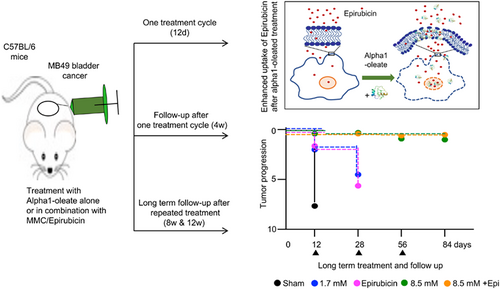
Long-term prevention of bladder cancer progression by alpha1-oleate alone or in combination with chemotherapy
Abstract
Bladder cancer is common and one of the most costly cancer forms, due to a lack of curative therapies. Recently, clinical safety and efficacy of the alpha1-oleate complex was demonstrated in a placebo-controlled study of nonmuscle invasive bladder cancer. Our study investigated if long-term therapeutic efficacy is improved by repeated treatment cycles and by combining alpha1-oleate with low-dose chemotherapy. Rapidly growing bladder tumors were treated by intravesical instillation of alpha1-oleate, Epirubicin or Mitomycin C alone or in combination. One treatment cycle arrested tumor growth, with a protective effect lasting at least 4 weeks in mice receiving 8.5 mM of alpha1-oleate alone or 1.7 mM of alpha-oleate combined with Epirubicin or Mitomycin C. Repeated treatment cycles extended protection, defined by a lack of bladder pathology and a virtual absence of bladder cancer-specific gene expression. Synergy with Epirubicin was detected at the lower alpha1-oleate concentration and in vitro, alpha1-oleate was shown to enhance the uptake and nuclear translocation of Epirubicin, by tumor cells. Effects at the chromatin level affecting cell proliferation were further suggested by reduced BrdU incorporation. In addition, alpha1-oleate triggered DNA fragmentation, defined by the TUNEL assay. The results suggest that bladder cancer development may be prevented long-term in the murine model, by alpha1-oleate alone or in combination with low-dose Epirubicin. In addition, the combination of alpha1-oleate and Epirubicin reduced the size of established tumors. Exploring these potent preventive and therapeutic effects will be of immediate interest in patients with bladder cancer.
Graphical Abstract
What's new?
The tumoricidal complex alpha1-oleate is active as a bladder cancer therapeutic and has shown efficacy in patients without evidence of toxicity. Here, the authors tested whether repeated treatment with alpha1-oleate, combined with low-dose chemotherapy, would improve long-term treatment efficacy. They treated fast-growing bladder tumors in mice with alpha1-oleate, alone or combined with Epirubicin or Mitomycin C. The first dose stopped tumor progression, providing protection lasting at least 4 weeks. Repeated treatment extended the protection. Protection was measured by bladder pathology but also expression of cancer-related genes.
Abbreviations
-
- DAPI
-
- 4′,6-diamidine-2′-phenylindole
-
- BrdU
-
- 5-bromo-2-deoxyuridine
-
- BCG
-
- Bacillus Calmette-Guérin
-
- H&E
-
- hematoxylin-eosin
-
- MMC
-
- Mitomycin C
-
- TUNEL
-
- terminal deoxynucleotidyl transferase dUTP nick end-labeling
1 INTRODUCTION
Combination therapy is widely used to improve the efficacy of cancer therapy and reduce side effects by administering lower, less toxic doses of each drug.1 The tumoricidal peptide-fatty acid complex HAMLET is broadly active against a range of cancer cells in vitro2-4 and therapeutic efficacy has been demonstrated in a murine bladder cancer model.5 Complexes formed by the N- or C-terminal alpha-helical peptides of alpha-lactalbumin show efficient tumoricidal activity and alpha1-oleate is active as a bladder cancer therapeutic with dose-dependent effects in the murine MB49 model.6 Treatment effects have further been demonstrated in patients with nonmuscle invasive bladder cancer, where intravesical alpha1-oleate instillations have been shown to trigger rapid shedding of tumor cells and tumor cell fragments into the urine, apoptosis in the tumor and a reduction in tumor size, without evidence of drug-associated toxicity.7 Cancer-related gene expression was reduced by about 80% in the treated tumors, including genes associated with bladder cancer.7 The beneficial toxicity profile suggested that alpha1-oleate might be suitable for combination therapy, to enhance the effect of established, more toxic compounds at a lower dose.
Nonmuscle invasive bladder cancer is considered as an unmet medical need by the Food and Drug Administration, due to high recurrence rates and a lack of effective new treatment options.8, 9 Adjuvant treatment is recommended after transurethral resection, to reduce the risk of progression to muscle-invasive disease.8, 10 Immediate intravesical instillation of chemotherapy is recommended in patients with a low recurrence risk, while in high-risk patients, Bacillus Calmette-Guérin (BCG) instillations are recommended for at least 1 year after a first intravesical instillation of chemotherapy.8 In intermediate-risk patients, the guidelines recommend either further intravesical instillations of chemotherapy or a minimum of 1 year of BCG treatment after a first instillation of chemotherapy.8, 11-13
Our study investigated if combining alpha1-oleate with a low dose of Epirubicin or Mitomycin C (MMC) might improve therapeutic efficacy. MMC and Epirubicin are widely used for intravesical chemotherapy of bladder cancer, affecting recurrence rates and prolonging disease-free intervals.14-18 MMC targets DNA and induces interstrand and intrastrand cross-links, suppressing DNA synthesis primarily during late G1 and S phases19-21 and inhibiting tumor growth in animal models and clinic studies.14, 15, 18 Like MMC, Epirubicin acts by intercalating DNA strands and inhibiting DNA and RNA synthesis. Epirubicin also triggers DNA cleavage by topoisomerase II that leads to cell death. Moreover, Epirubicin generates free radicals that cause DNA damage and cell death.14, 15, 18, 22 Like Epirubicin and MMC, alpha1-oleate targets tumor cell nuclei and interacts with histones and spliceosome constituents,23 suggesting a possible molecular basis for synergy with Epirubicin and MMC. In our study, alpha1-oleate alone or in combination with Epirubicin or MMC was shown to maintain a near healthy bladder phenotype long-term, suggesting the potential for efficient prevention of tumor progression using these approaches.
2 MATERIALS AND METHODS
2.1 Chemicals and antibodies
Oleic acid (Croda, batch number: 0001120439), poly-l-lysine solution (Sigma, Cat no RNBF4239), Richard-Allan Scientific Signature Series Hematoxylin and Eosin-Y (Thermo Scientific, Cat no 7211 and 7111), Epirubicin (Sigma-Aldrich, Cat no E9406) and Mitomycin C (Sigma-Aldrich, Cat no M0440), rabbit polyclonal anti-VEGF (Abcam, Cat no ab46154), rabbit monoclonal anti-Cyclin D1 (Thermo Fisher, Cat no sc-8396), FITC-dextran 70 kDa (Sigma, Cat no 46945-100MG-F).
2.2 Peptide synthesis and complex generation
For our study, Fmoc solid phase chemistry was used to synthesize the 39 amino acid peptide (aa 1-39, Ac-KQFTKAELSQLLKDIDGYGGIALPELIATMFHTSGYDTQ-OH), achieving >95% purity (Polypeptide group, France). To form the alpha1-oleate complex, the peptide was mixed with sodium oleate at a 1:5 molar ratio as previously described.6, 24 The stock solution was diluted in PBS to the appropriate concentration for each experiment.
2.3 Bladder cancer model
Female C57BL/6 mice from 7 to 12 weeks old were bred at the Department of Laboratory Medicine, Lund University, and were anesthetized by intraperitoneal injection of a mixture of ketamine (1.48 mg in 100 μL of 0.9% NaCl solution, Intervet) and xylazine (0.22 mg in 100 μL of 0.9% NaCl solution, Vetmedic). MB49 (RRID:CVCL7076) mouse bladder carcinoma cells were provided by Sara Mangsbo, Uppsala University, Sweden. MB49 bladder cancer was established as previously described.5 On day 0, the bladder was emptied and preconditioned by intravesical instillation of 100 μL of poly-l-lysine solution (0.1 mg/mL) through a soft polyethylene catheter (Clay Adams) with an outer diameter of 0.61 mm. After 30 minutes, MB49 mouse bladder carcinoma cells (2 × 105 cells in 50 μL cell media) were instilled. Mice were randomly assigned to the different treatment groups and subjected to 100 μL instillations with alpha1-oleate (1.7 or 8.5 mM) or chemotherapeutic drugs (MMC 25 μg, Epirubicin 25 μg), alone or in combination on days 3, 5, 7, 9 and 11. The administration dose and timing interval of alpha1-oleate were chosen based on the previously published dose-escalation study.6 To detect the synergy of these chemotherapeutic drugs with alpha1-oleate, we used two doses of alpha1-oleate and lower doses of Epirubicin and MMC than in most publications.25, 26 Sham treated mice received PBS. The catheter was left in place for about 1 minute, but as the mice remained under anesthesia, the time to voiding was 2 to 3 hours. Groups of 5 to 6 mice were used for each treatment and two independent experiments were performed for each condition. All experiments were performed with mycoplasma-free cells.
2.4 Macroscopic examination and histology
Gross pathology was quantified by inspection of the bladders at sacrifice, and quantifying bladder size, bladder weight, edema and hyperemia (scale 0-10). Bladders assigned a 0 were indistinguishable from healthy controls and bladders assigned a 10 were large, tumor filled, edematous and hyperemic. Scoring was blinded and performed in an unbiased manner.
For histology, bladders were fixed in 10% (v/v) neutral buffered formalin and then processed for paraffin-embedding. Successive 5 μm sections were collected from the center of each tumor and sections capturing the largest tumor circumference were selected for histology and immunohistochemistry. For Hematoxylin-Eosin (H&E) staining, Hematoxylin 7211 was used followed by Eosin-Y 7111 for counterstaining. Images were captured using the AX10 microscope (Carl Zeiss). Tumor areas were calculated using the ImageJ software.
2.5 Immunohistochemistry
For immunohistochemistry, paraffin sections were deparaffinized, and rehydrated, followed by an antigen retrieval step (DAKO Target Retrieval Solution, S1699), permeabilized (0.25% Triton X-100 in PBS) for 30 minutes, and blocking with 5% fetal calf serum (FCS) in PBS for 1 hour at room temperature. The sections were then incubated with primary antibodies to Cyclin D1 (1:50, Santa Cruz Biotechnology, sc-8396), or VEGF (1:200, Abcam, ab46154) overnight at 4°C, followed by Alexa-Fluor 488- or 568-labeled secondary antibodies (1:200, Invitrogen, 11034 and A11036) incubation for 1 hour at room temperature. Nuclei were counterstained with DAPI (4′,6-diamidine-2′-phenylindole, 1:1000, Abcam, ab228549) for 15 minutes at room temperature; and examined by LSM 900 confocal microscopy (Carl Zeiss). Fluorescence quantification was done using ImageJ software.
2.6 Gene expression analysis
Frozen bladder tissue was pulverized using liquid nitrogen, as previously described.27 Total RNA was extracted with on-column DNA digestion (RNeasy Mini kit, Qiagen). Exactly 100 ng of total RNA was amplified using the Affymetrix WT PLUS Reagent Kit and then fragmented. Next, labeled antisense RNA (aRNA) was hybridized onto Clariom S Mouse HT or Mouse Genome 430 PM arrays (all Affymetrix) for 16 hours at 45°C, washed, stained (Applied Biosystems, ThermoFisher Scientific) and scanned using the GeneTitan or GeneAtlas system. Relative expression levels were calculated against bladders from untreated healthy control mice and genes with an absolute fold change ≥4.0 and 2.0 were considered as differentially expressed. Heat-maps were constructed using the Gitools 2.1.1 software. Differentially expressed genes were functionally characterized using the Ingenuity Pathway Analysis (IPA, Qiagen) software. Three mice were included for sham and two mice for treatment and healthy groups.
2.7 Epirubicin and FITC-dextran (70 kDa) imaging in vitro
Epirubicin is auto-fluorescent and shows a broad absorption maximum at 480 nm.28 To visualize uptake by tumor cells, Epirubicin (15 μM) was added to A-549 cells (RRID:CVCL0023) grown on μ-slide coverslips (2 × 105 cells, Ibidi, Cat no 80606). The fluorescence signal was monitored for 10, 20 and 60 minutes at 37°C. To address if alpha1-oleate affects Epirubicin uptake, cells were pretreated with alpha1-oleate or PBS for 20 minutes, washed and exposed to Epirubicin. To visualize the uptake by tumor cells, FITC-dextran (final concentration 0.3 mg/mL) was added to A-549 cells grown on μ-slide coverslips (2 × 105 cells, Ibidi, Cat no 80606) in the presence or absence of alpha1-oleate pretreatment (20 minutes, room temperature). The FITC-dextran uptake was quantified after 5 and 20 minutes, comparing the extracellular to the intracellular staining intensity, using ImageJ software.
2.8 Labeling of the alpha1-oleate complex and co-localization with Epirubicin
Alpha1 peptide was N-terminally labeled with the photo-stable dye JF549 and the complex with oleic acid was generated as described.7 A-549 cells grown on μ-slide coverslips (2 x 105cells) were pretreated with labeled alpha1-oleate for 20 minutes, followed by exposure to Epirubicin for 10 minutes. Cells were fixed with 2% paraformaldehyde, mounted with ProLong Glass antifade mountant (Thermofisher, Cat no P36980) and imaged using a confocal microscope (Zeiss LSM 900) with 488 and 561 nm excitation wavelengths. Fluorescence signal intensities, inside the cells and the nucleus, were quantified using ImageJ software.
2.9 Cell proliferation
The proliferation of A-549 cells was quantified using the 5-bromo-2-deoxyuridine (BrdU) ELISA assay. A-549 cells (1 × 104 cells/well) were seeded on 96-well plates (Tecan Group Ltd) and cultured overnight. Cells were treated with alpha1-oleate (7 and 21 μM), Epirubicin (15 μM) or Mitomycin C (15 μM), alone or in combination, and incubated in serum free medium for 1 hour at 37°C, with 5% CO2. BrdU labeling solution (Cell Proliferation ELISA, BrdU kit, Roche, Cat no 11647229001) was added and incubated for 2 hours at 37°C. After fixation, cells were incubated with anti-BrdU antibodies, followed by substrate solution, and BrdU staining was quantified by measuring absorbance at 450 nm (reference wavelength: 690 nm), using a microplate reader (Infinite F200, Tecan). The assay was done in technical triplicate and repeated twice.
2.10 DNA fragmentation
DNA fragmentation was detected using the terminal deoxynucleotidyl transferase dUTP nick end-labeling (TUNEL) assay (Click-iT TUNEL Alexa Fluor 488 imaging assay kit, ThermoFisher). A-549 cells were seeded in 8-well chamber slide (2 × 104 cells/well) cultured overnight (37°C, 5% CO2) and incubated with alpha1-oleate (21 μM) or Epirubicin (15 μM) in serum-free RPMI-1640 at 37°C. FCS was added after 1 hour and cells were fixed after a total of 3 hours (2% PFA, 15 minutes), permeabilized (0.25% Triton X-100 in PBS, 20 minutes) and incubated with TUNEL reaction mixture containing TdT for 60 minutes at 37°C. After the TUNEL reaction, cells were incubated with Click-iT reaction mixture for 30 minutes. Cells were counterstained with DAPI (20 μg/mL, 10 minutes), mounted in Fluoromount aqueous mounting media, and examined by LSM 900 confocal microscopy (Carl Zeiss). Fluorescence signal intensities were quantified using ImageJ software.
2.11 Colony assay
Long-term effects on cell viability were quantified using the colony assay.7 A-549 cells were seeded on 24-well plates (1000 cells/well in 1 mL RPMI with FCS), allowed to adhere for 24 hours, washed in PBS and treated with alpha1-oleate (1 or 7 μM), Epirubicin (5, 10 or 50 nM) or Mitomycin C (5, 10 or 50 nM), alone or in combination, in 1 mL of serum-free RPMI for 1 hour. After addition of FCS (5%) the cells were incubated at 37°C in 5% CO2 for 7 days or until visible colonies were present in the PBS control. Plates were fixed in cold methanol and stained with crystal violet for 1 hour. Staining intensity and percent colony area was quantified using ImageJ software.
2.12 Statistical analysis
Results are presented as means ± SEMs. The D'Agostino & Pearson normality test was used to determine if the data followed a Gaussian distribution. Statistical differences were determined by Mann-Whitney U test (two group comparisons) or One-way ANOVA (multiple comparisons) with Holm-Šídák's multiple comparisons test. Statistical significance was determined by GraphPad Prism version 7.
3 RESULTS
3.1 Short-term effects in the murine MB49 bladder cancer model of alpha1-oleate, Epirubicin or MMC alone or in combination
Bladder cancer was established by intravesical instillation of MB49 cells (Figures 1 and 2).5 Female mice were used for technical reasons and 100% of the mice developed tumors. In sham treated mice, receiving PBS, tumor progression was rapid, and the mice were sacrificed on day 12. Bladders were enlarged, edematous and hyperemic compared to healthy controls (11/11 mice; Figures 1B, 2A, S1 and S2). Large tumors were visible, and the tumor areas were quantified in H&E-stained whole-bladder tissue sections (Figures 1G, 2F and S3). A gross pathology score was assigned to individual mice, based on the bladder weight, bladder size, edema, hyperemia and tumor area. Bladders assigned a 0 were indistinguishable from healthy controls and bladders assigned a 10 were large, tumor filled, edematous and hyperemic. Scoring was blinded and performed in an unbiased manner. In the sham treated group, a median gross pathology score of six was recorded after 12 days (range 4-10; Figures 1C-F and 2B-E). The tumor area was further evaluated by histopathology of whole bladders.
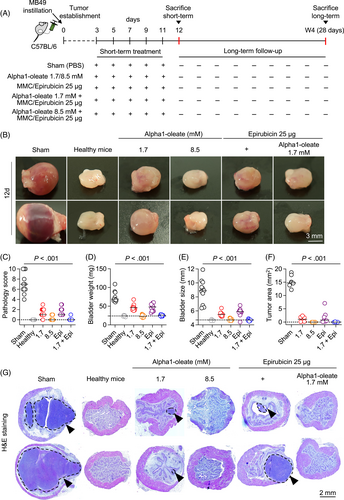
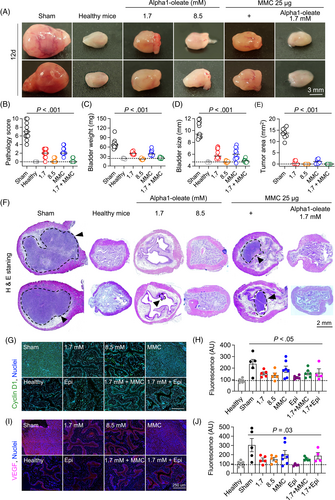
Treatment was administered intravesically, on days 3, 5, 7, 9 and 11 and mice were sacrificed on day 12 (Figure 1A). Tumor progression was delayed in mice receiving alpha1-oleate, Epirubicin or MMC, respectively (P < .001 compared to sham; Figures 1B-G, 2A-F and S1-S3). The higher dose of alpha1-oleate (8.5 mM) was the most efficient single treatment (P < .001 compared to sham).
Combination therapy was administered, using the same protocol (Figure 1A). Intravesical instillation of alpha1-oleate (1.7 mM) was followed by Epirubicin or MMC instillation after 2 hours (25 μg in 100 μL/dose). Combination treatment prevented tumor progression, as shown by the absence on day 12 of gross pathology, compared to healthy, tumor free controls. Tumor tissue was not detected in H&E-stained whole bladder sections (P < .001 compared to sham; Figures 1G, 2F and S3). The higher dose of alpha1-oleate alone (8.5 mM) was as efficient as the combination of alpha1-oleate (1.7 mM) with Epirubicin or MMC (P < .001 compared to sham). In contrast, mice treated with the lower dose of alpha1-oleate (1.7 mM), Epirubicin or MMC alone developed small tumors. The results suggest that alpha1-oleate delays bladder cancer development alone or in combination with Epirubicin or MMC.
Treatment was accompanied by a reduction in tumor biomarkers Cyclin D1 and vascular endothelial growth factor (VEGF) (Figure 2G-J). A further comparison of whole bladder mounts detected a difference in tissue distribution of these markers. In the sham group, Cyclin D1 and VEGF were present in the epithelial layer, the lamina propria and the muscular layer of the bladders, indicating an invasive growth pattern. In treated mice, in contrast, staining was restricted to the epithelium, potentially suggesting less invasive growth of cells expressing these markers.
3.2 Long-term protection against tumor progression
Based on the positive effects of the first treatment round, mice were followed for 4 weeks without further intervention (Figures 3 and S4). Mice treated with alpha1-oleate (8.5 mM) or alpha1-oleate (1.7 mM) combined with Epirubicin or MMC were protected from tumor progression and did not differ from healthy mice. In contrast, groups that had received the low dose of alpha1-oleate alone (1.7 mM), or Epirubicin or MMC alone, showed evidence of tumor progression (not significant compared to sham; Figure 3A-J).
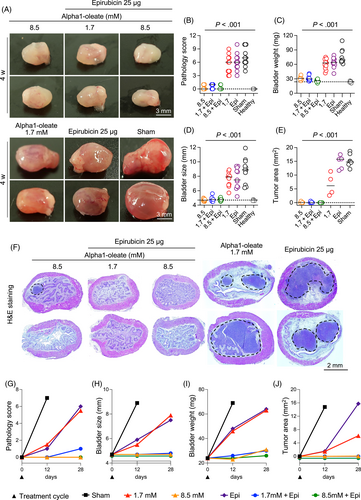
To delay tumor development even further, mice were subjected to repeated treatment rounds at 4-week intervals. Each treatment round included five instillations (Figure 4A) and the treatment effects were evaluated after 8 and 12 weeks, respectively (Figure 4B-P). Strong protective effects were detected in mice receiving alpha1-oleate alone (8.5 mM) or in combination with Epirubicin or MMC (Figures 4B-P, S5 and S6). The pathology scores, bladder sizes and bladder weights did not differ from those of healthy mice after 8 or 12 weeks (Figure 4Q-T).
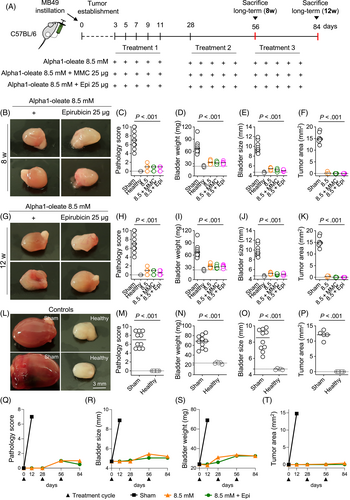
3.3 Effects on cancer-related gene expression
To understand the molecular basis of these strong protective effects, whole-bladder RNA was subjected to gene expression analysis. Sham-treated mice showed markedly elevated gene expression (heat maps in Figure 5A). More than 1000 genes were upregulated and more than 2000 suppressed, compared to healthy mice (Figures 5B and S7). Biofunctions such as tumor growth, cell movement and cell death were strongly upregulated, confirming the transcriptional overactivity of growing tumors (Figure S8).
In contrast, cancer-related gene expression was virtually absent in mice treated with alpha1-oleate (8.5 mM) alone or in combination with Epirubicin or MMC (Figures 5A,B, S7 and S8). By Principal Component Analysis (PCA) analysis, these treatment groups were shown to cluster adjacent to healthy tissue, consistent with the lack of tumor development and low expression of cancer-related genes in these mice. Sham-treated mice, in contrast, formed a cluster distant from healthy tissue and groups receiving Epirubicin alone were clustered closer to the sham and more distant from the healthy group, consistent with a moderate regrowth of the tumor and expression of cancer-related genes in these groups (Figure 5C).
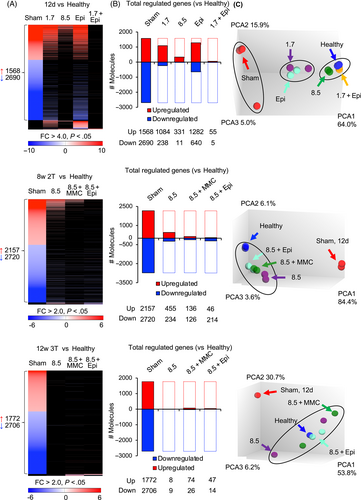
Treatment further inhibited the expression of genes specifically associated with bladder cancer (Figure 6A). Osteopontin (SPP1), which triggers the release of vascular endothelial growth factor (VEGF) and matrix metallo-proteinases (MMP), were strongly inhibited, as well as the proliferation marker Ki67 and survivin (BIRC5). Survivin is an inhibitor of apoptosis and Wnt signaling, and highly expressed in the sham group compared to healthy controls (Fold change 285; Figure 6B).
A synergistic effect on gene expression was detected on day 12 in mice receiving the low dose of alpha1-oleate (1.7 mM) and Epirubicin or MMC. The combinations showed a more pronounced effect on cancer gene expression than each treatment alone (Figure 6C,D). After 4 weeks and during long-term follow-up for 8 or 12 weeks, the protective effect of the higher dose of alpha1-oleate (8.5 mM) alone was too pronounced for synergy to be detected.
3.4 Treatment of established tumors
We further addressed if, in addition to delaying tumor progression, alpha1-oleate also reduces the size of established tumors. Treatment was initiated on day 5 or 7 after the instillation of MB49 cells, when macroscopically visible tumors had been formed. To define the treatment effect, bladder weights, bladder sizes and pathology scores were assessed in mice sacrificed before the onset of treatment on day 5 or 7 and at sacrifice on day 15 or 17, respectively (Figure S9).
Tumor progression was arrested by alpha1-oleate (8.5 mM) treatment alone or in combination with Epirubicin or MMC (Figure S9). Importantly, treatment also reduced the tumor size and pathology score compared to the onset of treatment, suggesting that treatment removes pre-existing tumor and prevents further tumor development (Figure S9).
3.5 Alpha1-oleate enhances tumor cell uptake and nuclear translocation of Epirubicin
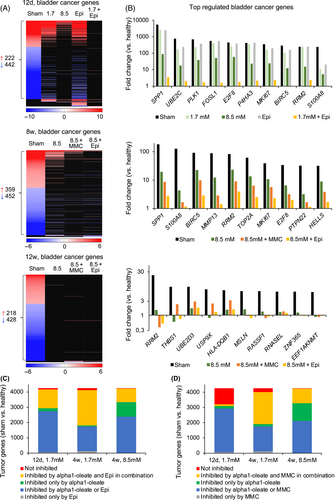
The synergistic effects in the bladder cancer model suggested that, in addition to directly killing tumor cells, alpha1-oleate may enhance the antitumor effects of Epirubicin and MMC (Figure 7A).
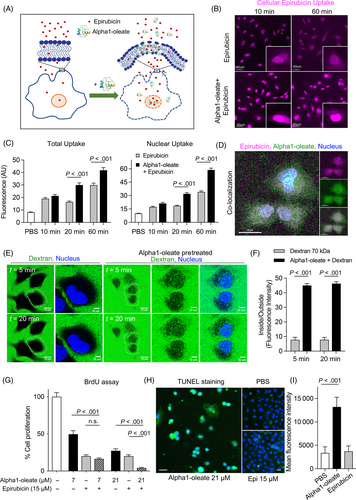
Alpha1-oleate has previously been shown to interact with the plasma membrane of tumor cells and affect membrane structure.24 To examine if alpha1-oleate facilitates the uptake of Epirubicin, lung carcinoma cells (A-549) were pretreated with alpha1-oleate (21 μM, 20 minutes), followed by Epirubicin (15 μM, 10 or 60 minutes; Figure 7B,C). A significant, time dependent increase in cellular Epirubicin levels was detected, compared to cells not exposed to alpha1-oleate (Figure 7C). Furthermore, increased nuclear translocation of Epirubicin was detected in alpha1-oleate treated cells and significant co-localization suggested a basis for a combined effect (Figure 7D and S10). The effect on membrane permeability was confirmed using fluorescein-labeled dextran particles (70 kDa), which were taken up more efficiently by cells pretreated with alpha1-oleate than by cells exposed to dextran alone (live cell imaging; Figure 7E,F). No further increase was observed after 5 minutes suggesting an immediate effect of alpha1-oleate on membrane permeability. A similar effect of alpha1-oleate on Epirubicin uptake was observed for the MB49 bladder cancer cells, which were used in the animal model to establish bladder cancer (Figure S11).
Epirubicin achieves broad toxicity by intercalating DNA strands.21, 23, 29 Acute effects on cell proliferation were therefore examined, using BrdU as a read out after 3 hours. Each compound inhibited BrdU incorporation in a dose-dependent manner and the combination of alpha1-oleate and Epirubicin was more efficient than either compound alone suggesting a combined effect on cell proliferation and death (P < .001 and P = .0034; Figure 7G). MMC was less potent than Epirubicin, alone or in combination with alpha1-oleate (P < .001; Figure S12). DNA strand breaks defined by the TUNEL assay were detected in alpha1-oleate treated cells after 3 hours but not in Epirubicin treated cells (Figure 7H,I).
4 DISCUSSION
The alpha1-oleate complex kills bladder cancer cells in vitro and has shown safety and therapeutic efficacy in animal models of bladder cancer.6 Recently, promising antitumor effects were demonstrated in a placebo-controlled bladder cancer trial, without drug-related toxicity.7 Here, alpha1-oleate was shown to delay tumor progression long-term, in the MB49 bladder cancer model. Treatment was highly efficient, both short-term and long-term, after repeated treatment rounds. The therapeutic effect was further enhanced, by combining alpha1-oleate with low doses of Epirubicin or MMC, especially at 4-week follow-up after the initial treatment cycle, suggesting that alpha1-oleate may increase the efficacy of chemotherapeutic drugs at lower, less toxic concentrations. In addition to the morphological evidence, the gene expression profiles of treated tissues shifted toward a healthy phenotype in mice treated with alpha1-oleate alone or in combination with Epirubicin or MMC. The findings suggest that tumor cells are targeted and removed by this treatment regimen and point to a possible combination therapy approach in this patient group, for substances whose effects are increased by alpha1-oleate.
The effect of combination therapy was mainly observed in the group followed for 4 weeks after the initial treatment cycle, consistent with the alpha1-oleate specific tumor cell priming, facilitating the cellular uptake and nuclear translocation of Epirubicin. Alpha1-oleate has high affinity for the plasma membrane, triggering membrane blebbing and tubulation in tumor cells.7, 30 Potent membrane effects of alpha1-oleate and the alpha2-oleate complex, formed by the C-terminal alpha-lactalbumin peptide, have also been observed in giant unilamellar vesicles (GUVs), including the division of single GUVs in a protocell model.31 The facilitated uptake of Epirubicin by tumor cells may thus reflect increased membrane permeability induced by alpha1-oleate. Nuclear translocation of Epirubicin was facilitated, and strong effects of both alpha1-oleate, Epirubicin and MMC on tumor cell proliferation suggested that alpha1-oleate may facilitate the access of these compounds to the nuclei. Alpha1-oleate has been shown to bind to histones and spliceosomes affecting chromatin structure and transcriptional activity.22 Epirubicin and MMC are well known inhibitors of RNA, DNA and protein synthesis.32 Furthermore, the ability of alpha1-oleate to trigger apoptosis-like cell death has been documented earlier as alpha1-oleate triggered an apoptotic response in tumor biopsies from alpha1-oleate-treated patients with bladder cancer,7 suggesting combined and independent effects of the compounds. Importantly, Epirubicin alone was not protective under these experimental conditions.
Our study suggests that tumor progression can be delayed by repeated intravesical treatment with alpha1-oleate alone or in combination with low doses of Epirubicin or MMC. The data supports two scenarios: prevention of tumor progression or treatment of an established tumor. Early treatment and repeated treatment cycles prevented tumor development short-term and tumor progression long-term, resulting in a near healthy phenotype at follow-up after 8 to 12 weeks. In contrast, the sham-treated mice only survived until day 12. Treatment effects on established tumors were also documented. The therapeutic effects were further resolved and confirmed by transcriptomics technology, revealing general inhibition of cancer-related genes and functions. The expression of bladder cancer-related genes was markedly reduced in the treated mice and the effect increased after repeated rounds of treatment, confirming the extended protective effect. By PCA analysis, the treated tissues were shown to resemble healthy tissues, as cancer-related gene expression was turned off.
The therapeutic options are limited in patients with nonmuscle-invasive bladder cancer (NMIBC) and recurrence rates are high.33-38 In a recent review of potential new treatment options in nonmuscle-invasive bladder cancer, Steinberg et al. mentioned that only two treatments have been approved in the past 30 years for this indication, including Valrubicin (anthracycline) in September 1998 and pembrolizumab (antiprogrammed cell death protein-1 [PD-1]) in January 2020.39 The authors mention that “lack of consensus on clinical trial endpoints and appropriate control arms has slowed research advancements, along with the challenges of enrolling patients in early-stage clinical trials. Following BCG failure in high-risk NMIBC, the standard of care is radical cystectomy. Given the BCG shortage and the morbidities of radical cystectomy, there is an urgent need for new therapies for high-risk NMIBC.”39
New therapeutic options for this patient group are actively being explored using a range of technologies, particularly in patients with disease recurrence after BCG treatment. Device-assisted hyperthermia was shown to increase the efficacy of intravesical chemotherapy, but side effects reduced compliance.13, 40, 41 Intravesical, oncolytic virus-based therapy was recently reported to achieve a complete response in 53.4% of patients with BCG-unresponsive carcinoma in situ, in a phase III trial.38 The authors discuss the development of biomarkers to select patients suitable for this therapy. A 41% response rate to Pembrolizumab was reported in patients with BCG unresponsive disease, but side effects were prevalent, limiting compliance.42
The present study identifies alpha1-oleate as an active drug candidate in the murine bladder cancer model with short-term and long-term effects. The complex appeared to remove emergent tumor cells, thereby preventing the formation of a significant tumor mass. In addition, alpha1-oleate treatment reduced the size of established tumors, confirming a direct, tumoricidal effect. These two scenarios may be clinically relevant to reduce the size of existing tumors and prevent or delay recurrences. Results of a placebo-controlled Phase I/II trial have provided evidence of rapid antitumor effects of alpha1-oleate after repeated intravesical instillations during 1 month without significant side effects. A subsequent dose-finding study detected a dose-dependent increase in treatment efficacy. In view of the low toxicity, it might be feasible to use alpha1-oleate as a general approach to early treatment of nonmuscle invasive bladder cancer. The potential of increasing tumor access of other drugs at lower, less toxic concentrations points to an adjuvant treatment scenario, in addition to the stand-alone treatment. These options should be further explored in future clinical trials.
AUTHOR CONTRIBUTIONS
Tran Thi Hien: Conceptualization, data curation, formal analysis, methodology, writing-original draft, writing-review and editing. Ines Ambite: Data curation, formal analysis, methodology, writing-original draft, writing-review and editing. Murphy Lam Yim Wan: Data curation, formal analysis, writing-original draft, writing-review and editing. Michele Cavalera: Data curation, tissue staining and analysis. Parisa Esmaeili: Data curation, formal analysis. Arunima Chaudhuri: Data curation, formal analysis. Samudra Sabari: Data curation. Marek Babjuk: Conceptualization of combination therapy, methodology, writing-review. Catharina Svanborg: Conceptualization, resources, supervision, methodology, project administration, writing and editing. The work reported in the article has been performed by the authors, unless clearly specified in the text.
ACKNOWLEDGEMENTS
The authors thank Daniel Butler for his expertise in tissue physiopathology and his help in assessing bladder pathology scores.
FUNDING INFORMATION
The Swedish Research Council, the Swedish Cancer Society (Cancerfonden), the Royal Physiographic Society in Lund, HAMLET Pharma and the European Union's Horizon 2020 research and innovation program under grant agreement No. 954360.
CONFLICT OF INTEREST STATEMENT
Catharina Svanborg holds shares in HAMLET Pharma, as a representative of scientists in the HAMLET group. Patents have been filed to protect the use of alpha1-oleate complex in combination therapy. Other authors do not have a conflict of interest to declare.
ETHICS STATEMENT
Experiments were approved by the Malmö/Lund Animal Experimental Ethics Committee at the Lund District Court, Sweden (nos M118-16, 4528-20 and 2211-21). Animal care and protocols followed institutional, national and European Union guidelines and were governed by the European Parliament and Council Directive (2016/63, EU), the Swedish Animal Welfare Act (Djurskyddslagen 1988:534), the Swedish Welfare Ordinance (Djurskydssförordningen 1988:539) and Institutional Animal Care and Use Committee (IACUC) Guidelines.
Open Research
DATA AVAILABILITY STATEMENT
The microarray data has been deposited in the NCBI's Gene Expression Omnibus repository under accession number GSE209883. All other data that support the findings of our study are available from the corresponding author upon reasonable request.