisai, and Merck Sharp & Dohme, grants from Cepheid, personal fees from Lilly, Pierre Fabre, SeaGen, Roche, Agendia, Sanofi Aventis, Gilead, Mylan, Menarini, Medac, Veracyte, GuardantHealth, during the conduct of the study. HT reports honoraria, consulting fee, expert testimony with Lilly, Novartis, Roche, GSK, Seagen, Pfizer, AstraZeneca, Daiichi-Sankyo, Gilead, EXACT Science. JK is an employee of Novartis. GG is an employee and stock owner of Novartis. Other authors (FS, AS, CU, TF, CM, OS, WB, and FF) have no conflict to disclose.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, upon reasonable request.