Revealing genes associated with cervical cancer in distinct immune cells: A comprehensive Mendelian randomization analysis
Abstract
Human papillomavirus can be contracted by sexually active women. However, only a small proportion of these infections persist and have the potential to progress into cervical cancers, indicating a significant involvement of the immune system in cervical cancer development. Despite this, our understanding of the precise contributions of genes from different immune cell types in cervical cancers remains limited. Therefore, the primary objective of our study was to investigate the potential causal relationships between specific immune cell genes and the development of cervical cancers. By accessing expression quantitative trait loci datasets of 14 distinct immune cell types and genome wide association study of cervical cancers, we employed the summary data-based Mendelian randomization (SMR) along with multi-single nucleotide polymorphism (SNP)-based SMR to identify significant genes associated with cervical cancers. Colocalization analysis was further conducted to explore the shared genetic causality. A total of 10 genes across 11 immune cell types (26 significant gene-trait associations) were found to be associated with cervical cancers after false discovery rate correction. Notably, the ORMDL3, BRK1 and HMGN1 gene expression levels showed significant association with cervical cancer in specific immune cell types, respectively. These associations were supported by strong evidence of colocalization analyses. Our study has identified several genes in different immune cells that were associated with cervical cancer. However, further research is necessary to confirm these findings and provide more comprehensive insights into the association between these gene expressions and cervical cancer risk.
Graphical Abstract
What's new?
Only a small proportion of human papillomavirus infections persist and may progress into cervical cancers, indicating a significant involvement of the immune system in cervical cancer development. However, the precise contributions of genes from different immune cell types to cervical cancers remain poorly understood. Here, by conducting summary data-based Mendelian randomization, the authors identified 10 genes across 11 immune cell types that showed significant associations with cervical cancer after false-discovery rate correction, with ORMDL3, BRK1 and HMGN1 gene expression levels showing notable associations in specific immune cell types.
1 INTRODUCTION
Cervical cancer ranked as the fourth most prevalent cancer in women worldwide, with ~604,000 new cases and 342,000 deaths reported in 2020.1 It was the most frequently diagnosed cancer in some developing countries, primarily located in sub-Saharan Africa, Melanesia, South America and South-Eastern Asia.1 Almost all cervical cancers (99%) are related to genital infection with the human papillomavirus (HPV).2 HPV 16 and 18 types account for almost 50% of high-grade cervical cancer.2 More than 70% of sexually active individuals may contract HPV at some point in their lives, and some may experience multiple infections.3 In the majority of cases, the infection typically resolves spontaneously within a short period, whereas only a small percentage of infections with specific types of HPV can persist and lead to cervical cancer,4 suggesting that the immune system plays a vital role in cervical cancers.5 A meta-analysis across Africa, Asia, Europe and North America6 showed that women living with HIV were six times more likely to develop cervical cancers compared to women without HIV, which suggested that variances in immune system function may account for why certain individuals who contract HPV go on to develop cancers, whereas others do not.
The immune system is a complex network comprising various organs, cells and proteins that work together to protect the body against bacteria and viruses. Different types of immune cells have distinct roles in mounting an effective immune response.7 Previous studies have revealed that the immune cell infiltration was significantly different in cervical cancer compared to normal cervix uteri samples.8, 9 However, the precise gene expression in specific immune cells that impact the development of cervical cancer has not yet been fully elucidated. A recent study utilizing single-cell RNA sequencing data identified independent cis-expression quantitative trait loci (eQTLs) in distinct immune cells and suggested cell type-specific effects on gene expression.10 Therefore, it is vital to systematically explore the associations of gene expression in specific immune cells with cervical cancer, which will help us to better understand the underlying pathways or mechanisms involved in the development of cervical cancers. By exploring these associations, we aim to enhance our understanding of cervical cancer and potentially identify new targets for prevention and treatment strategies.
Mendelian randomization (MR) is a natural experiment that is similar to randomized controlled trials and uses genetic variants as instrumental variables to establish causality between exposures and outcomes. This approach can help minimize the effects of potential confounding and reverse association.11 Hence, to better understand whether gene expression in distinct immune cell types could affect cervical cancer, we leveraged a summary data-based Mendelian randomization (SMR) using summary data to identify potential associations.
2 MATERIALS AND METHODS
This MR study evaluated the potential causality of immune cell type-specific gene expression on cervical cancer using eQTL data from Yazar et al.10 and genome-wide association studies (GWASs) from the FinnGen consortium.12
2.1 Data sources for exposure
Summary statistics of cis-eQTL studies revealed the effects of genetic variants on immune cell type-specific genes expression. The eQTL data were retrieved from the article by Yazar et al.,10 which utilized single-cell RNA sequencing data obtained from 1.27 million peripheral blood mononuclear cells collected from 982 donors in the OneK1K cohort to classify 14 immune cell types and map the genetic effects on gene expression. The selection of genetic variants was based on the OneK1K eQTLs across the 14 cell types including CD4+ naive and central memory T cells (CD4NC), CD4+ T cells with an effector memory or central memory phenotype (CD4ET), CD4+ T cells expressing SOX4 (CD4SOX4), CD8+ T cells with an effector memory phenotype (CD8ET), CD8+ naive and central memory T cells (CD8NC), CD8+ T cells with expression of S100B (CD8S100B), natural killer cell (NK), NK recruiting cells (NKR), plasma cells, memory B cells (BMem), immature and naive B cells (Bin), classical monocytes (MonoC), nonclassical monocytes (MonoNC) and dendritic cell (DC).
2.2 The selection of genetic instruments
The instrumental variable was chosen from the cis-region of a gene that exhibited the highest correlation with the expression of the gene. Only genes that had at least one cis-eQTL having PeQTL <5.0 × 10−8 were considered for analysis. Besides, we also excluded SNPs with an allele frequency difference >0.2 between the GWAS summary data and eQTL summary data. Finally, the top-associated SNP with gene expression was selected as the genetic instrument. The genetic instruments were selected using SMR software (version 1.03, https://yanglab.westlake.edu.cn/software/smr/#Download).
2.3 Data sources for outcomes
We obtained publicly available GWAS for cervical cancer with 2913 cases and 149,394 controls in women. The identification of cervical cancers was based on the International Classification of Diseases (ICD-10) code of C53. The controls were cancer-free individuals. The GWAS was obtained from FinnGen consortium (https://r8.finngen.fi/). The FinnGen cohort is a collaboration between public and private partners that combines genetic data from nine Finnish biobanks with digital health record data from Finnish Health Registries.12
3 STATISTICAL ANALYSES
3.1 Summary data-based Mendelian randomization and heterogeneity in dependent instrument test
SMR is an approach integrating summary data from cis-eQTL study and GWAS to infer causality between gene expression and outcomes. SMR command line was used to automatically identify effect alleles and other alleles to align eQTL statistics with GWAS data. SMR analysis was first performed to calculate effect values (β) and standard errors of cervical cancers associated with cell type-specific gene expression.13 The effect size estimate from SMR analysis represented the effects for cervical cancer per one standard deviation increase in gene expression. The heterogeneity in dependent instruments (HEIDI) test was leveraged to distinguish pleiotropy from linkage (i.e., the observed significant association may be attributed to the presence of two variants in strong linkage disequilibrium [LD] with each other, rather than being driven by a single causal variant). To perform the HEIDI test, SMR excluded SNPs that exhibited either strong LD (r2 > .9) or weak LD (r2 < .05), and conducted the test only if the number of SNPs was ≥3.13 Associations of PHEIDI < .05 meant the existence of pleiotropy and hence were excluded. The exclusion of SNPs and the HEIDI test were conducted by using “smr” command in SMR software.
3.2 Assess F-statistic
The strength of the genetic instruments was calculated using the mean F-statistic described by Bowden et al.14 (F-statistic = β2/SE2). If the F-statistic of the genetic variable was >10, it indicated that the weak instrument bias was minimized.
3.3 Enrichment analysis
To better understand the causal mechanisms linking gene expression with cervical cancer, we conducted gene function annotation for identified genes. The metascape website (https://metascape.org/gp/index.html#/main/step1)15 was used for pathway enrichment analysis. The corpus was from KEGG, GO, Reactome Gene Sets, Canonical Pathways, CORUM, WikiPathways and PANTHER Pathway. A P < .05 was defined as significant enrichment.
3.4 Multi-SNP-based SMR
Given that more than one SNP may be involved in regulating the expression of a gene, using a single eQTL as the proxy could result in biased findings and an increased risk of false positives. Furthermore, relying solely on a single variant does not allow for the differentiation between associations that arise from causality and those that are a result of horizontal pleiotropy.16 Therefore, we leveraged multi-SNP-based SMR through the command “smr-multi” in SMR software to improve the statistical power and validate the results. We selected all SNPs with P < 5.0 × 10−8 and removed the SNPs in high LD with the top associated SNP (r2 > .9) as genetic instruments to perform multi-SNP-based SMR analysis. Some genes have only one SNP that satisfied the above criteria, which was not included in the multi-SNP-based SMR. Because the output results of multi-SNP-based SMR had no effect size, the estimation of effect size was done based on the inverse variance weighted method using TwoSampleMR package (version 0.5.6) in R (version 4.1.2).
3.5 Colocalization analysis
To assess confounding by LD, we applied a Bayesian colocalization method17 through the “coloc” (version, 5.1.0.1) R package in R (version, 4.1.2) to test the posterior probability that the genetic instrument of gene expression was shared with the outcome. For each significant gene (PSMR < .05 and PHEIDI > .05) in different cell types, we analyzed all SNPs with minor allele frequency >0.01 within 1 MB of the significant genes. The default prior probabilities (PP.H1 = 1 × 10−04, PP.H2 = 1 × 10−04, PP.H3 = 1 × 10−05) were used for analysis. If the posterior probability for the colocalization hypothesis is above 80%, it indicates that gene expressions and outcomes are highly likely to be colocalized within the test region.
3.6 Evaluate horizontal pleiotropy
A single genetic variant may be related to more than one gene expression, which could result in SNP being associated with outcomes via other genes expression. Specifically, for each instrumental variable we extracted the associations with all other nearby genes (within a 2 MB window) to detect whether there were significant correlations. If genetic variants were related to other genes, SMR analysis was performed to determine whether the expression of other genes was related to outcomes.
False discovery rate (FDR) correction was applied across all immune cell types. Suggestive evidence was defined as P < .05 and FDR >0.05. Strong evidence was defined as FDR < 0.05. Data were analyzed using R v4.1.2 and SMR v1.03 (https://yanglab.westlake.edu.cn/software/smr/#Download).
4 RESULTS
4.1 SMR and HEIDI tests
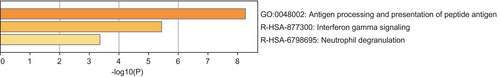
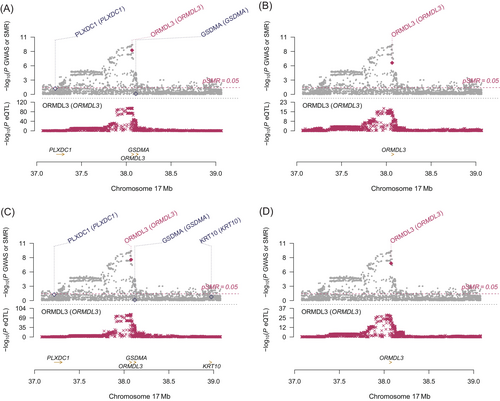
Using a threshold of PSMR < .05 and PHEIDI > .05, we found a total of 186 genes associated with cervical cancer. The majority of these associations were found on CD4NC, CD8ET, NKR, CD8NC and CD4ET cells (Tables S1–S14 and Figure S1). Table S15 presents significant genes related to cervical cancer in each specific cell type. After FDR correction, a total of 10 genes across 11 immune cell types (26 significant gene-trait associations) were identified. Specifically, HLA-B, MRPL21, MICA and IGHMBP2 in CD4NC; HLA-B, ORMDL3 and MRPL21 in CD4ET; ORMDL3 and MRPL21 in CD8ET; HLA-B and MRPL21 in CD8NC; HLA-B and ORMDL3 in CD8S100B; HLA-B and HLA-C in NK; ORMDL3 and MRPL21 in NKR; ORMDL3, HLA-DQB2 and MRPL21 in BMem; ORMDL3, C6orf48 and MRPL21 in Bin; MRPL21 in MonoC; HMGN1 and BRK1 in MonoNC were significantly associated with cervical cancer (Figures 1 and 2, plotted by using the website: https://www.bioinformatics.com.cn/plot_basic_Manhattan_Plot_by_CMplot_110_en). Significantly, ORMDL3 and MRPL21 emerged as the genes strongly associated with cervical cancer in multiple immune cell types, such as in CD4NC (MRPL21: β = 6.28, SE = 1.43, PFDR = 0.004), CD4ET (ORMDL3: β = 8.88, SE = 1.79, PFDR = 1.92 × 10−4; MRPL21: β = 6.64, SE = 1.76, PFDR = 0.03), CD8ET (ORMDL3: β = 4.73, SE = 0.80, PFDR = 3.52 × 10−6; MRPL21: β = 10.35, SE = 2.67, PFDR = 1.04 × 10−4), CD8NC (MRPL21: β = 6.89, SE = 1.65, PFDR = 0.01), CD8S100B (ORMDL3: β = 5.42, SE = 1.10, PFDR = 1.17 × 10−4), NKR (ORMDL3: β = 4.91, SE = 0.87, PFDR = 9.59 × 10−6; MRPL21: β = 10.39, SE = 2.76, PFDR = 0.047), BMem (ORMDL3: β = 5.79, SE = 1.07, PFDR = 9.13 × 10−6; MRPL21: β = 6.75, SE = 1.85, PFDR = 0.02), Bin cells (ORMDL3: β = 6.81, SE = 1.25, PFDR = 1.39 × 10−5; MRPL21: β = 8.61, SE = 2.40, PFDR = 0.04) and MonoC (MRPL21: β = 4.16, SE = 1.19, PFDR = 0.03). These findings underscore the pivotal role of ORMDL3 and MRPL21 in the development and progression of cervical cancer across specific immune cell types. The enrichment pathway analysis showed that the 10 genes associated with cervical cancer were possibly involved in antigen processing and presentation of peptide antigen, interferon gamma signaling and neutrophil degranulation (Figure 3).
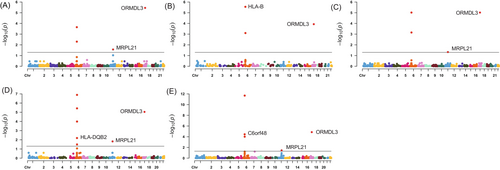
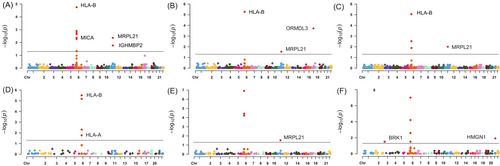
4.2 Sensitivity analyses
The F-statistics of genetic variants ranged from 29.72 to 1957.52, which means that weak instrument bias was excluded (Tables S1–S14). We conducted multi-SNP-based SMR analysis and inverse variance weighted analysis to verify the significant associations in SMR analyses after FDR correction and found the results still were significant for those genes with multiple instruments. For the remaining 14 significant associations, we did not perform multi-SNP-based SMR analyses because there was only one genetic instrument (Table S16).
4.3 Colocalization analysis
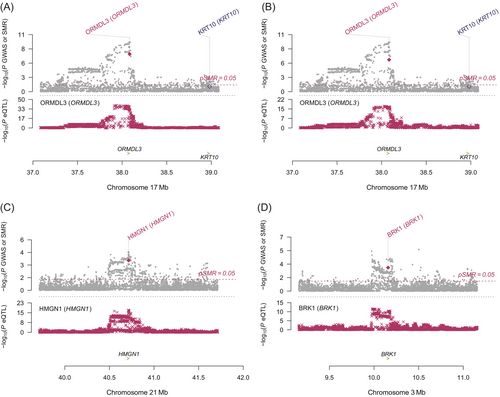
Colocalization analysis suggested a genetic causality of ORMDL3 expression levels in CD4ET (PP.H4 = 0.924), CD8ET (PP.H4 = 0.962), CD8S100B (PP.H4 = 0.916), NKR (PP.H4 = 0.845), BMem (PP.H4 = 0.954) and Bin (PP.H4 = 0.924) in cervical cancers (Table 1). Simultaneously, strong evidence of colocalization between MRPL21 in CD4NC (PP.H4 = 0.923), CD4ET (PP.H4 = 0.909), CD8NC (PP.H4 = 0.921), CD8ET (PP.H4 = 0.918), NKR (PP.H4 = 0.916), Bmem (PP.H4 = 0.916), Bin (PP.H4 = 0.911) and MonoC (PP.H4 = 0.907) and cervical cancers existed. Additionally, the posterior probability that the expression levels of IGHMBP2, HMGN1, GATS and BRK1 in specific cells shared genetic variants with cervical cancer were >0.8.
| Gene | Cell type | β | SE | P-value | FDR | PHEIDI | PP.H4 |
|---|---|---|---|---|---|---|---|
| ORMDL3 | CD8ET | 4.73 | 0.80 | 3.40 × 10−9 | 3.52 × 10−6 | .83 | .962 |
| CD8S100B | 5.42 | 1.10 | 8.63 × 10−7 | 1.17 × 10−4 | .58 | .916 | |
| NKR | 4.91 | 0.87 | 1.42 × 10−8 | 9.59 × 10−6 | .88 | .845 | |
| BMem | 5.79 | 1.07 | 6.88 × 10−8 | 9.13 × 10−6 | .79 | .954 | |
| Bin | 6.81 | 1.25 | 5.39 × 10−8 | 1.39 × 10−5 | .75 | .924 | |
| CD4ET | 8.88 | 1.79 | 6.94 × 10−7 | 1.92 × 10−4 | .10 | .924 | |
| MRPL21 | CD4NC | 6.28 | 1.43 | 1.21 × 10−5 | 4.32 × 10−3 | .36 | .923 |
| CD4ET | 6.64 | 1.76 | 1.58 × 10−4 | 0.029 | .42 | .909 | |
| CD8ET | 10.35 | 2.67 | 1.04 × 10−4 | 0.027 | .23 | .918 | |
| CD8NC | 6.89 | 1.65 | 3.13 × 10−5 | 0.010 | .40 | .921 | |
| NKR | 10.39 | 2.76 | 1.65 × 10−4 | 0.047 | .27 | .916 | |
| BMem | 6.75 | 1.85 | 2.59 × 10−4 | 0.015 | .62 | .916 | |
| Bin | 8.61 | 2.40 | 3.42 × 10−4 | 0.035 | .28 | .911 | |
| MonoC | 4.16 | 1.19 | 4.89 × 10−4 | 0.030 | .25 | .907 | |
| IGHMBP2 | CD4NC | −50.84 | 12.72 | 6.44 × 10−5 | 0.017 | .29 | .921 |
| CD8ET | −44.37 | 12.65 | 4.54 × 10−4 | 0.094 | .10 | .903 | |
| GATS | Bin | 32.05 | 9.59 | 8.34 × 10−4 | 0.061 | .88 | .843 |
| HMGN1 | MonoNC | −1.31 | 0.36 | 2.85 × 10−4 | 0.012 | .86 | .899 |
| BRK1 | MonoNC | 1.82 | 0.55 | 9.00 × 10−4 | 0.031 | .65 | .808 |
- Note: β means the effect of genes on cervical cancer using SMR method.
- Abbreviations: Bin, immature and naive B cells; BMem, memory B cells; CD4ET, CD4+ T cells with an effector memory or central memory phenotype; CD4NC, CD4+ naive and central memory T cells; CD8ET, CD8+ T cells with an effector memory phenotype; CD8NC, CD8+ naive and central memory T cells; CD8S100B, CD8+ T cells with expression of S100B; FDR, false discovery rate; MonoC, classical monocytes; MonoNC, nonclassical monocytes; NKR, natural killer recruiting cells; PHEIDI, the P-value of the heterogeneity in dependent instruments; PP.H4, the posterior probability of hypothesis 4; SE, standard error; SMR, summary data-based Mendelian randomization.
4.4 Evaluation of horizontal pleiotropy
The selected genetic instruments for ORMDL3 expression were also related to adjacent genes (GSDMB, WIPF2, PNMT, STAC2, RP11-94L15.2, RAPGEFL1) at P < .05, including one gene in CD8ET at P < 1.0 × 10−5 (GSDMB; Table S17). Because there were no significant SNPs (P < 5.0 × 10−8) associated with these genes as instrumental variables, SMR analysis could not be conducted. Consequently, we searched diseases and traits associated with these genes by PhenoScanner (http://www.phenoscanner.medschl.cam.ac.uk/) and found no association with cervical cancers,18 suggesting limited evidence for horizontal pleiotropy. The genetic variants as proxy of MRPL21 were associated with the expression levels of IGHMBP2 and IGHMBP2 which also showed association with cervical cancer, therefore we could not fully rule out the existence of pleiotropy. The SNP rs1628077 is instrumental variable of GATS and is adjacent to the gene GPC2. We observed the association between GPC2 expression and the death from cervical cancer in the PhenoScanner website, which indicated that GATS possibly was not the causal gene of outcome. HMGN1 (β = −1.31, SE = 0.36, PFDR = .01) and BRK1 expression (β = 1.82, SE = 0.55, PFDR = .03) in MonoNC were associated with cervical cancer and showed no evidence having pleiotropy.
Based on the evidence from the above comprehensive analysis, we concluded that ORMDL3, HMGN1 and BRK1 genes were causally associated with cervical cancer in specific immune cell types, as shown in Figures 4 and 5.
5 DISCUSSION
In this comprehensive MR study, we aimed to identify genes within distinct immune cell types that are associated with cervical cancer. We assessed a total of 14 immune cell types measured in the blood. Through SMR and multi-SNP-based SMR analyses, we identified 10 genes across 11 immune cell types that showed significant associations with cervical cancer after FDR correction. Notably, ORMDL3 emerged as the gene with the strongest association with cervical cancer in multiple immune cell types. HMGN1 and BRK1 expression in MonoNC were also associated with cervical cancer.
To our best of knowledge, this is the first study to explore the genetic contributions of various genes across 14 immune cell types to cervical cancer. We leveraged the extensive single-cell RNA sequencing data obtained from over 1 million peripheral blood mononuclear cells to investigate the hereditary effects on gene expression within each immune cell type. This analysis encompassed a comprehensive examination of 26,597 independent eQTLs in distinct immune cell types on cervical cancer. It is important to acknowledge that genetic variants influencing gene expression may be specific to particular cell types and absent in others. Therefore, by focusing on distinct immune cell types, we were able to examine gene-trait associations in a cell type-specific manner. To ensure the reliability of our findings, we employed strict statistical standards and emphasized genes that exhibited significant associations after FDR correction. While this approach helps minimize false positive findings, it is crucial to recognize that false negatives cannot be avoided. By considering these methodological considerations and limitations, we aimed to provide a comprehensive and robust analysis of the genetic contributions of immune cell-specific gene expression to cervical cancer. Further research and validation studies are needed to confirm and expand upon our findings.
The ORMDL3 gene is located on chromosome 17 from 39,921,041 to 39,927,601 (https://www.ncbi.nlm.nih.gov/gene/94103). ORMDL3 is an endoplasmic reticulum membrane protein, which plays an important role in several processes, including oxidative stress, inflammation, negative regulation of the B-cell apoptotic process, negative regulation of ceramide biosynthetic process, and sphingolipid metabolism.19, 20 It can promote autophagy in human bronchial epithelium to influence asthma.21-23 Consistent with our findings, a case–control study conducted by Hu et al.24 found the SNPs located at the intron of ORMDL3 might be the causal variants contributing to cervical cancer risk in the Han Chinese population. The specific roles of immune cells may differ depending on the stage of the disease, such as during early tumor formation, metastatic transmission and therapeutic intervention.25 Cytotoxic immune cells such as NK and CD8+ T cells recognized and eliminated more immunogenic cancer cells in the early stages of tumor development.25 During the stage of metastatic dissemination, regulatory T cells, regulatory B cells and immature DC exerted the influence of immune tolerance.25 Therefore, we speculated that ORMDL3 had a significant effect on the early stage of cervical cancer development as its expression was significantly associated with cervical cancer in CD8ET, CD8S100B, NKR, BMem and Bin cells.
HMGN1 stands for high mobility group nucleosome binding domain 1 and plays the vital role in the regulation of histone modification and tumor immunity.26, 27 Previous study indicated that HMGN1 modulated several estrogen-regulated genes by interacting with DNA-binding transcription factors.28 A systemic administration of low doses of HMGN1 and anti-CD4 depleting antibody reversed T-cell exhaustion and resulted in tumor regression and rejection in monitored mice.29 To our knowledge, there has not been any study investigating the association between HMGN1 and cervical cancer, further experiments and studies are highly needed.
BRK1 (BRICK1 subunit of SCAR/WAVE actin nucleating complex) is located at 3p25.3 and involved in Rac protein signal transduction and positive regulation of cellular component organization. Studies on the association between BRK1 and cervical cancer are lacking, but a study has shown that BRK1 was required for cell transformation and proliferation.30
Our study had some particular strengths. First, recognizing the diverse functions of genes within specific immune cell types, we specifically examined the causal role of gene expression in each immune cell type in relation to cervical cancer. This approach will allow for insights into cell-specific mechanisms underlying the disease. Second, to address concerns of horizontal pleiotropy or confounding by LD, we employed various statistical analyses such as the HEIDI test, multi-SNP-based SMR and colocalization analysis. These rigorous methods enhanced the reliability of our findings by accounting for potential biases and supported the validity for the causal associations.
This study does also have certain limitations that should be acknowledged. First, the focus on the European population limits the generalizability of our findings to other populations. Genetic and environmental factors can vary across different ethnicities and geographical regions, thus caution should be exercised when extrapolating these results to diverse populations. Second, the eQTL analysis utilized a sample size of 982 donors, which is relatively small. As a consequence, the standard errors in our study may be relatively large, potentially affecting the precision of our estimates. Larger sample sizes would enhance statistical power and provide more precise estimates of gene expression associated with cervical cancer. It is important to consider these limitations when interpreting the results of our study and to recognize the need for further research involving larger and more diverse populations to validate and extend our findings.
In summary, our study revealed significant associations between specific genes in distinct immune cells and cervical cancer. Notably, we observed associations between high expression levels of ORMDL3 in CD8ET, CD8S100B, NKR, BMem, Bin and CD4ET cells and the risk of cervical cancer. Additionally, HMGN1 and BRK1 expression in MonoNC were also associated with cervical cancer. It is important to note that immune cells can exhibit dual roles in tumor progression, promoting or inhibiting cancer, and it remains unclear regarding the potential roles of these observed genes in cervical cancer. The robustness of these findings was confirmed through sensitivity analyses. Further research is warranted to validate these results in independent cohorts and to elucidate the underlying mechanisms that link ORMDL3, HMGN1 and BRK1 expression to cervical cancer. These findings contribute to our understanding of the potential role of immune cell-specific genes in cervical cancer development and may pave the way for targeted interventions or therapies in the future.
AUTHOR CONTRIBUTIONS
Ning Li: Data curation; formal analysis; methodology; software; visualization; roles/writing—original draft. Huan Yi: Methodology; validation; writing—review and editing. Wen Sun: Methodology; software; validation; writing—review and editing. Jan Sundquist: Writing—review and editing. Kristina Sundquist: Writing—review and editing. Xiaoyu Zhang: Formal analysis; supervision; writing—review and editing. Deqiang Zheng: Conceptualization; funding acquisition; writing—review and editing. Jianguang Ji: Conceptualization; data curation; funding acquisition; writing—review and editing. The work reported in the article has been performed by the authors unless clearly specified in the text.
ACKNOWLEDGMENTS
We acknowledge and greatly appreciate the contributions of Seyhan Yazar et al. for providing the eQTL data used in this study, as well as the FinnGen consortium for making their GWAS data available.
FUNDING INFORMATION
This work was supported by the Beijing Municipal Health System Special Funds of High-Level Medical Personnel Construction (2022-3-042) to Deqiang Zheng, the Swedish Research Council (2021-01187), MAS Cancer and 111 project (no. B21024) to Jianguang Ji and Fujian Research and Training Grants for Young and Middle-aged Leaders in Healthcare; Joint Funds for the Innovation of Science and Technology, Fujian Province (Grant numbers: 2021Y9169 and 2021Y9180); Special Health Subidy of Fujian Provincial Finance Department (Grant number: 210020650502302); Fujian Natural Sciences Foundation (Grant number: 2023J011223); Youth and Middle-aged Training of Fujian Provincial Health Technology Project (Grant number: 2021GGB014) and 111 project (no. B21024). The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
ETHICS STATEMENT
Our analysis used only summary statistics and the written informed consent and approval were obtained by the respective institutional ethical review committees.
Open Research
DATA AVAILABILITY STATEMENT
The GWAS summary statistics of cervical cancer are available through the FinnGen consortium (http://r8.finngen.fi/) with phenotype code “C3_CERVIX_UTERI_EXALLC.” The cis-eQTL data that support the findings of this study are available from the authors, Yazar et al., entitled “Single-cell eQTL mapping identifies cell type-specific genetic control of autoimmune disease.” Further details and other data that support the findings of this study are available from the corresponding author upon request. The code was deposited in Github (https://github.com/CCMULiNing/Immune-Genes-and-Cervical-Cancer).