International Anal Neoplasia Society's consensus guidelines for anal cancer screening
Megan A. Clarke and Ashish A. Deshmukh contributed equally to this study.
Disclaimer: The authors alone are responsible for the views expressed in this paper and they do not necessarily represent the views, decisions, or policies of the institutions with which they are affiliated.
Abstract
The International Anal Neoplasia Society (IANS) developed consensus guidelines to inform anal cancer screening use among various high-risk groups. Anal cancer incidence estimates by age among risk groups provided the basis to identify risk thresholds to recommend screening. Guided by risk thresholds, screening initiation at age 35 years was recommended for men who have sex with men (MSM) and transgender women (TW) with HIV. For other people with HIV and MSM and TW not with HIV, screening initiation at age 45 years was recommended. For solid organ transplant recipients, screening initiation beginning from 10 years post-transplant was recommended. For persons with a history of vulvar precancer or cancer, screening initiation was recommended starting within 1 year of diagnosis of vulvar precancer or cancer. Persons aged ≥45 years with a history of cervical/vaginal HSIL or cancer, perianal warts, persistent (>1 year) cervical HPV16, or autoimmune conditions could be considered for screening with shared decision-making, provided there is adequate capacity to perform diagnostic procedures (high-resolution anoscopy [HRA]). Anal cytology, high-risk (hr) human papillomavirus (HPV) testing (including genotyping for HPV16), and hrHPV-cytology co-testing are different strategies currently used for anal cancer screening that show acceptable performance. Thresholds for referral for HRA or follow-up screening tests are delineated. These recommendations from IANS provide the basis to inform management of abnormal screening results, considering currently available screening tools. These guidelines provide a pivotal foundation to help generate consensus among providers and inform the introduction and implementation of risk-targeted screening for anal cancer prevention.
Graphical Abstract
What's new?
Recently, treatment of anal high-grade squamous intraepithelial lesions (HSIL) was found to reduce anal cancer risk in people living with HIV. However, there are no current comprehensive anal cancer screening guidelines. The International Anal Neoplasia Society (IANS) recommendations provide evidence-based consensus guidance for which populations should be offered anal cancer screening and for management of abnormal screening results, considering currently available screening tools. The IANS guidelines provide a basis for expansion of anal cancer screening infrastructure to all at-risk populations.
1 INTRODUCTION
Almost all squamous cell carcinomas of the anus (hereinafter, anal cancers) are caused by human papillomavirus (HPV) and are preceded by high-grade squamous intraepithelial lesions (HSIL), which are screening-detectable precancerous lesions. Worldwide, nearly 30,500 new anal cancer cases occurred in 2020.1 Although rare among the general population, anal cancer disproportionately affects several specific populations, including people with HIV (PWH), men who have sex with men (MSM), solid organ transplant recipients (SOTR), and women with a history of vulvar cancer or precancer.2 The elevated anal cancer incidence among these and other high-risk groups warrants the development of risk-targeted anal cancer screening recommendations.
Screening guidelines for anal cancer prevention have been developed by several organizations worldwide, largely targeting PWH (see Supporting information, Appendix 1). These guidelines differ with respect to whom to screen, at what age to initiate screening, preferred screening test(s), and how to manage abnormal results. Most of these recommendations were developed before the recently published Anal Cancer/HSIL Outcomes Research (ANCHOR) study addressed a key uncertainty regarding the effectiveness of treatment for anal precancer by showing that treating anal HSIL reduces anal cancer risk among PWH.3 In addition to the ANCHOR study findings, more data on the risk of anal cancer among other groups and the performance of anal cancer screening tests have accumulated over time, providing a critical foundation to generate evidence-based anal cancer screening guidelines.2, 4, 5
The International Anal Neoplasia Society (IANS) is the leading professional organization committed to providing the highest quality evidence, recommendations, and standards of care for the prevention and early detection of anal cancer. To develop consensus guidelines for anal cancer prevention and early detection, IANS convened a task force to assess the needs, develop evidence-based guidance, and address knowledge gaps for anal cancer screening.
2 METHODS
In 2018, IANS assembled a task force (TF) of 17 international experts representing 6 countries with a wide range of professional expertise including epidemiology, decision science, pathology, public policy, infectious diseases, gynecology, colorectal surgery, and high resolution anoscopy (HRA) providers. The IANS TF determined three priority areas for guidelines development which became the basis to convene three working groups that evaluated: (1) anal cancer incidence by risk group to identify populations who would benefit from screening, (2) current clinical practice, including screening tools, HRA and treatment of HSIL, and (3) the performance of available anal cancer screening tests and approaches. The initial findings were presented at the IANS Scientific Meeting in June 2019 and subsequently evolved into three recent publications.2, 4, 6 Based on these results, the IANS TF deliberated in a further series of meetings and online surveys among IANS members to establish (1) which populations to screen based on the evaluation of anal cancer incidence in different groups (2) which screening tools to recommend, and (3) management of results and threshold for HRA referral. To include more diverse representation by discipline, geography, providers of under-represented populations, and community advocates, the task force was expanded to 60 persons representing 19 countries (Supporting information, Appendix 2). The IANS TF assigned recommendation strength (A–E) and quality of evidence (I–III) using the same grading system applied to the US multi-organizational cervical cancer screening and management guidelines where applicable (see Supporting information, Appendix 3).7 The finalized guidelines were sent to the IANS' membership and other stakeholders for a public comment period of 2 weeks in March 2023. These guidelines were then approved by the IANS task force committee members and the IANS Board of Directors.
3 RESULTS
3.1 Populations to screen and timing of screening
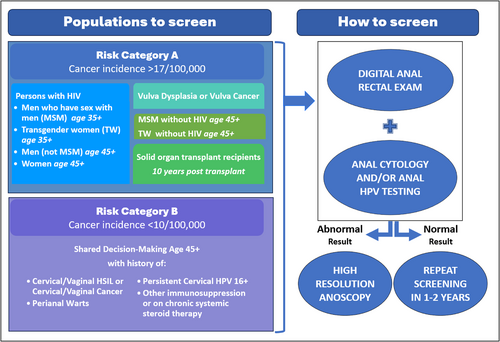
Anal cancer incidence estimates by risk group and age were evaluated to identify populations and age ranges to recommend for screening (Table 1).2, 5 The observed anal cancer incidence estimates from the United States (US) and other countries were categorized into two groups by the TF. Risk Category A included high-risk groups with an incidence of at least 17 per 100,000 (defined as 10-fold greater incidence compared with the general US population—1.7 per 100,000 person-years [py]).8 A second tier (Risk Category B) were those groups with higher incidence compared with the general US population but who did not reach the Category A incidence threshold. The TF developed specific recommendations for Risk Category A groups. Recommendations for Risk Category B are currently limited to shared decision-making, but updates will be considered when more data on screening outcomes, particularly, harms and benefits, in these populations become available.
| Population—Risk category | When | Anal cancer incidence2, 5 per 100,000 person-years |
|---|---|---|
| Risk Category A (incidence ≥ 10-fold compared to the general population) | ||
| MSM and TW with HIV | Age 35 | >70/100,000 age 30–44 >100/100,000 age 45+ |
| Women with HIV | Age 45 | >25/100,00 age 45+ |
| MSW with HIV | Age 45 | >40/100,000 age 45+ |
| MSM and TW not with HIV | Age 45 | >18/100,000 age 45–59 >34/100,000 age 60+ |
| History of vulvar HSIL or cancer | Within 1 year of diagnosis | >40/100,000 |
| Solid organ transplant recipient | 10 years post-transplant | >25/100,000 |
| Risk Category B (incidence up to 10-fold higher compared to the general population) | ||
| Cervical/vaginal cancer | Shared decision age 45a | 9/100,000 |
| Cervical/vaginal HSIL | Shared decision age 45a | 8/100,000 |
| Perianal warts (male or female) | Shared decision age 45a | Unknown |
| Persistent cervical HPV 16 (>1 year) | Shared decision age 45a | Unknown |
| Other immunosuppression (e.g., Rheumatoid arthritis, Lupus, Crohn's, Ulcerative colitis, on systemic steroid therapy) | Shared decision age 45a | 6/100,000 |
| Incidence among the general population: 1.7 per 100,0008 | ||
- Abbreviations: HSIL, high grade squamous intraepithelial lesion; MSM, Men who have sex with men; MSW, Men who have sex with women; TW, Transgender women.
- a Shared decision-making is defined as the process in which a health care provider and patient work together to make a health care decision. The optimal decision considers evidence-based information regarding available options, the provider's knowledge and experience, and the patient's values and preferences.
3.1.1 Risk Category A (incidence ≥10-fold compared to the general population)
Persons with HIV
MSM and transgender women (TW) with HIV (age ≥35 years)
This risk group presents the highest anal cancer incidence, which begins to peak at a relatively younger age (>70 per 100,000 py among persons aged ≥35 years).2 Strong evidence on effectiveness of anal HSIL treatment among PWH aged ≥35 years and initial data suggesting that anal HSIL treatment before age 35 may cause more harm than benefit, supports the recommendation for screening initiation starting at age 35 years.3, 9, 10 While data for TW are currently lacking, analogous clinical recommendations were considered appropriate by the TF.
Men who have sex with women (MSW) and women with HIV (age ≥45 years)
Among MSW and women with HIV, anal cancer incidence increases with age, reaching >10-fold compared with the general population at age ≥45 years,2 supporting the recommendation of screening initiation at age 45 years for this population.
MSM and TW not with HIV (aged ≥45 years)
Among MSM not with HIV, anal cancer incidence peaks at age ≥60 years (34 per 100,000 py) but begins to increase at 45 years (reaching ~10-fold compared with the general population [i.e., ≥18 per 100,000 py]), supporting the recommendation for screening initiation at age 45 years.5 While data for TW are currently lacking, analogous clinical recommendations were considered appropriate by the TF.
History of vulvar cancer or precancer
Anal cancer incidence is high among individuals with a history of HPV-associated vulvar cancer (absolute incidence of 48 per 100,000 py) and those with a history of HPV-associated vulvar precancer (42 per 100,000 py).2 Screening initiation within 1 year after diagnosis of HPV-associated vulvar precancer and cancer was considered appropriate.
SOTR (10-year post-transplantation)
Among SOTR recipients, anal cancer incidence exceeds the risk threshold of ≥10-fold after 10 years from transplantation (incidence of 24.5 per 100,000 py among men and 49.6 per 100,000 py among females),2 supporting the recommendation for screening initiation 10 years post-transplantation. Although it was recognized that the incidence for women with SOTR increases earlier than for men with SOTR, the TF recommended a singular simplified strategy for this risk group.
3.1.2 Risk Category B (incidence less than 10-fold higher than the general population)
Persons with a history of cervical/vaginal HSIL or cancer, perianal warts, persistent (>1 year) cervical HPV16, and autoimmune conditions (including but not limited to rheumatoid arthritis, Systemic Lupus Erythematosus, Crohn's Disease, Ulcerative Colitis on systemic therapy)11 have incidences of anal cancer that are less than 10 per 100,000 but elevated compared with the general population.11, 12 These risk groups (Risk Category B), which did not reach the benchmark (≥10-fold the general population), could be included for screening with shared decision-making, provided there is adequate capacity for HRA. The TF voted not to include women with a history of vulvar warts in risk category B. For additional comments on the screening populations, see Supporting information, Appendix 4a.
3.2 Screening tests for anal HSIL and cancer
Screening tests and management strategies for the detection of anal HSIL are summarized in Tables 2 and 3. Based on findings from the systematic review and meta-analysis of diagnostic studies, conducted primarily among PWH, anal cytology, hrHPV testing (including genotyping for HPV16), and hrHPV-cytology co-testing are all strategies currently used for anal cancer screening and show acceptable performance.4 A positive screening result can be further stratified with triage strategies including sending abnormal cytology specimens for hrHPV testing, or sending hrHPV positive specimens for cytology, though data were sparse for some of these test combinations. Data on longitudinal performance (beyond 2–3 years) of screening were also lacking. We include management options for settings with limited HRA capacity using higher specificity thresholds, ensuring that individuals with the highest risk of anal HSIL are prioritized for immediate referral to HRA. The TF defined sufficient HRA capacity as the availability of HRA evaluation within 6 months of an abnormal screening test for the eligible population; whereas limited capacity indicates a longer wait time until HRA can be provided. These screening strategies are intended for populations with access to HRA. In the absence of HRA availability, screening should be limited to digital anal rectal exam (DARE) for detection of anal cancer.13, 14 For additional comments on screening tools, see Supporting information, Appendix 4b.
| Primary screening test | Triage test | Level of evidence | Special considerations |
|---|---|---|---|
| Cytology | None | BII | Anal cytology is the most widely used and evaluated test for anal cancer screening. Providers may consider using different thresholds for referral to HRA depending on capacity (see Table 3). |
| hrHPV (with or without genotyping) | CII | hrHPV testing to triage ASC-US cytology (or other results, see Table 3) could be used to reduce HRA referral rates. This strategy has not been widely evaluated in the literature. | |
| hrHPV (with or without genotyping) | None | BII | The efficiency of primary testing with a pooled hrHPV test is limited in populations with high HPV prevalence (e.g., MSM with HIV). This strategy could be considered in settings with no cytology infrastructure, or to reduce HRA (for patients testing hrHPV negative) in practices providing HRA on all patients. In most settings, additional triage will be needed for individuals who test hrHPV positive. Use of hrHPV genotyping, specifically for HPV16, may help identify patients with high risk of HSIL or cancer. Performance does not seem to improve with the addition of HPV18.4 hrHPV testing may not be available in many settings. |
| Cytology | CII | Triage of hrHPV-positive results with cytology (e.g., at an ASC-US or worse threshold) can improve specificity of hrHPV-testing and reduce HRA referral. However, observational data on this approach are lacking in the literature. | |
| Cytology/hrHPV co-test (with or without genotyping) | None | BII | Current available data suggest that anal co-testing does not provide any benefit over primary hrHPV testing for anal HSIL. However, anal co-testing may be especially beneficial for its negative predictive value. Co-testing may be less efficient in populations with high hrHPV prevalence. |
| Digital anal rectal exam (DARE) | None | BII | All populations at-risk for anal cancer receive DARE at time of screening tests (or in lieu of screening tests in absence of HRA availability). |
- Abbreviations: ASC-US, atypical squamous cells of undetermined significance; hr, high risk; HRA, high resolution anoscopy; HSIL, high grade squamous intraepithelial lesion; MSM, men who have sex with men.
| Primary screening test | Triage test | Test results | Management | Modification for low HRA capacitya |
|---|---|---|---|---|
| Cytology | None | NILM | Repeat screening 12 months | Repeat 12–24 months |
| ASC-US or worse | HRA referral | ASC-US/LSIL—repeat 12 months HSIL and ASC-H—HRA referral |
||
| hrHPV testing of ASC-US or worse | ASC-US/hrHPV negative | Repeat screening 12 months | Repeat 24 months | |
| LSIL/hrHPV-negative | Provider discretion—either HRA referral or repeat screening in 12 months | Repeat 12 months | ||
| ASC-US or LSIL/hrHPV positive | HRA referral | ASC-US/LSIL/hrHPV positive (non 16)—repeat 12 months hrHPV16 positive (regardless of cytology)—HRA referral |
||
| ASC-H/HSIL (regardless of HPV) | HRA referral | HRA referral |
||
| hrHPV testing [HPV16 genotyping] | None | hrHPV negative | Repeat screening 12–24 months | Repeat 24 months |
| hrHPV positive | HRA referral | hrHPV positive (non16)– repeat 12 months HPV16 positive—HRA referral |
||
| Cytology of hrHPV positive | NILM/hrHPV positive [hrHPV positive (non16)] |
Provider discretion—either HRA referral or repeat screening in 12 months | Repeat 12 months | |
| ASC-US or worse/hrHPV positive [HPV16 positive/regardless of cytology] | HRA referral | ASC-US/LSIL/hrHPV positive (non16)—repeat 12 months HSIL, ASC-H (regardless of hrHPV)—HRA referral hrHPV16 positive (regardless of cytology)—HRA referral |
||
| Cytology/hrHPV co-testing [HPV16 genotyping] | None | NILM/hrHPV negative | Repeat screening 12–24 months | Repeat 24 months |
| ASC-US/hrHPV negative | Repeat screening 12 months | ASCUS/hrHPV negative—repeat 24 months | ||
NILM/hrHPV positive [NILM/hrHPV positive (non16)] |
Provider discretion—either HRA referral or repeat screening in 12 months | Repeat 12 months | ||
| LSIL/hrHPV negative | Provider discretion—either HRA referral or repeat screening in 12 months | Repeat 12–24 months | ||
ASC-US or LSIL/hrHPV positive HSIL, ASC-H (regardless of HPV) [HPV16 positive, regardless of cytology] |
HRA referral | ASC-US/LSIL/hrHPV positive (non16)—repeat 12 months HSIL, ASC-H (regardless of hrHPV)—HRA referral hrHPV16 positive (regardless of cytology)—HRA referral |
- Abbreviations: ASC-H, atypical squamous cells cannot exclude high grade; ASC-US, atypical squamous cells of undetermined significance; hr, high risk; HRA, high resolution anoscopy; HSIL, high grade squamous intraepithelial lesion; LSIL, low grade squamous intraepithelial lesion; NILM, negative for intraepithelial lesion or malignancy.
- a Low HRA capacity is defined as greater than 6 month wait for HRA referral for an abnormal screening test.
3.2.1 Cytology alone or with hrHPV triage
Anal cytology alone is acceptable for anal cancer screening (quality of evidence BII). Immediate HRA referral is recommended for individuals with cytologic diagnosis of atypical squamous cells of undetermined significance (ASC-US) or worse cytology (ASC-US+); repeat cytology screening in 12 months is recommended for individuals with negative for intraepithelial lesions or malignancy (NILM) cytology. It is recommended to repeat unsatisfactory anal cytology.15 In settings with limited HRA capacity, it is acceptable to only refer individuals with high-grade cytology (HSIL) or atypical squamous cells, cannot exclude HSIL (ASC-H) to immediate HRA, with repeat testing in 12 months recommended for individuals with low-grade cytology (LSIL) or ASC-US, and repeat testing in 12–24 months with NILM results.
Triage using hrHPV testing of individuals with ASC-US or LSIL cytology can reduce the need for immediate referral to HRA and is acceptable for anal cancer screening (CII). HPV testing is not necessary for individuals with ASC-H or HSIL cytology. HRA referral is recommended for individuals with ASC-US or LSIL who test positive for hrHPV, and those with ASC-H or HSIL cytology results regardless of HPV results. Repeat screening in 12 months is recommended for individuals with ASC-US who test hrHPV negative. Management of LSIL with hrHPV negative test results is at the discretion of the provider—either HRA referral or repeat screening in 12 months are acceptable options (see Table 3 and/or Supporting information, Appendix 5a–e).
3.2.2 hrHPV alone or with cytology triage
hrHPV alone is acceptable for anal cancer screening (BII). Immediate HRA referral is recommended for individuals testing hrHPV positive; repeat screening within 12–24 months is recommended for individuals testing hrHPV negative. Triage of individuals testing hrHPV positive using cytology can reduce immediate HRA referral and is acceptable for anal cancer screening (CII). Immediate HRA referral is recommended for individuals with hrHPV positive test results with ASC-US+ cytology. Management of individuals testing hrHPV positive with NILM cytology is at the discretion of the provider—either HRA referral or repeat screening in 12 months are acceptable options. If HPV genotyping is provided by the screening test, immediate HRA referral is recommended for individuals testing HPV16 positive, regardless of cytological diagnosis.
In settings with limited HRA capacity, it is acceptable to only refer individuals testing positive for HPV16 to immediate HRA, with repeat testing in 12 months recommended for individuals testing positive for other hrHPV types. Repeat screening in 24 months is recommended for individuals testing hrHPV negative.
3.2.3 Cytology and hrHPV co-testing
Cytology and hrHPV co-testing is acceptable for anal cancer screening (BII). Immediate HRA referral is recommended for (1) individuals with ASC-US+ cytology and hrHPV positive test results (2) ASC-H or HSIL cytology, regardless of hrHPV result and (3) individuals testing HPV16 positive, regardless of cytology. Repeat screening in 12 months is recommended for individuals with ASC-US cytology testing hrHPV negative, and in 12–24 months for those with NILM cytology testing hrHPV negative. Management of NILM cytology with a hrHPV positive result is at the discretion of the provider—either HRA referral or repeat screening in 12 months are acceptable options. Similarly, management of LSIL cytology with a hrHPV negative result is also at the discretion of the provider. In settings with reduced HRA capacity, immediate referral to HRA would be recommended for ASC-H or HSIL cytology, regardless of hrHPV result and individuals testing HPV16 positive, regardless of cytology. Management of other results are outlined in Table 3 and Supporting information, Appendix 5a–e. For management of repeat screening results, see Supporting information, Appendix 6.
3.2.4 Digital anal rectal exam
The DARE should be performed at all screening visits following the collection of samples for cytology and/or hrHPV testing. The DARE provides screening for early anal cancers that may be detectable by palpation. If HRA referral is not available, it is recommended that the DARE be performed routinely in populations identified for anal cancer screening.
4 DISCUSSION
The IANS recommendations provide evidence-based consensus guidance for which populations should be offered anal cancer screening and for management of abnormal screening results, utilizing currently available screening tools. Historically, the controversy regarding anal cancer screening focused on the rarity of anal cancer in the general population and the lack of evidence on the effectiveness of treatment for anal precursor lesions to prevent progression to anal cancer. The ANCHOR study confirmed that the detection and treatment of HSIL in PWH is effective for anal cancer prevention.3 While the low incidence of anal cancer does not support screening in the general population, several recent studies defined other specific populations at elevated risk and assessed the performance of various screening tests, providing the necessary foundation to generate this evidence- and risk-based screening guidance.2, 4, 5, 11
These guidelines primarily focus on populations with an anal cancer incidence ≥10-fold compared with the general population. These recommendations also provide guidance for other populations with increased incidence of anal cancer but less than a 10-fold increased incidence (Risk Category B, e.g., women with a history of cervical precancer or cancer or patients with auto-immune diseases). For these populations, given their relatively lower risk, the TF recommended shared decision-making as an approach for individuals concerned about their potential risk, provided there is adequate HRA capacity. Risk category B also included populations with an unknown incidence of anal cancer, for example, perianal warts. Recommendations for these groups were made by consensus expert opinion.
Several screening approaches, including cytology and hrHPV testing with different triage strategies have been evaluated for anal cancer screening in different populations. Currently, there are not enough data on comparative effectiveness or evaluating the harms and benefits of these strategies to recommend a preferred option. Further, longitudinal studies evaluating different screening approaches are lacking, making it difficult to recommend evidence-based intervals for screening and management. For example, most anal cancer screening studies have been cross-sectional and the few prospective studies were limited to 2–3 years follow-up with varying designs and cohorts unlike the robust longitudinal data (e.g., 10 or more year outcomes for cervical cytology and HPV testing results) available for cervical cancer screening.7 This precludes recommending screening intervals beyond the observation periods of 1–3 years. Ultimately, with more data, a risk-based approach with clinical action thresholds can be developed for anal screening similar to the current approach for cervical screening.16, 17 As more long-term clinical follow-up data and evidence from mathematical modeling studies become available, anal cancer prevention guidelines will be updated.
It is critical to emphasize the implementation aspects of anal cancer screening programs. An effective screening program would rely on initial screening with DARE and anal swab collection (for cytology and/or hrHPV) provided by primary care providers, gynecologists, HIV providers, or other specialists who interface with at-risk populations. Cytopathologists with expertise in cervical pathology should be able to accommodate the increase in anal cytology and biopsy specimen volume. Screening providers should have access to HRA clinics and adequate follow-up to ensure patients' successful participation in their care. The impact of screening on preventing anal cancers will be largely dependent on achieving high compliance with screening recommendations, follow-up treatment, and surveillance.18
Differences in the organization of national health care systems, economic resources and cultural barriers pose particularly difficult challenges to standardize screening practices for the prevention of anal cancer worldwide. Screening strategies implemented in regions lacking resources or infrastructure should be tailored to ensure that those at the highest risk of anal cancer receive the immediate and the best available approach while developing strategies to improve access for others.
Even among high resource settings, the major challenge to the implementation of anal cancer screening guidelines is the limited availability of HRA infrastructure for referral of patients with abnormal screening results. Currently, most HRA providers are located in large cities in the United States,19 Western Europe and Australia; and much of the world has very limited (if any) access to HRA providers. HRA requires significant training and costly equipment.14 Advocacy for sufficient funding and capacity-building is critical for clinics to support the necessary infrastructure for creating or expanding HRA practices. In particular, equitable distribution of screening resources targeted toward the highest risk groups will be important to prevent disparities in anal cancer prevention.
We recognize that knowledge gaps exist regarding the harms and benefits of different screening algorithms (e.g., age to initiate/terminate screening, different screening intervals), the tradeoff between disease detection and resource utilization (i.e., HRA capacity), and the impact of incorporating emerging technologies in screening. We also recognize that the impact of screening may differ by HPV vaccination status. In addition, robust data on the clinical performance of screening strategies among risk groups other than those with HIV are lacking. Despite these limitations, recent advances in anal cancer screening and epidemiology research have allowed us to generate current evidence- and consensus-based recommendations. Future studies addressing these questions will be important to provide continued guidance on optimal approaches for anal cancer screening and prevention. Likewise, guidelines for management following treatment for anal HSIL or cancer also need to be developed but were considered beyond the purview of these screening guidelines. IANS is committed to these goals and will continue to review emerging evidence necessary to provide further updates to these anal cancer prevention guidelines.
These consensus recommendations provide a pivotal framework to inform evidence-based anal cancer screening practices for several high-risk populations. Prevention of anal cancer through screening and treatment of anal HSIL has been proven to be effective among high-risk individuals. Of note, in many regions and countries, scarcity of screening and HRA resources may preclude implementation of these guidelines and there will be a need to develop the necessary infrastructure to provide screening and HRA services. In addition, as studies mature, longitudinal data regarding harms and benefits of screening will provide the basis for risk-based screening and management recommendations. These guidelines serve as a foundation for advocacy and expansion of HRA and screening infrastructure, thereby ensuring that anal cancer prevention becomes accessible to all at-risk populations.
AUTHOR CONTRIBUTIONS
Conceptualization: Jay, Stier, Clifford, Palefsky, Goldstone, Hillman, Barroso. Methodology: Jay, Stier, Clarke, Deshmukh, Wentzensen, Palefsky. Formal analysis: Stier, Jay, Clarke, Deshmukh, Wentzensen, Clifford, Palefsky. Visualization: Jay, Stier, Clifford, Palefsky, Goldstone, Hillman. Writing (original draft): Stier, Jay, Clarke, Deshmukh. Writing (Review and editing): Stier, Clarke, Deshmukh, Wentzensen, Liu, Poynten, Cavallari, Fink, Cuming, Rosa-Cunha, La Rosa, Plotzker, Roberts, Jay. The work reported in the paper has been performed by the authors, unless clearly specified in the text.
CONFLICT OF INTEREST STATEMENT
AAD is a consultant for Merck Inc. and Value Analytics Lab and received an honorarium from NIH. SEG is an investigator and consultant for Merck, consultant for THD America, and investigator for Frantz Viral Technologies. VF received honoraria from MSD. LLR is a consultant for MSD. JMP reports potential personal conflicts of interest with Vir Biotechnologies, Roche Diagnostics, Merck and Abbott and reports potential financial conflicts of interest with Sotlight Pharmaceuticals, Atila Biosystems, and Barinthus Biotherapeutics. The other authors declare no conflict of interest.