Premature ovarian insufficiency and chance of pregnancy after childhood cancer: A population-based study (the Fex-Can study)
Abstract
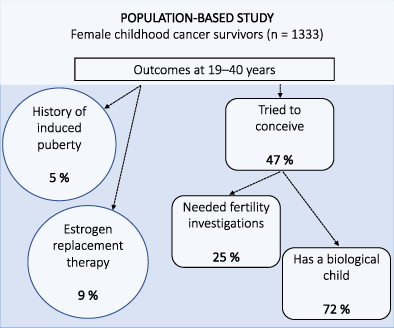
Endocrine complications are a common late effect after childhood cancer. Our study assessed the prevalence and predictors of premature ovarian insufficiency (POI) and prospects of pregnancy in young female survivors. This nationwide study combined registry and survey data for female childhood cancer survivors aged 19 to 40 years, identified through the National Quality Registry for Childhood Cancer in Sweden. Of 1989 approached young women, 1333 (67%) participated by completing a survey. Median age at diagnosis 1981 to 2017 was 6 (range 0-17) and at study 28 (19-40) years. There were two indicators of POI, induced puberty reported in 5.3% and estrogen replacement therapy (ERT) in 9.3% at assessment. In separate logistic regression analyses (P < .001), induced puberty and ERT were significantly predicted by hematopoietic stem cell transplantation (HSCT), abdominal irradiation, central nervous system irradiation and chemotherapy. ERT was also associated with older age at diagnosis. Of the 626 women (48% of responders) who had tried to become pregnant, 25% had undergone fertility investigations and 72% reported having a biological child. Treatment with HSCT was associated with 5.4 times the odds of needing fertility investigations (P < .001). Having a biological child was associated with non-HSCT treatment, but also with ever having had a partner and older age at the time of study (all P < .001). In conclusion, the majority of those female childhood cancer survivors who had tried to conceive were able to successfully give birth. However, a small identifiable group of female survivors are at risk of subfertility and early menopause.
Graphical Abstract
What's new?
Premature ovarian insufficiency (POI) and infertility are known treatment-related late effects following childhood cancer. However, prevalence rates of POI in recent patient cohorts are lacking. This population-based study in Sweden analyzed registry and survey data for female childhood cancer survivors who were diagnosed between 1981 and 2017. Investigations show that 9.3% of female survivors of childhood cancer ages 19 to 40 had ongoing estrogen replacement therapy, indicating POI. Of survivors who had tried to conceive, 72% were successful. The identified prevalence rates are consistent with findings from other studies and suggest that late sequelae remain problematic for some survivors.
Abbreviations
-
- AOF
-
- acute ovarian failure
-
- CNS
-
- central nervous system
-
- ERT
-
- estrogen replacement therapy
-
- HSCT
-
- hematopoietic stem cell transplantation
-
- POI
-
- premature ovarian insufficiency
-
- TBI
-
- total body irradiation
1 INTRODUCTION
A growing number of children who have been treated for cancer survive and enter adulthood. However, chronic health conditions are common after cancer treatments.1 For young adult survivors, treatment-related premature ovarian insufficiency (POI) and associated infertility are particularly important issues.
POI occurs in two forms before the age of 40. First, POI can occur as acute ovarian failure (AOF) during the years following cancer treatment as either the cessation of menstruation or the failure to achieve natural menarche. Second, POI can manifest as premature menopause in females who initially retain their ovarian function after cancer treatment. AOF has been reported in ~6% of childhood cancer survivors2 and nonsurgical premature menopause, when excluding patients with AOF, in 9%.3 POI in general has been reported in 11% to 13% of childhood cancer survivors.4, 5 POI affects fertility negatively and leads to a narrower time window of fertility.3 Consequently, in a population-based study, the incidence of pregnancy was 28% to 31% lower in female childhood cancer survivors compared to the general population.6
Risk factors that have uniformly been associated with the development of POI and infertility include exposure to alkylating agents, ovarian/pelvic irradiation, total body irradiation (TBI) and hematopoietic stem cell transplantation (HSCT) regimens.3, 5, 7, 8 It is generally considered a risk factor for premature menopause to be diagnosed postpubertally compared to the prepubertal period.9 Due to the diminishing ovarian reserve, the risk of premature menopause after treatment also increases by age.3, 5
Compared to the previous studies on POI, our study presents data from more recently treated patients and assessed a national population which included all female survivors aged 19 to 40 years treated at the pediatric oncology units in Sweden. Thus, results can be used to inform presently treated patients about their future risk for POI and fertility prospects. The first aim of our study was to assess the prevalence and predictors of POI in young women who have survived childhood cancer. The second aim was to assess the chance of pregnancy and its predictors after childhood cancer.
2 MATERIALS AND METHODS
2.1 Participants
Our study is part of the national population-based Fex-Can Childhood project addressing the prevalence and predictors of sexual dysfunction and fertility-related distress in young adult childhood cancer survivors.10 It included all survivors in the National Quality Registry for Childhood Cancer in Sweden who were 19 to 40 years of age and residents in Sweden at the time of enrolment. The registry included patients diagnosed with cancer before the age of 18 years; however, during the 1980s and part of the 1990s only patients diagnosed before the age of 16 were treated at the child oncology units and, hence, included in the registry. Patients were excluded from the study if they were unable to read/write in Swedish or had cognitive impairment that compromised study participation. The survivors were identified through the National Quality Registry for Childhood Cancer. A total of 2030 possible female participants were identified, whereof 41 were excluded due to unknown address (n = 23), living outside Sweden (n = 1), cognitive dysfunction (n = 14) and administrative failure (n = 3). Finally, of the 1989 eligible participants, 1333 (67%) participated in the study. They had been diagnosed with cancer 1981 to 2017. There were no significant differences in demographic or clinical characteristics between female participants and nonparticipants.11
2.2 Procedure
Clinical data about primary cancer diagnoses, date of diagnosis, cancer treatments, disease relapses and second malignancies were retrieved from the National Quality Registry for Childhood Cancer. Information about TBI was not available. Abdominal irradiation possibly affecting the gonads and cranial irradiation included only the local irradiation doses. Diagnoses were classified according to the International Classification of Childhood Cancer, 3rd revision.12 Each child's treatment was categorized by a pediatric oncologist using the Intensity of Treatment Rating scale (ITR-3.0), a psychometrically validated measure of treatment intensity of current treatment protocols in pediatric oncology.13 Different disease and/or treatment modalities were classified according to one of the four intensity levels, from level 1 (minimally intensive; including treatments such as surgery only or chemotherapy for Wilms' tumor Stages 1 and 2) to level 4 (most intensive; including treatments such as HSCT or chemotherapy for acute myeloid leukemia).
Sociodemographic data, medication use and information on menstruation and fertility were retrieved from a survey consisting of validated self-report instruments and study-specific items. The survey was sent to all eligible participants together with a letter describing the study.10 The survey could be completed on paper, Web or by telephone interview. Two reminders were sent out to the nonresponders. Data was collected August 2019 to February 2020.
2.3 Measures
The outcome measures in our study were self-reported need of induced puberty, ongoing use of medication with ERT, attempt to conceive, having undergone fertility investigations and having a biological child. This data was retrieved from the following five study-specific questions in the survey: (a) Have you needed medication to induce puberty (no/I do not know or remember/yes)? (b) During the past 2 years, have you used female sex hormone to replace estrogen during a minimum of 1 month (no/I do not know or remember/yes)? (c) Have you had difficulties becoming pregnant (no/some difficulty/yes/have not tried)? (d) Have you undergone any investigations due to difficulties becoming pregnant, for example, blood sample or ultrasound (no/I do not know or remember/yes)? (e) Do you have a biological child (yes/no)? Participants had the possibility to also write an open-ended response.
For background characteristics, the following questions from the survey were included: Have you ever cohabitated or been married to a partner (no/yes/do not want to specify)? Have you ever needed injections with growth hormone (no/I do not know or remember/yes)? During the past 2 years, have you used medication for thyroid function/diabetes during a minimum of 1 month (no/I do not know or remember/yes)? What is your menstrual status right now?
Of those 1297 females who indicated their current menstrual status, 41 (3.2%) considered themselves to be menopausal, 177 (13.6%) reported that they had no menstruation the past 6 months, 300 (23.1%) had irregular menses and 779 (60.1%) regular menses. However, based on a review of the participants multiple-choice responses and written comments, for example, based on the names of medications the participants had stated, there were discrepancies in the responses. Some who reported no menstruation were pregnant or lactating or had no menstruation due to hormonally induced amenorrhea. After this review, ERT was considered the best indicator of ovarian failure. However, also in ERT there were some contradictory information, why 37/1295 (2.9%) responses were re-classified. In most of these cases (n = 21) the survivor had indicated the use of ERT, or ERT and contraception, to avoid pregnancy. The re-classified cases were revised to “no current use of ERT” if the patient indicated hormone replacement therapy but described medication for in vitro fertilization, nongonadal hormonal deficiencies, endometriosis, polycystic ovary syndrome or contraceptive implant to avoid pregnancy.
2.4 Statistical analyses
For continuous variables, sociodemographic characteristics are presented as median (range), as the data was not normally distributed. Categorical variables are presented as frequencies and percentages. Group comparisons of the background variables were undertaken with the Mann-Whitney U-test and Fisher's exact χ2-test.
Five binary logistic regression analyses were undertaken to examine predictors associated with the outcome variables: the need of induced puberty, current use of ERT, attempt to conceive, having undergone fertility investigations and having a biological child. Independent variables were treatment-related variables (chemotherapy, irradiation potentially exposing the gonads, central nervous system [CNS] irradiation, HSCT; yes/no), age at diagnosis, age at study and partner status (if the participant had ever been married or cohabitated with a partner; yes/no). These predictors were chosen a priori based on their theoretical importance. Employment status (yes/no) was initially added as a predictor but was removed since it did not add predictive value. Cancer diagnosis or ITR-3.0 were not used in the regression analyses since they are strongly correlated with the cancer treatment variables.
To obtain a cutoff value for the CNS and abdominal irradiation doses, the outcome variables were subjected to AUC analysis of ROC curves for the participants with a known irradiation dosage. For AUC, 0.8 is considered acceptable.14 Hence, only significant AUC analyses with AUC ≥ 0.8 are presented. Statistical calculations were carried out using IBM SPSS Statistics 28.0. All tests of significance were two-sided (P < .05).
3 RESULTS
3.1 Sociodemographic characteristics
Demographic characteristics are presented in Table 1. The survivors were a median of 28 years at the time of the study. The majority of survivors (86.0%) were working or studying full- or part-time or were on parental leave. The most common education was a university degree. Approximately two-thirds had at some point cohabitated with a partner or been married.
| Median (range) | |
|---|---|
| Age at diagnosis, years | 6 (0-17) |
| Age at study, years | 28 (19-40) |
| Follow-up time, years | 21 (1-38) |
| n (%) | |
| Treatment era | |
| 1980-1989 | 237 (17.8%) |
| 1990-1999 | 585 (43.9%) |
| 2000-2017 | 511 (38.3%) |
| Age at study, years | |
| 19-25 | 455 (34.1%) |
| 26-30 | 345 (25.9%) |
| 31-35 | 281 (21.1%) |
| 36-40 | 252 (18.9%) |
| Country of birth (n = 1328) | |
| Sweden (vs other) | 1272 (95.8%) |
| Partner status (n = 1318) | |
| Ever cohabitated or been married | 889 (67.5%) |
| Highest level of education (ongoing or finished, n = 1327) | |
| High school | 47 (3.5%) |
| Secondary level | 479 (36.1%) |
| Tertiary level | 745 (56.1%) |
| Other | 56 (4.2%) |
| Employment (n = 1333) | |
| Full-time employment | 670 (50.3%) |
| Part-time employment | 227 (17.0%) |
| Student | 250 (18.8%) |
| Unemployed | 61 (4.6%) |
| Sick leave, early retirement | 119 (8.9%) |
| Other | 6 (0.5%) |
3.2 Clinical characteristics
Outcome variables are presented by diagnosis and treatment modality in Table 2. The most common diagnoses were hematological cancers in 45.3% of the survivors and the most common treatment modality was moderately intensive.
| n (%) | Outcome variables | ||||||
|---|---|---|---|---|---|---|---|
| Induced puberty | ERT | Tried to conceive | Fertility investigations | Biological children | |||
| Total sample | Tried to conceive | ||||||
| All diagnoses | 1333 (100%) | 69/1293 (5.3%) | 121/1295 (9.3%) | 626/1321 (47.4%) | 205/1309 (15.7%) | 459/1333 (34.4%) | 451/626 (72.0%) |
| Hematological cancers | |||||||
| Acute lymphoblastic leukemia | 362 (27.2%) | 15/346 (4.3%) | 32/354 (9.0%) | 171/358 (47.8%) | 48/357 (13.4%) | 126/362 (34.8%) | 125/171 (73.1%) |
| Standard/intermediate risk | 299 (82.6) | 11/290 (3.8%) | 21/294 (7.1%) | 138/295 (46.8%) | 35/295 (11.9%) | 102/299 (34.1%) | 101/138 (73.2%) |
| High risk | 63 (17.4%) | 4/56 (7.1%) | 11/60 (18.3%) | 33/63 (52.4%) | 13/62 (21.0%) | 24/63 (38.1%) | 24/33 (72.7%) |
| Myeloid leukemia | 56 (4.2%) | 13/55 (23.6%) | 21/54 (38.9%) | 27/55 (49.1%) | 15/54 (27.8%) | 17/56 (30.4%) | 16/27 (59.3%) |
| Lymphoma | 148 (11.1%) | 0/147 (0%) | 13/144 (9.0%) | 77/147 (52.4%) | 37/144 (25.7%) | 58/148 (39.2%) | 57/77 (74.0%) |
| Nonhigh stage | 110 (74.3%) | 0/109 (0%) | 8/106 (7.5%) | 61/110 (55.5%) | 22/107 (20.6%) | 49/110 (44.5%) | 48/61 (78.7%) |
| High stage | 38 (25.7%) | 0/38 (0%) | 5/38 (13.2%) | 16/37 (43.2%) | 15/37 (40.5%) | 9/38 (23.7%) | 9/16 (56.3%) |
| Histiocytosis | 37 (2.8%) | 1/35 (2.9%) | 2/37 (5.4%) | 21/37 (56.8%) | 4/37 (10.8%) | 19/37 (51.4%) | 19/21 (90.5%) |
| Neuroblastoma, sarcoma, gonadal tumors | |||||||
| Neuroblastoma | 55 (4.1%) | 10/54 (18.5%) | 10/53 (18.9%) | 20/54 (37.0%) | 14/53 (26.4%) | 12/55 (21.8%) | 12/20 (60.0%) |
| Nonhigh stage | 33 (60.0%) | 0/33 (0%) | 0/32 (0%) | 12/33 (36.4%) | 7/33 (21.2%) | 8/33 (24.2%) | 8/12 (66.7%) |
| High stage | 22 (40.0%) | 10/21 (47.6%) | 10/21 (47.6%) | 8/21 (38.1%) | 7/20 (35.0%) | 4/22 (18.2%) | 4/8 (50.0%) |
| Sarcoma | 123 (9.2%) | 9/119 (7.6%) | 11/119 (9.2%) | 57/122 (46.7%) | 26/122 (21.3%) | 43/123 (35.0%) | 41/57 (71.9%) |
| Germ cell and gonadal tumors | 68 (5.1%) | 2/68 (2.9%) | 4/65 (6.2%) | 38/68 (55.9%) | 11/68 (16.2%) | 28/68 (41.2%) | 28/38 (73.7%) |
| Other non-CNS tumors | |||||||
| Renal tumors | 84 (6.3%) | 4/84 (4.8%) | 5/82 (6.1%) | 40/84 (47.6%) | 12/84 (14.3%) | 33/84 (39.3%) | 33/40 (82.5%) |
| Nonhigh stage | 66 (78.6%) | 0/66 (0%) | 3/65 (4.6%) | 33/66 (50.0%) | 9/66 (13.6%) | 28/66 (42.4%) | 28/33 (84.8%) |
| High stage | 18 (21.4%) | 4/18 (22.2%) | 2/17 (11.8%) | 7/18 (38.9%) | 3/18 (16.7%) | 5/18 (27.8%) | 5/7 (71.4%) |
| Hepatic tumors | 11 (0.8%) | 0/10 (0%) | 0/10 (0%) | 2/11 (18.2%) | 0/11 (0%) | 2/11 (18.2%) | 2/2 (100.0%) |
| Retinoblastoma | 32 (2.4%) | 0/32 (0%) | 1/32 (3.1%) | 12/32 (37.5%) | 3/32 (9.4%) | 5/32 (15.6%) | 5/12 (41.7%) |
| Carcinoma | 36 (2.7%) | 1/35 (2.9%) | 2/34 (5.9%) | 18/36 (50.0%) | 3/35 (8.6%) | 12/36 (33.3%) | 12/18 (66.7%) |
| Miscellaneous tumorsa | 11 (0.8%) | 0/11 (0%) | 0/11 (0%) | 4/11 (36.4%) | 3/11 (27.3%) | 4/11 (36.4%) | 4/4 (100.0%) |
| CNS tumors | |||||||
| Low-grade CNS tumors | 204 (15.3%) | 3/198 (1.5%) | 7/197 (3.6%) | 103/201 (51.2%) | 20/199 (10.1%) | 76/204 (37.3%) | 74/103 (71.8%) |
| High-grade CNS tumors | 106 (8.0%) | 11/99 (11.1%) | 13/103 (12.6%) | 36/105 (34.3%) | 9/102 (8.8%) | 24/106 (22.6%) | 23/36 (63.9%) |
| Treatment modality | |||||||
| Intensity of treatment (IRT-3) | |||||||
| Minimally intensive | 156 (11.7%) | 0/154 (0%) | 3/153 (2.0%) | 80/156 (51.3%) | 19/156 (12.2%) | 65/156 (41.7%) | 65/80 (81.3%) |
| Moderately intensive | 669 (50.2%) | 11/659 (1.7%) | 21/649 (3.2%) | 335/663 (50.5%) | 82/660 (12.4%) | 261/669 (39.0%) | 257/335 (76.7%) |
| Very intensive | 330 (24.8%) | 20/313 (6.4%) | 36/317 (11.4%) | 140/327 (42.8%) | 59/322 (18.3%) | 98/330 (29.7%) | 95/140 (67.9%) |
| Most intensive | 178 (13.4%) | 38/167 (22.8%) | 61/176 (34.7%) | 71/175 (40.6%) | 45/171 (26.3%) | 35/178 (19.7%) | 34/71 (47.9%) |
| Chemotherapy | 945/1326 (71.3%) | 65/911 (7.1%) | 112/918 (12.2%) | 430/936 (45.9%) | 162/926 (17.5%) | 313/945 (33.1%) | 307/430 (71.4%) |
| Any local irradiation | 282 (21.2%) | 33/268 (12.3%) | 46/273 (16.8%) | 120/277 (43.3%) | 51/274 (18.6%) | 90/282 (31.9%) | 86/120 (71.7%) |
| Local cranial irradiation | 154 (11.6%) | 16/140 (11.4%) | 23/147 (15.6%) | 65/151 (43.0%) | 21/148 (14.2%) | 51/154 (33.1%) | 49/65 (75.4%) |
| Local irradiation possibly affecting the gonads | 15 (1.1%) | 8/15 (53.3%) | 8/15 (53.3%) | 3/15 (20.0%) | 5/14 (35.7%) | 1/15 (6.7%) | 1/3 (33.3%) |
| Surgery | 504 (37.8%) | 24/490 (4.9%) | 30/489 (6.1%) | 225/500 (45.0%) | 58/495 (11.7%) | 167/504 (33.1%) | 163/225 (72.4%) |
| HSCT with or without TBI | 83 (6.2%) | 31/77 (40.3%) | 47/82 (57.3%) | 38/82 (46.3%) | 31/79 (39.2%) | 14/83 (16.9%) | 13/38 (34.2%) |
| Allogeneic | 57 (4.3%) | 22/54 (40.7%) | 34/56 (60.7%) | 27/56 (48.2%) | 23/56 (41.1.%) | 10/57 (17.5%) | 9/27 (33.3%) |
| Autologous | 26 (2.0%) | 9/23 (39.1%) | 13/26 (50.0%) | 11/26 (42.3%) | 8/23 (34.8%) | 4/26 (15.4%) | 4/11 (36.4%) |
| Relapse therapy | 131 (9.8%) | 16/127 (12.6%) | 28/127 (22.0%) | 51/128 (39.8%) | 25/127 (19.7%) | 31 /131 (23.7%) | 30/51 (58.8%) |
| Therapy for second malignancyb | 20 (1.5%) | 2/16 (12.5%) | 7/19 (36.8%) | 10/20 (50.0%) | 7/19 (36.8%) | 7/20 (35.0%) | 7/10 (70.0%) |
- Note: Diagnostic groups were based on ICCC-3 classification, but some groups were combined due to a low number of observations. Data indicated as observed n/n for all replies on that item (%). Outcome variables denote the prevalence of reported hormonal deficiencies and pregnancy prospects per diagnostic group and per treatment modality. Numbers of survivors who have mothered a biological child are given for the whole population as well as for those survivors who have tried to conceive.
- Abbreviations: CNS, central nervous system; ERT, estrogen replacement therapy; TBI, total body irradiation.
- a Eleven survivors had miscellaneous tumors (6 malignant melanomas and 5 other rare malignant tumors).
- b The data was collected from the National Quality Registry for Childhood Cancer and, hence, only includes second malignancies diagnosed before the age of 17 years.
Of the 1333 female participants, one had undergone hysterectomy and was surgically sterile. Additionally, 10 females had undergone a unilateral oophorectomy. Of the 10 females who had undergone oophorectomy (median age at study 29.5, range 19-37 years), only one had ongoing ERT. Further, six indicated that they had not tried to become pregnant while four had biological children without having undergone fertility investigations.
3.3 Premature ovarian insufficiency
Of those 1234 females who had indicated their menarchal status at the time of diagnosis, 967 (78.4%) female patients were premenarchal before cancer diagnosis. Of these, spontaneous menarche post-treatment was reported by 898 participants (92.9%) at a median age of 13.0 (range 8-18) years. Spontaneous menarche was seen in all females with lymphoma, nonhigh stage neuroblastoma or renal tumors, hepatic tumors, retinoblastoma and in survivors with minimally intensive treatment (Table 2). Hormone-induced puberty was reported in 69 (7.1%) of the females who were premenarchal at the time of diagnosis. In the whole population the prevalence was 5.3%. Induced puberty was common after myeloid leukemia, high-stage neuroblastoma or renal tumors, as well as in survivors with the most intensive treatments (Table 2).
The need of hormone-induced puberty among females who were premenarchal before cancer diagnosis was significantly predicted by all treatment variables included in the logistic regression model, χ2 (5) = 122.31, P < .001 (Table 3). The ROC analysis showed significant predictive power of the abdominal irradiation dose to predict the need for induced puberty (n = 13, AUC 0.925, P = .013). The analysis included only the local irradiation doses possibly affecting the gonads. Information about TBI was not available. A local abdominal irradiation dose of 36.0 Gy had high sensitivity (100%) and specificity (80%) to predict the need of induced puberty. A cutoff at 24.0 Gy had lower sensitivity (63%) but better specificity (100%). The ROC analysis with local CNS irradiation dose was not significant.
| Predictors | Outcome variables | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Induced puberty (n = 963) | Estrogen replacement therapy (n = 1288) | Tried to conceive (n = 1301) | Fertility investigations (n = 610) | Biological children (n = 614) | ||||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Chemotherapy | 3.91 (1.36-11.24) | .012 | 3.63 (1.77-7.42) | <.001 | 1.09 (0.80-1.49) | .581 | 1.35 (0.88-2.08) | .170 | 1.20 (0.78-1.84) | .401 |
| Cranial irradiation | 4.02 (2.02-7.99) | <.001 | 3.00 (1.71-5.25) | <.001 | 0.59 (0.37-0.93) | .024 | 1.11 (0.60-2.06) | .748 | 0.84 (0.44-1.61) | .606 |
| Abdominal irradiation | 11.04 (2.74-44.52) | <.001 | 5.27 (1.50-18.51) | .009 | 0.27 (0.06-1.26) | .096 | 4.45 (0.38-51.88) | .234 | 0.24 (0.01-4.32) | .330 |
| HSCT | 14.61 (7.74-27.58) | <.001 | 18.47 (10.75-31.76) | <.001 | 1.43 (0.75-2.70) | .277 | 5.36 (2.64-10.87) | <.001 | 0.13 (0.06-0.29) | <.001 |
| Age at diagnosis, years | 1.05 (0.98-1.12) | .194 | 1.09 (1.04-1.13) | <.001 | 1.01 (0.98-1.04) | .467 | 1.02 (0.98-1.05) | .416 | 0.98 (0.95-1.02) | .389 |
| Age at study, years | NA | NA | 1.00 (0.96-1.03) | .824 | 1.21 (1.17-1.24) | <.001 | 1.02 (0.98-1.06) | .436 | 1.14 (1.10-1.19) | <.001 |
| Ever cohabitated | NA | NA | NA | NA | 9.13 (6.28-13.27) | <.001 | 1.77 (0.76-4.14) | .189 | 5.85 (2.80-12.20) | <.001 |
- Note: For all outcome variables and all predictors except for age: 0 = no, 1 = yes. There were some missing data for most of the outcome and predictor variables. The number of responses analyzed in each regression analysis is stated beneath the outcome variable.
- Abbreviations: CI, confidence interval; HSCT, hematopoietic stem cell transplantation; OR, odds ratio.
Of 1295 females, 121 (9.3%) indicated current use of ERT. The need of ERT was common after myeloid leukemia, high-stage neuroblastoma as well as in survivors with the most intensive treatments including relapse therapy and therapy for second malignancy. Accordingly, more than half of the survivors who had undergone HSCT or abdominal irradiation possibly affecting the gonads had ongoing ERT (Table 2). Of the 121 females with ERT, 27 (22.3%) had one additional hormone deficiency and 30 (24.8%) had two or more additional hormone deficiencies (thyroid, growth hormone) or diabetes. ERT together with two or more hormone deficiencies was most prevalent in patients with high-grade CNS tumors (12/121, 9.9%), patients treated with CNS irradiation (19/121, 15.7%) or HSCT (10/121, 8.3%). In the regression model, χ2 (6) = 182.17, P < .001, ongoing ERT was significantly predicted by all the treatment variables included as well as older age at diagnosis, but not with age at study (Table 3). However, ROC analyses were not significant and, hence, cutoffs for local CNS or abdominal irradiation doses could not be estimated.
3.4 Chance of pregnancy
Of 1321 respondents, 626 (47.4% of responders) reported that they had tried to become pregnant. Possible relationships between the wish to conceive and treatments, particularly CNS irradiation through its effect on sexual functions and partnering, were explored. In a logistic regression, χ2 (7) = 598.99, P < .001, survivors who had never cohabitated with a partner, who were younger at assessment and who had undergone CNS irradiation were less likely to have tried to conceive (Table 3). Chemotherapy, abdominal irradiation, HSCT and age at diagnosis were not significantly associated with the attempt to conceive in the regression analysis.
Of the 626 women who had tried to conceive, 251 women (40.1%) reported that they had at least some difficulties to become pregnant and 158 (25.2%) that they had undergone fertility investigations. Need of fertility investigations was common after high-stage lymphoma and neuroblastoma. Further, more than a third of the survivors who had needed a fertility investigation had received abdominal irradiation, HSCT and/or had undergone therapy for a second malignancy (Table 2). In a logistic regression, χ2 (7) = 32.93, P < .001, women who had undergone HSCT had 5.4 times the odds of needing fertility investigations. No other treatment-related variable or factors in the current situation were significant predictors for the need of fertility investigations.
Finally, of the 626 women who had tried to conceive, 451 women (72.0%) had a biological child. High frequencies of pregnancies were reported in survivors of histiocytosis, nonhigh stage renal tumors and hepatic tumors. It was less common to have a biological child after high-stage neuroblastoma, retinoblastoma or after the most intensive treatment modality (Table 2). Similarly, a lower proportion of women with subsequent need of ERT had a biological child. This proportion was 10.7% (13/121) among all women with ongoing ERT and 29.5% (13/44) in women with ERT who had tried to conceive. In a logistic regression model, χ2 (7) = 108.94, P < .001, having had a partner, older age at assessment and non-HSCT treatment predicted having a biological child (Table 3).
4 DISCUSSION
In this population-based study, all young women who had survived childhood cancer diagnosed between 1981 and 2017 in Sweden were invited. The study combined national registry and self-report survey data from 1333 survivors. It is to our knowledge the most comprehensive study on POI in survivors from the most recent treatment era. ERT, an indicator of POI, was reported by 9.3% of the young adult survivors. Moreover, a significant number of survivors with ERT reported simultaneously other hormone deficiencies, indicating that females suffering from POI often have a larger range of hormonal late effects. Of the survivors who had tried to conceive a biological child, one fourth underwent fertility investigations due to difficulties becoming pregnant yet as many as 72% had to date been able to successfully give birth to a biological child. Treatment burden, particularly HSCT, was associated with poorer outcome for POI and the chance of pregnancy. As an independent predictor, also the ability to find a partner was important for the chance of pregnancy, reflecting the multitude of biological, psychological and social factors involved in fertility and partnering.
The prevalence of POI in the general population in Sweden is 1.9%,15 indicating a significantly increased risk for POI after childhood cancer treatment. Although our study represents a slightly younger survivor population, aged 19 to 40 years, from a more recent treatment era, the prevalence of self-reported ERT in our study (9.3%) was similar to the frequency of POI reported in previous studies of childhood cancer survivors. For instance, our results are well in line with the reported prevalence of 10.9% in the St Jude Lifetime Cohort4 and 13% in a recent study by Netterlid et al (2021) of childhood cancer survivors in southern Sweden.5 In those studies, POI was defined based on self-reported menstrual history, supplemented with ovarian hormone concentrations. On the other hand, the Childhood Cancer Survivor Study defined premature menopause as the cessation of menses for at least 6 months beginning 5 years after cancer diagnosis. Although they excluded survivors with AOF, the prevalence of premature menopause by the age of 40 (9.1%) was almost identical to our study results and may reflect the older treatment era (1970-1986).3 Similarly, the number of survivors with the need of induced pubertal onset (5.3%) in our study was similar to the 6% prevalence of AOF reported by the Childhood Cancer Survivor Study.2, 16
Concerning the variables in defining POI, patient self-reports on menstrual status and menopause were detected to be somewhat unreliable in our study. The use of ERT was almost three times more common than self-reports of menopause (3.2%). In a previous study, POI was defined based on survivors' self-reports of menopausal status in questionnaires. Similar to our self-report data, they found a 2.1% incidence of nonsurgical premature menopause.9 Hence, self-reports of menopausal status per se, may lead to a gross underestimation of POI. Further, if the absence of menstruation is used as a definition for menopause, pregnant or lactating women and women with hormonally induced amenorrhea should be identified and excluded from the menopausal group. In our study, self-reported ERT together with a review of present medications was considered a reliable definition. In sum, although we defined POI somewhat differently compared to previous studies, as the need of ERT and/or induced puberty, the prevalence rates were comparable to earlier studies, particularly when considering the younger median age of our study participants. In Sweden, health care is free, and medications subsidized. Hence, all women with a clinical indication for induced puberty or ERT can be assumed to have received the treatments, which further supports their use as a definition of POI in a Scandinavian setting.
Since estrogen deficiency is known to have significant consequences on bone density, cardiovascular health, physical fitness and quality of life,4, 5 POI and the need of ERT can potentially affect self-reported distress. In future survey studies, estrogen deficiency is an important variable to take into account when assessing, for example, quality of life and physical fitness. Moreover, we identified that almost half of the survivors with ERT needed one or more additional hormone therapies, possibly further increasing the burden of late sequelae. Similarly, Netterlid et al (2021) recently reported higher numbers of other hormonal replacement therapies in survivors with POI. In our study, ERT together with two or more hormone deficiencies was seen especially after a diagnosis of high-grade CNS tumor, treatment with CNS irradiation or HSCT. ERT was also more common in survivors who had undergone relapse therapy or a second malignancy, reflecting increased treatment burden.
Concerning the chance of pregnancy, fertility is on group-level lower in survivors of childhood cancer compared to controls.7, 17 In the present national population, 47% of the childhood cancer survivors had tried to conceive. Of these, 25% had undergone fertility investigations. Finally, 72% of those survivors who had tried to conceive had been able to successfully give birth to a biological child. Highest percentage of biological children was seen in survivors of histiocytosis, nonhigh stage lymphoma or renal tumors, germ cell and gonadal tumors and survivors who have undergone the least intensive treatments. In a diagnostically similar population of childhood cancer survivors in the Netherlands, the DCOG LATER-VEVO study, similar frequencies were reported; 44% had tried to become pregnant and 82% managed to become pregnant, irrespective of the outcome of the pregnancy.7 However, van Dijk et al observed that survivors, compared to controls, were younger at their first pregnancy, which may mitigate the effect of treatment and be a consequence of fertility education due to cancer treatments.7 Younger age at the birth of their firstborn has also been observed in a population-based study of reproductive patterns in childhood cancer survivors in Sweden.18 Due to their lower fertility rates, this is particularly important for women who develop subsequent need of ERT.3
Based on the current findings, HSCT is the strongest predictor of POI and the need of fertility investigations. Our study had a much higher number of patients who had undergone HSCT compared to the St Jude Lifetime or Childhood Cancer Survivors Studies,2, 3 but more comparable to the study by Netterlid et al.5 This reflects differences in the distribution of diagnoses between the cohorts as well as the more recent treatment era of the participants in the present study when HSCT has been increasingly used. Although conventional treatments have improved, HSCT remains the only curative therapy in many severe hematological cancers. This result may also reflect the awareness of fertility-related issues after HSCT, as a high number of referrals to infertility investigations in survivors treated with HCST was observed in our study. Although a strikingly low number of females who had undergone HSCT had a biological child (17%), attempting pregnancy and having a biological child were in the regression analyses more strongly associated with the ability to find a partner and older age at study than therapy intensity. This observation reflects the multitude of bio-psycho-social factors associated with partnering and having children. The importance of being able to form long-lasting relationships for the chance of pregnancy is clinically significant considering that previous reports have found cancer survivors to be less likely to partner in adulthood.11, 19
Further, females who had received chemotherapy, local irradiation to the abdomen or CNS and who were older at the time of diagnosis were at increased risk of POI. These findings are well in line with the harmonized recommendations for surveillance of pubertal development, POI and/or counseling about fertility preservation in females who have received alkylating agents, HSCT and/or radiotherapy potentially exposing the ovaries or CNS.20, 21 In the present study a local abdominal irradiation dose of 24 to 36 Gy predicted the need of induced puberty. A similar cutoff of 20 to 35 Gy has earlier been described with a 22% fertility deficit in female survivors of childhood cancer.22 In the ROC analyses, the abdominal irradiation dose possibly affecting the gonads only predicted the risk for induced puberty but not the need of ERT before the age of 40 years. This is in line with the recent report by Clark et al (2020) who stated that predicting premature menopause is more challenging than predicting AOF due to the added aspect of time.2
Due to a lack of research, no recommendations regarding the risk of POI were given by the International Late Effects of Childhood Cancer Guideline Harmonization Group for females who have undergone unilateral oophorectomy.20 In a later recommendation, low-quality evidence for an increased risk of POI was presented.21 The research findings are mixed; some studies have not associated unilateral oophorectomy with an increased risk of POI,3, 4 while in a study of older survivors (median age 36 years), females with unilateral oophorectomy developed POI 7 years earlier than other survivors.9 In the present study, survivors of oophorectomy did not appear to have higher frequencies of POI or fertility issues. Hence, qualitatively it seems like survivors who had undergone unilateral oophorectomy had good outcomes in young adulthood. However, more research with longer follow-up times is needed. Similarly, survivors of low-grade CNS tumors had good endocrine and fertility outcomes, indicating that their risk of ovarian insufficiency is low. This notion is important as these patients have most often been excluded in previous studies2-4, 22 and may not receive follow up at the child oncology departments.
4.1 Methodological considerations
Our study presents a large national sample with a high response rate. No systematic response bias was detected, and, hence, the sample seems representative for the population. However, self-reports were in some cases observed not to be reliable. Some survivors mixed up medications and the indications for these medications, or menstrual cycle and bleeding for cyclic ERT, leading to contradictory information. Since comprehensive information was available, data could in these cases be corrected. In future survey studies, study-specific questions need to be constructed in a very precise manner taking this into account. Moreover, the study lacked laboratory data to confirm menopausal status.
Due to the cross-sectional design, timing of the outcome variables could not be assessed. Moreover, since the sample consists of young adults many have not yet tried to conceive a child. However, the desire of the survivors to become pregnant was known. Hence, only females who had stated that they had tried to conceive were included in analyses concerning infertility investigations and having a biological child.
Concerning the treatment variables used, large registry data was available. Nevertheless, we did not have information on which patients treated with HSCT had received TBI. Hence, we could not analyze the effect of TBI based conditioning regimens compared to non-TBI based regimens. All irradiation variables were presented as local irradiation only. Further, specific doses for alkylating agents were not available in the National Quality Registry for Childhood Cancer. Hence, drug- or dose-specific risks could not be assessed. However, based on the available data survivors could be categorized into four different groups based on the intensity of treatment.
5 CONCLUSIONS
We present a cross-sectional overview of ovarian function in a population-based cohort of young adult survivors of childhood cancer treated during the recent decades. In all, 95% of the survivors had a spontaneous menarche despite having undergone cancer treatment and the majority of survivors who had attempted pregnancy had successfully mothered a biological child. The risk of subfertility and early menopause was associated with a higher treatment burden, particularly HSCT or irradiation exposing the ovaries, and with postpubertal age at the time of diagnosis. At particular risk were survivors after myeloid leukemia, high-stage neuroblastoma or renal tumors, and a second cancer. On the other hand, females with mild to moderate treatment regimens had reassuring endocrine and fertility outcomes. The present overview will help clinicians to counsel female childhood cancer patients and their families about the possible need for ovarian hormone replacement and subfertility after completion of cancer therapy.
AUTHOR CONTRIBUTIONS
Anu Haavisto: Conceptualization; Formal analysis; Methodology; Validation; Visualization; Writing - original draft. Lena Wettergren: Conceptualization; Data curation; Funding acquisition; Investigation; Methodology; Project administration; Resources. Claudia Lampic: Conceptualization; Data curation; Funding acquisition; Investigation; Methodology; Project administration; Resources. Päivi M. Lähteenmäki: Data curation; Investigation; Methodology. Kirsi Jahnukainen: Conceptualization; Data curation; Funding acquisition; Investigation; Methodology; Project administration; Validation; Visualization; Writing - original draft. Software: N/A; Supervision: N/A. Writing - review and editing: All authors. The work reported in the article has been performed by the authors, unless clearly specified in the text.
ACKNOWLEDGEMENTS
We would like to thank all study participants for their valuable contributions. We would also like to thank the Swedish National Quality Registry for Childhood Cancer for identifying potential participants for our study and providing the data.
FUNDING INFORMATION
The Swedish Cancer Society (CAN 2013/886), the Swedish Childhood Cancer Foundation (TJ2014-0050, TJ2019-0045, PR2014-0177, PR2016-0075, PR2017-0037 and KP2020-0012), the Swedish Research Council for Health, Working Life and Welfare (2014-4689, 2019-00839), the Swedish Research Council (2017-01530), the Birgitta and Carl-Axel Rydbeck's Research Grant for Pediatric Research (2020-00335 and 2021-00079), The Cancer Foundation Finland and the Finnish Foundation of Pediatric Research.
CONFLICT OF INTEREST STATEMENT
The authors report no conflict of interest.
ETHICS STATEMENT
The Regional Ethical Review Board in Stockholm, Sweden, granted ethical approval (2015/1609-31; 2018/2688-32; 2019/01066; 2019/04603). Informed consent was obtained from all participants.
Open Research
DATA AVAILABILITY STATEMENT
Data used in the present study were population-based register data from the National Quality Registry for Childhood Cancer database as well as self-report surveys. Data is available upon reasonable request.