Reproduction patterns among classical Hodgkin lymphoma survivors treated with BEACOPP and ABVD in Sweden, Denmark and Norway—A population-based matched cohort study
Abstract
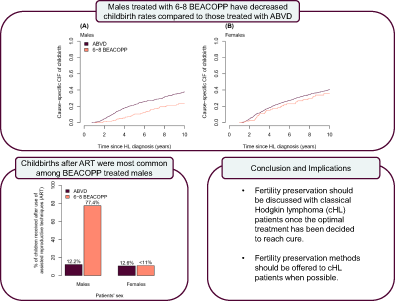
Childbirth rates in classical Hodgkin lymphoma (cHL) survivors have historically been reduced compared to the general population. Understanding if contemporary treatment protocols are associated with reduced fertility is crucial as treatment guidelines shift toward more liberal use of intensive chemotherapy. We identified 2834 individuals aged 18-40 years with cHL in Swedish and Danish lymphoma registers, and in the clinical database at Oslo University Hospital diagnosed 1995-2018, who were linked to national medical birth registers. Cox regression adjusted for stage, performance status, year, and age at diagnosis was used to estimate hazard ratios (HRs) and 95% confidence intervals (CI) contrasting time to first childbirth by treatment groups (ABVD, 2-4 BEACOPP, 6-8 BEACOPP) up to 10 years after diagnosis. Overall, 74.8% of patients were treated with ABVD, 3.1% with 2-4 BEACOPP and 11.2% with 6-8 BEACOPP. Adjusted HRs comparing childbirth rates in individuals treated with 6-8 BEACOPP, and 2-4 BEACOPP to ABVD were 0.53 (CI: 0.36-0.77) and 0.33 (CI: 0.12-0.91) for males, and 0.91 (CI: 0.61-1.34) and 0.38 (CI: 0.12-1.21) for females. Cumulative incidence of childbirths after 10 years was 19.8% (CI: 14.5%-27.0%) for males and 34.3% (CI: 25.8%-45.6%) for females treated with 6-8 BEACOPP. Proportions of children born after assisted reproductive technique (ART) treatments were 77.4% (CI: 60.2-88.6%) for males following 6-8 BEACOPP, and <11% for females. Among ABVD treated patients the corresponding proportions were 12.2% (CI: 8.5%-17.3%) and 10.6% (CI: 7.4%-14.9%). BEACOPP treatment is associated with decreased childbirth rates compared to ABVD in male, but not female, cHL patients, despite widespread access to ART in the Nordics.
Graphical Abstract
What's new?
While intensive chemotherapy with the combination regimen BEACOPP is increasingly used as first-line treatment for classical Hodgkin lymphoma (cHL), its impact on fertility among cHL survivors remains unclear. In this study, the authors compared childbirth rates among Scandinavian cHL patients treated with either ABVD, 2-4 BEACOPP cycles or 6-8 BEACOPP cycles. Whereas childbirth rates were similar for females treated with ABVD or BEACOPP, childbirth was reduced for males who underwent 6-8 BEACOPP cycles. The results highlight the need for timely fertility preservation for cHL survivors, particularly males, in order to alleviate treatment effects on future childbearing.
Abbreviations
-
- ABVD
-
- doxorubicin bleomycin vinblastine and dacarbazine
-
- ART
-
- assisted reproductive technique
-
- BEACOPP
-
- bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine and prednisone
-
- CCI
-
- Charlson comorbidity index
-
- cHL
-
- classical Hodgkin lymphoma
-
- CI
-
- confidence interval
-
- CIF
-
- cumulative incidence function
-
- DFs
-
- degrees of freedom
-
- FPM
-
- flexible parametric model
-
- HL
-
- Hodgkin lymphoma
-
- HR
-
- hazard ratio
-
- ICD-O
-
- International Classification of Disease for Oncology
-
- IVF
-
- in vitro fertilization
-
- LYFO
-
- Danish Lymphoma Register
-
- mDAG
-
- missingness directed acyclic graph
-
- OUH
-
- Oslo University Hospital
-
- SLR
-
- Swedish Lymphoma Register
1 INTRODUCTION
Treatment of advanced-stage classical Hodgkin lymphoma (cHL) has gradually shifted toward the use of more intensive chemotherapy regimens during the past two decades. Standard of care first-line treatment includes ABVD (doxorubicin, bleomycin, vinblastine and dacarbazine), and the more intensive BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine and prednisone) regime. Incorporating BEACOPP into first-line treatment has been demonstrated to improve progression-free survival in patients with advanced and early unfavorable disease, compared to ABVD-based regimens alone.1 The benefit in progression-free survival associated with BEACOPP treatment has increased its popularity as initial treatment for cHL patients.
Reduced fertility and subsequent lower childbirth rates are adverse side effects of the cHL disease and chemotherapy treatments.2, 3 Chemotherapy decreases both spermatogenesis,4-6 and the ovarian reserve in cHL survivors,5, 7, 8 which in turn might affect future fertility. Previous studies have shown conflicting results regarding childbirth rates in cHL survivors with some reporting decreased rates in cHL survivors,6, 9 and others reporting rates similar to those of the general population.3, 10 In spite of these positive findings of the two most recent studies among cHL patients overall, both studies indicated decreased childbirth rates in BEACOPP treated cHL survivors compared to the general population.3, 10 To date, studies that have aimed to contrast childbirth rates after BEACOPP with ABVD have been underpowered to determine the treatment-specific effect on infertility.1 Studies that investigate long-term toxicity, including infertility, after BEACOPP treatment should, however, be a priority given the high survival in patients with contemporary treatment protocols. The chance to have children after completing treatment is particularly important for cHL survivors since the disease affects many individuals at young age, and because impaired fertility and subsequent childlessness are associated with higher levels of emotional distress which can affect individuals throughout their whole life.11, 12
This Scandinavian multicountry study aims to advance the understanding of reproductive patterns among cHL survivors treated with ABVD and BEACOPP in Sweden, Denmark and the south-east region in Norway. Specifically, we report rates of childbirth after treatment completion, cumulative incidence by type of first-line chemotherapy, and use of assisted reproductive technique (ART) treatments.
2 MATERIALS AND METHODS
2.1 Study population
This is a population-based cohort study combining register data from Sweden, Denmark and Norway. In each country, registers were linked using each individual's unique identification number, and subsequently pooled for analyses. HL patients aged 18 to 40 years at diagnosis were identified based on their recorded diagnosis in the Swedish Lymphoma Register (SLR), the Danish Lymphoma Register (LYFO) and the clinical lymphoma database at Oslo University Hospital (OUH) during the years 2000-2018 (Sweden), 2000-2017 (Denmark) and 1995-2017 (Norway) (Table S1). The OUH database has been initiated in 1995 in contrast to the SLR and LYFO that started in 2000. While SLR and LYFO are national registers, the OUH database includes information on lymphoma patients diagnosed at OUH, which is the main referral hospital in the south-east health region in Norway, covering 55% of Norway's population. A total of 3072 HL patients were identified from the SLR (n = 1516), the LYFO (n = 984) and the clinical lymphoma database at OUH (n = 572) (Figure S1). Among these, patients with lymphocyte predominant HL (ICD-O 3ed 9659/3; n = 167), and patients who died, relapsed or had a child within 9 months after their HL diagnosis (start of follow-up) were excluded (n = 71). In a supplementary analysis, we additionally included 22 392 comparators from the general population matched on country, age and sex, who were free of lymphoma and alive at the time of matching. Ten comparators per cHL case were sampled with replacement in Denmark and Sweden whereas five comparators were selected for the Norwegian patients. Comparators who died within 9 months after matching date were excluded (n = 8).
2.2 Definition of first-line therapy
Patients were classified into four chemotherapy treatment groups based on their first-line treatment. Patients who received at least 2 cycles of BEACOPP treatment were categorized as either receiving 2-4 cycles (reflecting newer PET-guided treatment regimens),13-15 or 6-8 cycles of BEACOPP. Patients treated with ABVD only or with a combination that did not include more than 1 cycle of BEACOPP were classified as ABVD treated. BEACOPP treatment could include escBEACOPP or BEACOPP14. Other chemotherapy regimens including for example, AVD, EBVD, LVPP and MOPP were classified as other.
2.3 Definition of childbirth
Childbirths recorded in the Medical Birth Registers 9 months after cHL diagnosis or later contributed to the outcome definition. The registers include all live- and stillbirths from gestational week 12 in Norway and 28 in Denmark and Sweden (week 22 from 2008 onwards).
2.4 Confounders
Information on age at diagnosis (18-25 vs 26-40 years), Ann Arbor stage (I-IIA, IIB-IV, missing), WHO performance status (0, 1-4, missing) and year of diagnosis (1995-2000, 2001-2005, 2006-2010, 2011-2015, 2016-2018) was obtained from the lymphoma registers. Number of children pre-cHL was obtained from the Medical Birth Registers (0, ≥1), and Charlson Comorbidity Index (CCI) was calculated based on in- and outpatient diagnoses (excluding lymphoma diagnoses) from the National Patient Registers 5 years prior to cHL diagnosis date for patients diagnosed in Denmark or Sweden, and categorized as no comorbidity or at least one comorbidity.
2.5 ART treatment
Information on use of ART treatment was obtained for cHL patients with a childbirth after diagnosis using the Danish IVF registers and the Swedish Q-IVF register. Both registers include information on ART treatments, including in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI), conducted in private and public institutions with nationwide coverage since 1994 and 2007, respectively.
2.6 Follow-up time
Follow-up started 9 months after the date of cHL diagnosis (index date) and was accrued until the date of first childbirth, death or administrative censoring, whichever came first. Individuals were administratively censored on December 31st 2019 for Sweden, 2018 for Denmark and 2017 for Norway, or after a maximum follow-up of 10 years. Comparators were followed from 9 months after matching date, that is, diagnosis date, until first childbirth, death or administrative censoring. In a supplementary recurrent event analysis multiple childbirth during follow-up were considered, while otherwise using the censoring definitions described above.16 In a sensitivity analysis we additionally censored females at date of relapse/stem cell transplantation (SCT), given the strong effect of relapse treatment on fertility,17 and the limited possibility of ARTs for females in situations that call for urgent need of anticancer treatment.18
2.7 Statistical analysis
For estimating the total effect of chemotherapy on reproduction in cHL survivors, we identified confounders using a directed acyclic graph (DAG) (Figure S2). Age at diagnosis, performance status, lymphoma stage, calendar year of cHL diagnosis, parity and comorbidity were identified as potential confounders. Adjustment for comorbidity was only possible in cHL patients diagnosed in Sweden and Denmark due to lack of comorbidity data for Norwegian patients.
Cox proportional hazard models were used to estimate hazard rate ratios (HRs) and adjusted HRs (aHRs) of first childbirth comparing cHL survivors treated with 2-4 BEACOPP and 6-8 BEACOPP to those treated with ABVD. Cox models were adjusted for the a priori defined confounders and stratified by age at cHL diagnosis, lymphoma stage, parity, year of diagnosis and country of residence to adjust for nonproportional hazards. The proportional hazards assumption was investigated using the Grambsch-Therneau test.
All models included an interaction term between cHL treatment and sex to allow for effect modification. For the supplementary recurrent event analysis, we fitted an Andersen-Gill model to estimate the total effect of cHL treatment on all childbirths.16, 19
The Aalen-Johansen estimator was used to estimate the nonparametric cause-specific cumulative incidence functions (CIF) of first childbirths in the presence of death as competing event.20 Additionally, flexible parametric survival models (FPMs) were used to obtain model-based standardized cause-specific CIFs.21 The FPMs were fitted using three degrees of freedom (DFs) for the baseline hazard function, and two DFs for time-varying effects of age, stage and parity.
All individuals who were classified as receiving other chemotherapy treatments (n = 80), or with missing information on performance score, lymphoma stage or chemotherapy treatment (n = 298) were excluded from the adjusted and unadjusted time-to-event analysis. The complete case analysis was assumed to yield valid effect estimates for the complete study population based on the missingness directed acyclic graph (mDAG) shown in Figure S3.
A statistical analysis plan was prepublished on the Open Science Framework together with the main analysis programs (https://osf.io/eumy5/). Analyses were carried out using the statistical software package R and Stata.
3 RESULTS
We identified a total of 2834 eligible cHL patients from the SLR (n = 1416), LYFO (n = 892) and the clinical lymphoma database at OUH (n = 526). Among those, 2121 patients (74.8%) were treated with ABVD, 89 patients (3.1%) with 2-4 BEACOPP and 316 patients (11.2%) with 6-8 BEACOPP. ABVD was used as first-line treatment in 82.3%, 76.1% and 71.3% of patients diagnosed in Norway, Denmark and Sweden, respectively. Radiotherapy treatment was given to 24.6% of all advanced stage patients. Among female and male cHL patients 30.5% and 27.4% had at least one childbirth during follow up, respectively (Table 1). Average time from diagnosis to first childbirth were similar in the ABVD (4.3 years), 2-4 BEACOPP (4.4 years) and 6-8 BEACOPP group (5.0 years), respectively.
| By sex | ||||||
|---|---|---|---|---|---|---|
| Overall | Males | Females | ||||
| Characteristic | No. | (%) | No. | (%) | No. | (%) |
| Total | 2834 | (100.0) | 1439 | (100.0) | 1395 | (100.0) |
| Country | ||||||
| Sweden | 1416 | (50.0) | 690 | (47.9) | 726 | (52.0) |
| Denmark | 892 | (31.5) | 467 | (32.5) | 425 | (30.5) |
| Norway | 526 | (18.6) | 282 | (19.6) | 244 | (17.5) |
| Age at diagnosis, years | ||||||
| 18-25 | 1143 | (40.3) | 542 | (37.7) | 601 | (43.1) |
| 26-40 | 1691 | (59.7) | 897 | (62.3) | 794 | (56.9) |
| Lymphoma stages | ||||||
| Stage I-IIA | 1214 | (42.8) | 571 | (39.7) | 643 | (46.1) |
| Stage IIB-IV | 1590 | (56.1) | 850 | (59.1) | 740 | (53.0) |
| Missing | 30 | (1.1) | 18 | (1.3) | 12 | (0.9) |
| Performance status | ||||||
| 0 | 2147 | (75.8) | 1077 | (74.8) | 1070 | (76.7) |
| 1-4 | 613 | (21.6) | 316 | (22.0) | 297 | (21.3) |
| Missing | 74 | (2.6) | 46 | (3.2) | 28 | (2.0) |
| Nulliparous | ||||||
| No | 973 | (34.3) | 444 | (30.9) | 529 | (37.9) |
| Yes | 1861 | (65.7) | 995 | (69.1) | 866 | (62.1) |
| Year of diagnosis | ||||||
| 1995-2000a | 224 | (7.9) | 123 | (8.5) | 101 | (7.2) |
| 2001-2005 | 688 | (24.3) | 343 | (23.8) | 345 | (24.7) |
| 2006-2010 | 722 | (25.5) | 370 | (25.7) | 352 | (25.2) |
| 2011-2015 | 816 | (28.8) | 411 | (28.6) | 405 | (29.0) |
| 2016-2018 | 384 | (13.5) | 192 | (13.3) | 192 | (13.8) |
| Chemotherapy regime | ||||||
| ABVD | 2121 | (74.8) | 1043 | (72.5) | 1078 | (77.3) |
| 2-4 BEACOPP | 89 | (3.1) | 52 | (3.6) | 37 | (2.7) |
| 6-8 BEACOPP | 316 | (11.2) | 198 | (13.8) | 118 | (8.5) |
| Other | 80 | (2.8) | 27 | (1.9) | 53 | (3.8) |
| Missing | 228 | (8.0) | 119 | (8.3) | 109 | (7.8) |
| No. of children born ≥9 months after cHL diagnosis | ||||||
| 0 | 2014 | (71.1) | 1045 | (72.6) | 969 | (69.5) |
| 1 | 498 | (17.6) | 251 | (17.4) | 247 | (17.7) |
| 2 | 272 | (9.6) | 123 | (8.5) | 149 | (10.7) |
| >2 | 50 | (1.8) | 20 | (1.4) | 30 | (2.2) |
- a Patients from Sweden and Denmark are included from 2000 onwards.
Of the 2834 cHL patients, 2456 (49.0% females) were included in the time-to-event analyses. Median follow-up time was 5.8 years (IQR: 3.2-10.0), 3.9 years (IQR: 2.1-8.7) and 6.8 years (IQR: 3.7-10.0) for patients treated with ABVD, 2-4 BEACOPP and 6-8 BEACOPP, respectively. Overall, childbirth rates were higher among females compared to males (Table 2). Patients treated with ABVD had the highest childbirth rates per 1000 person-years among both females (51.8; 95% CI: 46.3-57.6) and males (48.1; 95% CI: 42.8-53.9). The rates in these groups were similar to those among the matched general population comparators (Table S2). The 6-8 BEACOPP treatment group had childbirth rates similar to the ABVD group among females (44.8; 95% CI: 30.6-63.2) but not among males (25.1; 95% CI: 17.4-35.1).
| No. (%) | No. with ≥1 childbirth (%) | Rate of first childbirtha (95% CI) | Crude HR (95% CI) | Adjusted HRb (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Females | ||||||||||
| ABVD | 1048 | (87.1) | 330 | (31.5) | 51.8 | (46.3-57.6) | 1 | (Ref.) | 1 | (Ref.) |
| 2-4 BEACOPP | 37 | (3.1) | ≤5c | (<16.2) | 17.7 | (3.7-51.8) | 0.38 | (0.12-1.17) | 0.38 | (0.12-1.21) |
| 6-8 BEACOPP | 118 | (9.8) | 32 | (27.1) | 44.8 | (30.6-63.2) | 0.87 | (0.60-1.25) | 0.91 | (0.61-1.34) |
| Males | ||||||||||
| ABVD | 1005 | (80.2) | 297 | (29.6) | 48.1 | (42.8-53.9) | 1 | (Ref.) | 1 | (Ref.) |
| 2-4 BEACOPP | 50 | (4.0) | ≤5c | (<12.0) | 14.6 | (4.0-37.5) | 0.31 | (0.12-0.84) | 0.33 | (0.12-0.91) |
| 6-8 BEACOPP | 198 | (15.8) | 34 | (17.2) | 25.1 | (17.4-35.1) | 0.51 | (0.36-0.73) | 0.53 | (0.36-0.77) |
- a Rates per 1000 person-years.
- b Model adjusted for year of cHL diagnosis and performance status, and stratified on age at cHL diagnosis, cHL stage, parity and country of residence.
- c Numbers ≤5 had to be masked to comply with data privacy regulations of Statistics Denmark.
HRs from the adjusted Cox models also showed no difference in reproductive patterns comparing female patients treated with 6-8 BEACOPP to those treated with ABVD (aHR 0.91; 95% CI: 0.61-1.34) (Table 2). Conversely, male patients treated with 6-8 BEACOPP had an estimated 47% lower childbirth rate compared to male patients treated with ABVD (aHR 0.53; 95% CI: 0.36-0.77, P-value for interaction: 0.04). In the 2-4 BEACOPP group, significantly lower childbirth rates were observed among males (aHR 0.33; 95% CI: 0.12-0.91) but not females (aHR 0.38; 95% CI: 0.12-1.21, P-value for interaction: 0.85) compared to those treated with ABVD. There was no evidence against the proportional hazard assumption (Table S4).
Including multiple childbirths into the analysis did not alter the direction or strength of the associations (Table S5). The results were also robust when only including Danish and Swedish data with additional adjustments for education, comorbidity and emigration, and when limiting the analysis to cHL survivors who were nulliparous prior to their diagnosis (Tables S6 and S7).
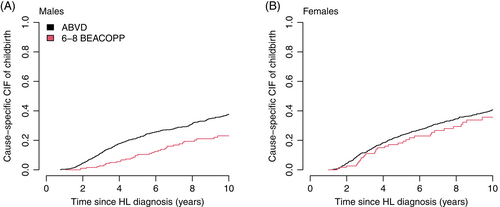
Results were consistent when accounting for competing risks (Table 3). The CIFs also supported a clear difference in the probability of having children after cHL when comparing males treated with 6-8 BEACOPP to ABVD (Figures 1, 2 and S4). After 2 years the standardized CIF of childbirth after 6-8 BEACOPP was 1.7% (CI: 1.1%-2.5%) for males and 3.2% (CI: 2.2%-4.8%) for females. The corresponding 10-year estimates were 19.8% (CI: 14.5%-27.0%) and 34.3% (CI: 25.8%-45.6%) for males and females, respectively. These results were robust when additionally censoring at relapse and date of SCT among female patients (Figure S5). Cause specific CIFs were also similar for patients treated with 2-4 ABVD and 6-8 ABVD among both males and females (Figure S7).
| CIF of childbirth after cHL diagnosis (%) with 95% CI | |||||||
|---|---|---|---|---|---|---|---|
| At 2 years | At 5 years | At 10 years | |||||
| Females | |||||||
| ABVD | 3.7 | (3.1-4.5) | 22.3 | (20.0-24.8) | 38.9 | (35.5-42.7) | |
| 6-8 BEACOPP | 3.2 | (2.2-4.8) | 19.5 | (14.1-26.8) | 34.3 | (25.8-45.6) | |
| Males | |||||||
| ABVD | 3.3 | (2.7-4.0) | 20.2 | (18.0-22.6) | 35.7 | (32.3-39.4) | |
| 6-8 BEACOPP | 1.7 | (1.1-2.5) | 10.6 | (7.6-14.8) | 19.8 | (14.5-27.0) | |
- Note: Average proportions were estimated as the standardized adjusted marginal estimate of the cause-specific CIF using flexible parametric survival models.
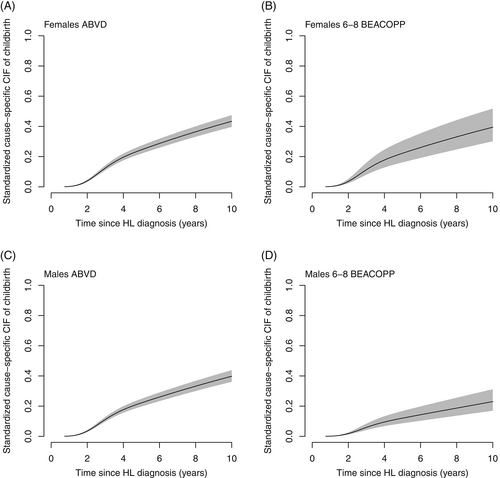
Including the general population comparators further showed that the cause-specific CIFs and HRs in male and female patients treated with ABVD were similar to those estimated for the matched comparators (Figure S6 and Table S3).
The use of ART treatments differed between males and females. Among males treated with 6-8 BEACOPP who had a child after diagnosis, 77.4% (CI: 60.2%-88.6%) utilized ART treatments, whereas <11% of the females in this patient category did. Among children born to males and females treated with ABVD 12.2% (CI: 8.5%-17.3%) and 10.6% (CI: 7.4%-14.9%) were born after ART treatments, respectively (Table S8). Nearly all male patients diagnosed in Sweden that used ART were treated with ICSI.
4 DISCUSSION
In this population-based study utilizing combined data from three Nordic countries, we show differences in reproductive patterns comparing male and female cHL patients treated with ABVD and BEACOPP. Encouragingly, female cHL patients treated with 6-8 BEACOPP had childbirth rates similar to those treated with ABVD. Conversely, male cHL patients treated with 6-8 BEACOPP had lower childbirth rates compared to ABVD treated males. The estimates for patients treated with 2-4 BEACOPP reflecting PET-guided treatments were limited by shorter follow-up, but also indicated decreased reproduction rates among male patients compared to after ABVD treatment. Childbirth after use of ART treatments were more common among male cHL patients compared to females.
The lower childbirth rate among male cHL survivors treated with 6-8 BEACOPP is in line with findings from previous studies.2, 3, 10 We have previously reported a 55% lower childhood rate comparing males treated with 6-8 BEACOPP to those treated with 6-8 ABVD in Denmark, but the study was not able to draw conclusions about specific chemotherapy regimens among females due to limited sample size and short follow-up.3 For males, our present findings are also in line with a supplementary analysis of the AHL2011 trial including 596 cHL patients which reported that patients treated with PET-guided ABVD had a lower risk of azoospermia and a higher likelihood of both recovery from treatment-related azoospermia and achieving pregnancy compared to patients treated with first-line BEACOPP.2 Albeit the authors reported similar patterns for premature ovarian insufficiency among females, they did not observe differences in conception proportions after treatment between females in those two groups.2 Conversely, decreased childbirth rates among BEACOPP treated female cHL patients were indicated in Sweden during the first 3 years after diagnosis for patients treated between 1992 and 2009 although lack of statistical power hampered a formal comparison.10 Results of our supplementary analysis concerning the comparison of cHL patients and the general population comparators were also in line with previous studies from the Nordic countries.3, 10
The observed difference in reproductive patterns among male and female cHL patients treated with ABVD and BEACOPP might be due to a combination of differences in treatment toxicities, access to fertility preservation and reproductive behavior. Reproduction is closely connected to fertility, which might be impaired through gonadotoxic properties of chemotherapy.4, 5, 7, 8 The high proportion of children born after ART treatment among partners of male cHL patients treated with 6-8 BEACOPP highlights the strong effect of intensive cHL treatment on fertility. A possibility to make future reproduction less dependent on posttreatment fertility is undergoing fertility preservation procedures at the time of diagnosis.22 Access to specialized care in oncology and reproduction is granted to all residents in the Nordics through state-financed social security systems. However, fertility preservation has traditionally been more accessible for males given the short time needed to acquire the specimen for cryopreservation.23 For women, banking of embryos and/or oocytes requires about 2 weeks of controlled ovarian stimulation.18 Since 2019, cryopreservation of ovarian tissue has been recognized as an established technique that does not require prior stimulation treatment and is accessible to women with sufficient ovarian reserves prior to anticancer treatment.18 Despite today's guidelines highlighting the importance of timely fertility counseling for patients facing potential infertility risk related to cancer treatment, it is still difficult to evaluate if access differs by gender, and many male patients might still not use the fertility preservation options available to them.23, 24 Nevertheless, the reported high childbirth rate among females treated with 6-8 BEACOPP is likely due to the availability and use of ART treatments. However, reproduction does not only depend on fertility, but also on reproductive behavior, which is likely affected by the impact of treatment intensity on psycho-social wellbeing.25
National treatment guidelines changed during the study period and differ slightly by country. Guidelines in the Nordic countries recommend 2-4 ABVD followed by radiotherapy for early stage cHL according to the HD10 and HD11 trial,26, 27 whereas guidelines in Norway and Denmark changed toward PET-guided additional use of BEACOPP in recent years following the HD14 and the PET-adapted EORTC H10 and HD17 trials.15, 28, 29 For advanced-stage cHL patients, guidelines originally recommended ABVD to most patients but have gradually shifted toward BEACOPP, at least for high-risk patients (EORTC20012 trial) or recommended PET-guided treatments with 2 cycles of ABVD upfront followed by eventual escalation to BEACOPP (RATHL trial).13, 30 Only more recently, upfront treatment with 2 escBEACOPP following the HD18 trial has been implemented in treatment guidelines across all Nordic countries.14 GnHR-agonists are not routinely used for cHL patients during chemotherapy throughout the Nordic countries and there are no general recommendations concerning the use of oral-contraceptives during treatment for these patients apart from a general recommendation to not become pregnant.
Strengths of the study include population-based data from multiple countries that includes both male and female cHL survivors and modern cHL chemotherapy treatments. Additionally, the use of registers with mandatory reporting and clinical databases with high coverage minimize bias due to loss of follow-up and patient selection.
Limitations of the study include some missing information on performance status, cHL stage and cHL treatment, which affected the power but unlikely the validity of our estimates. We were also unable to compare different variants of BEACOPP, that is, escalated and dose-reduced BEACOPP, or BEACOPP-14, to ABVD. Moreover, our study does not disentangle the direct effects of chemotherapy treatment, competing events, use of ART and changes in attitudes toward having children on childbirth rates. While such estimates might be relevant from an etiological perspective, the total effect of chemotherapy on reproduction is likely more relevant for clinical practice. Finally, we were not able to adjust for BMI at diagnosis which might be a confounder of the association between cHL chemotherapy treatment and subsequent reproduction. However, we assume that adjusting for BMI is unlikely to change the observed association. We assume that patients with high BMI levels are more likely to be treated with ABVD due to a potential lower tolerability of side effects of the BEACOPP regime. Hence, this would lead to an underestimation of the true childbirth rate in the ABVD group, given the negative impact of high BMI levels on fertility.31, 32 Thus, our comparison would be an underestimation of the true difference in childbirth rates between patients treated with ABVD and BEACOPP.
To conclude, we found that female cHL patients treated with 6-8 BEACOPP have similar childbirth rates as ABVD treated females, whereas male cHL patients treated with 6-8 BEACOPP have decreased childbirth rates compared to ABVD treated males. This difference between treatment groups and sex was observed despite a high access to ART treatments, and a high proportion of children born to partners of male patients that have been conceived after use of ART treatments. Thus, differences in treatment toxicities, and reproductive behavior might play an important role in explaining the observed differences between male and female patients. The presented results underscore the need for less gonadotoxic chemotherapy regimens for advanced stage cHL patients, which are currently under development, such as replacing oral procarbazine with intravenous dacarbazine.33 They further highlight the importance of discussing fertility issues with patients when the most optimal treatment has been decided to reach cure, and when possible providing access to fertility preservation counseling and procedures before and after treatment.
AUTHOR CONTRIBUTIONS
Conceptualization: All. Data curation: Joshua P. Entrop, Sandra Eloranta, Karin E. Smedby, Lasse H. Jakobsen, Knut B. Smeland. Formal analysis: Joshua P. Entrop, Sandra Eloranta. Funding acquisition: Sandra Eloranta, Karin E. Smedby, Tarec C. El-Galaly. Methodology: Joshua P. Entrop, Sandra Eloranta, Caroline E. Weibull, Lasse H. Jakobsen. Supervision: Sandra Eloranta. Writing—original draft: Joshua P. Entrop, Sandra Eloranta, Karin E. Smedby, Tarec C. El-Galaly. Review and editing: All. The work reported in the paper has been performed by the authors, unless clearly specified in the text.
ACKNOWLEDGEMENTS
This study has been supported by research grants from the Swedish, and Danish Cancer Society, the Nordic Cancer Union, Åke Wiberg Stiftelse and Karolinska Institutets Foundations. The authors would like to thank RKKP and all physicians who contributed to reporting data to LYFO.
FUNDING INFORMATION
Swedish, and Danish Cancer Society, Nordic Cancer Union, Åke Wiberg Stiftelse and Karolinska Institutets Foundations. The funders of this research were not involved in any parts of the research process for this study.
CONFLICT OF INTEREST STATEMENT
Caroline E. Weibull: Red Door Analytics: Membership on an entity's Board of Directors or advisory committees; Janssen Pharmaceutical NV: Research Funding; War On Cancer: Current Employment. Karin E. Smedby: Janssen Pharmaceutical NV: Research Funding. Lasse H. Jakobsen: Roche: Honoraria. Daniel Molin: MSD: Honoraria; Roche: Honoraria; Takeda: Honoraria; BMS: Honoraria. Ingrid Glimelius: Takeda: Research Funding; Janseen Cilag: Research Funding. Harald Holte: Nordic Nanovector: Honoraria, Safety committee; Novartis: Honoraria, Advisory board; Gilead: Honoraria, Advisory board; Incyte: Honoraria, Advisory board; Takeda: Honoraria, Advisory board; Genmab: Honoraria, Safety committee. Tarec C. El-Galaly: Roche: Ended employment in the past 24 months; Abbvie: Teaching in 2021. The other authors have no conflict to disclose.
ETHICS STATEMENT
The study has been approved by the Regional Ethical Review Board in Stockholm (No. 2014/1017-32), the Danish Data Protection Agency (No. 2018-88) and the Regional Committee for Medical Research Ethics South East Norway (No. 2018/2209). The need of obtaining informed consent was waived.
Open Research
DATA AVAILABILITY STATEMENT
A detailed analysis plan describing the data used for this study has been published online on the Open Science Framework together with the main statistical programs used for analysis (https://osf.io/eumy5/). Further information is available from the corresponding author upon request.