RETRACTED: Oxaliplatin activates P53/miR-34a/survivin axis in inhibiting the progression of gastric cancer cells
Qiang Guo and Xin-Yuan Wang contributed equally to this work.
Abstract
Introduction
The purpose of this research was to determine how the P53/microRNA-34a (miR-34a)/survivin pathway contributes to oxaliplatin-induced (L-OHP) cell inhibition in gastric cancer.
Methods
The BGC-823 gastric cancer cells were selected, and we examined their viability following treatment with L-OHP at different concentrations and time periods. The expression levels of miR-34a, P53, and survivin in the cells were determined.
Results
In the 12- and 24-h groups, drug concentration of 15 μg/cm² (p < .005 in both) significantly lowered cell viability. In comparison to the control group, miR-34a mRNA expression, P53 mRNA expression, and protein expression were all significantly greater in the 24-h group (p = .0324, p = .0069, p = .0260, respectively), but survivin mRNA and protein expressions were significantly lower than those in the control group (p = .0338, p = .0032, respectively). There was a significant decrease in gastric cancer cells in the miR-34a overexpression group (p = .0020), a significant increase in P53 mRNA and protein expression compared to the control group (p = .0080, p = .0121, respectively), and a significant decrease in survivin mRNA and protein expression compared to the control group. (p = .0213, p = .0069, respectively).
Conclusion
Oxaliplatin inhibits tumor growth, invasion, and metastasis by upregulating miR-34a, activating the expression of the upstream P53 gene, and driving the downregulation of survivin (P53/miR-34a/survivin axis) in BGC-823 gastric cancer cells.
1 INTRODUCTION
One of the most frequent forms of cancer worldwide, gastric cancer is a devastating disease. Second only to pancreatic cancer in China in terms of mortality rate, gastric cancer accounts for 40% of all cancer deaths in the country.1 Surgery, chemotherapy, and endoscopic therapy are the standard clinical therapies for stomach cancer at the moment. Patients with early-stage gastric cancer are good candidates for endoscopic therapy, but by the time they present to a doctor with clinical symptoms, the cancer has already progressed.2 Therefore, chemotherapy and radical surgical resection would be the only treatment options. When a primary tumor metastizes, it indicates the patient no longer has an option for surgery, and chemotherapy becomes the primary treatment.
Third-generation platinum derivatives like oxaliplatin (L-OHP) are the most widely used drugs for chemotherapy in clinical practice today. However, its precise molecular mechanism of action remains unclear. To provide novel clinical therapeutic ideas and solutions for progressive gastric cancer treatment and drug resistance, this study aims to understand the molecular mechanism of L-OHP in the treatment of gastric cancer.
Combinations of L-OHP with other antitumor medications such as leucovorin and 5-fluorouracil are occasionally used as first-line therapy for advanced gastric cancer.3 L-OHP is a new group of platinum compounds, and the primary mechanism by which they cause apoptosis and DNA damage is the formation of platinum DNA adducts.4 Furthermore, the platinum-based drug compounds targeted non-DNA molecules, resulting in cytotoxicity and proapoptotic effects.5, 6 miRNAs are a class of small noncoding RNAs that range in length from 20 to 22 nucleotides.7 Numerous biological processes such as chromatin modification, genomic imprinting, tumorigenesis, and cell differentiation have been revealed to involve miRNAs.8 Extensive investigations have shown that L-OHP is closely linked to the regulation of miRNAs in cancer.9, 10 However, the specific regulation axis of L-OHP in gastric cancer is still unknown.
The P53 gene is one of the most prevalent human antioncogenes. The wild-type P53 protein has a regulatory influence on the cell cycle and is thought to repair DNA (despite its short half-life).11 Tumor growth and metastasis are both exacerbated by mutant P53 because it loses its tumor-suppressing function and acquires new carcinogenic properties.12 Survivin or baculoviral inhibitor of apoptosis repeat-containing 5, is a recently discovered protein in this family. Tumor growth and its progression are linked to survivin's potential to suppress apoptosis (programmed cell death) and enhance cell proliferation.13 P53 protein has been shown to inhibit the G2 phase and down-regulate survivin, leading to an increase in apoptosis in tumor cells.12, 14 However, the action mechanism between P53 and L-OHP in gastric cancer remains unclear.
2 MATERIAL AND METHODS
2.1 Material and instruments
We purchased the BGC-823 cells from the Shanghai Institute of Cell Biology, Chinese Academy of Sciences.
The Roswell Park Memorial Institute (RPMI) 1640 Medium, trypsin (0.25%), phosphate-buffered saline, dimethyl sulfoxide, radioimmunoprecipitation assay (RIPA) lysate, bicinchoninic acid assay (BCA) protein concentration kit, protease inhibitor (PMSF), sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel preparation kit, enhanced chemiluminescence (ECL) plus ultrasensitive luminescent solution, 4× protein loading buffer (containing dithiothreitol [DTT]), 10× Running buffer, and 10× TBST buffer (a mix of tris-buffered saline and Polysorbate 20) were all purchased from Beijing Solarbio Technology Co., Ltd. The fetal bovine serum (FBS) was purchased from Hangzhou Sijiqing Bioengineering Materials Co. Ltd. The Opti-MEM I Reduced Serum Medium and Lipofectamine 2000 were purchased from Thermo Fisher Scientific (China) Co., Ltd. The cell proliferation and toxicity assay kit (Cell Counting Kit-8 [CCK-8]) was acquired from Dalian Meilun Biotechnology Co. The M221 direct-load color pre-stained protein marker was sourced from Beijing Kangrun Chengye Biotechnology Co., Ltd. Cell Signaling Technology Company supplied the rabbit monoclonal P53 antibody (#9282), rabbit monoclonal beta-actin (13E5) antibody (#4970), and rabbit monoclonal survivin (71G4B7) antibody (#2808). The horseradish-labeled goat anti-rabbit immunoglobulin G secondary antibody was sourced from Beijing Zhongshan Jinqiao Biotechnology Co. The RNA extraction and miRNA fluorescence polymerase chain reaction (PCR) kits were purchased from Shanghai GenePharma Co., Ltd.
Here are the instruments we used in the study: the cell incubator (Thermo Fisher Company), water purifier (Barnstead Company), Olympus CX31 microscope (Olympus Company), enzyme marker (Thermo Fisher Company), fluorescence inverted microscope TE2000-U (Nikon Company), electrophoresis electrotransfer equipment (Bio-Rad Company), NanoDrop 2000 spectrophotometer (Thermo Fisher Company), Applied Biosystems 7900 real-time PCR instrument (Applied Biosystems Company), small desktop high-speed refrigerated centrifuge (Eppendorf Company), and automatic digital gel image analysis system (Tanon Company).
2.2 Cell culture
The BGC-823 cells were routinely cultured in a cell culture incubator with 5% CO₂ at 37°C in a 100 mm culture dish with RPMI 1640 Medium containing 10% FBS. Microscopic examination revealed that inoculation ratios of 1:2 or 1:3 were used and that cell passages were completed when cell density reached 85%–90%.
2.3 Cell treated with oxaliplatin
At 37°C and 5% CO2, the cells were routinely grown in an incubator. After microscopically observing that the cell density in the culture had reached about 80%, a final concentration of 15 μg/cm² of the L-OHP was added to the culture. Cells treated with L-OHP were further incubated for another 12 h (called the 12-h group) and 24 h (24-h group) in this study.
2.4 Transfected cells
After an overnight culture in 96-well plates, cells were transfected with 5 pmol of the miR-34a and negative control (NC) fluorescent plasmids in 25 μL serum-free Opti-MEM medium. After incubating the serum-free Opti-MEM medium for 5 min at room temperature, 0.5 μL of Lipofectamine 2000 was added to the 25 μL of the medium and gently mixed. Lipofectamine 2000 was diluted, added to the diluted miR-34a and NC fluorescent plasmids, gently mixed, and incubated for 5 min at room temperature. Before adding the above-mentioned miR-34a and NC fluorescent plasmid containing Lipofectamine 2000, the original media of incubated cells in the 96-well plate was discarded. The cells were then allowed to grow in culture for 24–48 h. The medium of the transfection cells was changed every 4–6 h, and the cells were analyzed using an inverted fluorescence microscope. The +34a and NC groups represent the cells that were successfully transfected.
2.5 Analysis of cell viability with cell counting Kit-8
After preparing cell suspensions, 100 μL of the medium was used to inoculate 96-well plates, and the plates were incubated at 37°C with 5% CO₂ until the cells adhered to the surface of the well. Different concentrations of L-OHP (5, 10, 15, 20, and 25 μg/cm²) were introduced into the wells. A well devoid of L-OHP served as a control in the meantime. The L-OHP-treated cells were kept in the incubator for an additional 2, 6, 12, 24, and 48 h. After adding 10 μL of CCK-8 solution to the treated cells and gently tapping the dish, the cells were well combined. These cells remained in the incubator for a further 2 h at 37°C. Cell viability was determined by measuring the absorbance of each well at 490 nm.
2.6 Detection of protein expression of P53 and survivin using the Western blot
Each set of cells was isolated, and then 100 μl of RIPA lysate and 1 μL of PMSF were used to extract the total amount of cell protein. Protein concentration was determined with a BCA kit. Using the SDS-PAGE gel preparation kit, we made a 10% separation gel and a 5% concentration gel. Cell precipitates were placed onto gels according to the estimated volume with confirmed protein concentrations, and electrophoresis was performed. The gel was transferred via electrophoresis to a polyvinylidene difluoride (PVDF) membrane, which was left to dry overnight. The location of the target protein was identified by the protein marker. The trans-PVDF membrane was blocked with 5% skim milk, incubated at room temperature for 1.5 h, then washed twice with TBST following an overnight trans-membrane. Primary antibodies (P53, survivin, beta-actin) were diluted at 1:1000 and incubated in a shaker overnight at 4°C. Following that, we collected the primary antibodies and washed the PVDF membrane three times with TBST. After incubating the membrane at room temperature for 2 h with the appropriate secondary antibodies, diluted at 1:1000, the membrane was washed three times with TBST. ECL, an ultrasensitive luminescent solution, was used for chemiluminescence detection of protein signals.
2.7 Detection of messenger RNA expressions of P53 and survivin with a real-time polymerase chain reaction
Total RNA was isolated from the cells in each sample using an RNA extraction kit. Each group's RNA concentration was measured using a NanoDrop 2000 spectrophotometer. The Hairpin-itTM MicroRNAs Quantitation PCR Reverse Transcription Kit was used to measure miR-34a expression (the primer sequences are illustrated in Table 1). The miR-34a was first quickly reverted into copy DNA (cDNA), and then its expression levels were measured using real-time fluorescent quantitative PCR. In contrast, using the Universal Reverse Transcription Kit, we first converted the mRNA for the P53 and survivin genes into cDNA, and then we used the SYBR Green Real-time PCR Master Mix to identify the expression of these messenger RNAs. The approach was used to determine gene expression levels in relation to one another.
| Gene | Sequence (5′–3′) |
|---|---|
| U6-F | CGCTTCGGCAGCACATATAC |
| U6-R | TTCACGAATTTGCGTGTCATC |
| miR-34a -F | AGCCGCTGGCAGTGTCTTA |
| miR-34a -R | CAGAGCAGGGTCCGAGGTA |
| β-actin -F | GGCTGTATTCCCCTCCATCG |
| β-actin -R | CCAGTTGGTAACAATGCCATGT |
| P53-F | TTTTCCCCTCCCATGTGCTC |
| P53-R | CAGTCTGGCTGCCAATCCA |
2.8 Statistic analysis
All results in this study are presented as mean ± standard deviation, and statistical tests were conducted using a two-sided t test. The data were analyzed using SPSS 20.0 statistical software. If p < .05, then the result was considered statistically significant.
3 RESULTS
3.1 Effects of oxaliplatin on the viability of BGC-823 cells
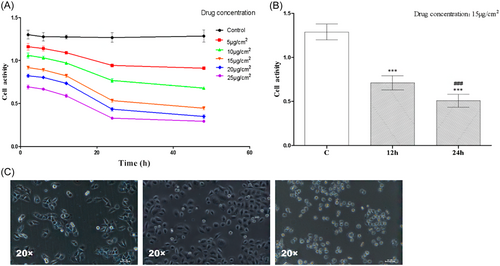
The CCK-8 results are displayed in Figure 1A. The viability of BGC-823 cells decreased after L-OHP injection, and this decline was most pronounced between 12 and 24 h after the L-OHP injection. Thus, in the subsequent experiments, groups receiving 12- and 24-h pharmacological treatments were chosen. The cell viability of BGC-823 gradually decreased as the medication concentration increased. However, because of the drug's side effects and toxicity to normal cells, a concentration of 15 μg/cm² was chosen for subsequent experiments, which was consistent with the drug-related instructions. The CCK-8 results are presented in Figure 1B. Both the 12-h and 24-h groups exposed to 15 μg/cm² of the drug, showed a declining trend in cell viability compared to the control group (C group). There was a statistically significant (p < .005) difference in cell expression levels between the C group (1.288 ± 0.02981), the 12-h group (0.7111 ± 0.02665), and the 24-h group (0.5082 ± 0.02445). There was a statistically significant (p < .005) decline in cell viability between the 12-h and 24-h groups). In Figure 1C, we can see the cell morphology in action. The BGC-823 cells in the C group grew larger, and more densely clustered, and their membrane boundaries were brighter and more defined. More bright cells developed in the 12-h group, and cell shrinkage was not noticeable. The BGC-823 cells in the 24-h group shrank substantially, lost any discernible cellular structure, and had a marked increase in bright cells.
3.2 Effects of oxaliplatin on the messenger RNA and protein expressions of P53 and survivin in BGC-823 cells
Figure 2A depicts the results of the real-time PCR. After 12 h, P53 mRNA expression began to increase, with expression rates in the C group and the 12-h group at 1.000 ± 0.05774 and 1.580 ± 0.6205, respectively; nevertheless, there was no statistically significant difference between the two groups. There was a statistically significant (p < .01) rise in mRNA expression of P53 after 24 h, with an mRNA expression of 2.563 ± 0.299. Figure 2B depicts the mRNA expression of survivin. The mRNA expression of survivin was shown to be essentially identical between the 12-h group and the C group. Neither the C group nor the 12-h group showed a statistically significant difference in their survivin mRNA expressions, with values of 1.000 ± 0.05774 and 0.9635 ± 0.1541, respectively. There was a statistically significant (p < .05) decline in surviving mRNA expression from 24 h, with an mRNA expression of 0.6403 ± 0.09769.
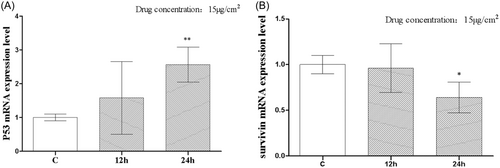
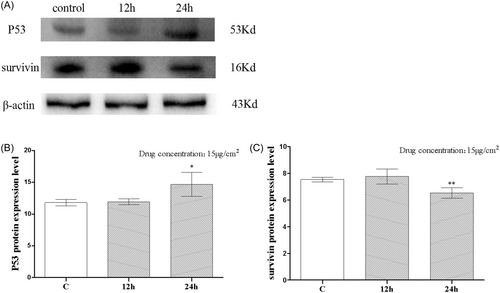
Figure 3A,B depict the outcomes of the WB. The P53 protein expression did not differ significantly between the 12-h and C groups. P53 protein expressions in the C group and the 12-h group were, respectively, 11.80 ± 0.2552 and 11.94 ± 0.2229, with no statistically significant difference between the two groups. At 24-h, there was a statistically significant (p < .05) increase in P53 protein expression, with levels reaching 14.68 ± 0.9468. In Figure 3C, we see survivin as a protein expression. There was no statistically significant change in surviving protein expression between the 12-h group and the C group. The protein expressions of survivin were 7.526 ± 0.08260 and 7.256 ± 0.08000 in the C group and the 12-h group, respectively, and there was no statistically significant difference. There was a statistically significant decline in protein expression of survivin (p < .01) beginning at 24 h, when the expression level was 6.522 ± 0.1957.
3.3 Effects of oxaliplatin on microRNA-34a messenger RNA expression and the effects of microRNA-34a overexpression on BGC-823 cell viability
Figure 4A displays the results of the real-time PCR. The 12-h group showed no discernible difference in miR-34a expression as compared to the C group. The expression levels were 1.000 ± 0.05774 and 1.005 ± 0.3807, respectively, and there was no statistically significant difference. The mRNA expression of miR-34a was significantly upregulated in the 24-h group, with an expression level of 3.695 ± 0.8362, and the difference was statistically significant (p < .05). Figure 4B displays real-time PCR results, showing expression levels of 1.000 ± 0.05774, 1.066 ± 0.2934, and 7.535 ± 0.6164 in the C, NC, and +34a groups, respectively. The difference between the C and NC groups was not statistically significant, while the difference between the C and +34a groups was statistically significant (p < .005). In Figure 4C, +34a and NC groups represent BGC-823 cells into which fluorescent miR-34a and NC plasmids were respectively transfected. Figure 4D displays the results of the CCK-8. There was no significant change in the viability of BGC-823 cells in the NC group compared with the C group. The cell expressions were 1.288 ± 0.02981 and 1.210 ± 0.03583 in the C and NC groups, respectively, and there was no statistically significant difference. The cell viability showed a decreasing trend in group +34a compared with the C group. The cell expression was 1.098 ± 0.04234 in group +34a, and the difference was statistically significant (p < .01).
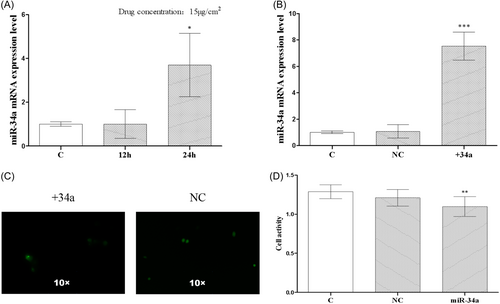
3.4 Effects of microRNA-34a overexpression on the expressions of P53, survivin messenger RNA, and protein in BGC-823 cells
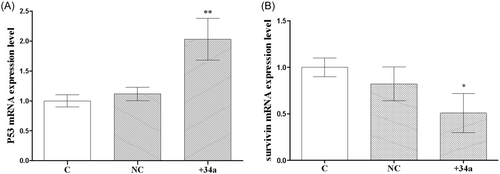
Figure 5A depicts the results of the real-time PCR. There was no discernible difference in the mRNA expression of P53 between the C and NC groups. The mRNA expressions of P53 in the C and NC groups were 1.000 ± 0.05774 and 1.116 ± 0.06426, respectively, with no statistically significant difference. The mRNA expression of P53 was higher in the +34a group compared with the C group. The mRNA expression of P53 was 2.030 ± 0.2021 in the +34a group, and the difference was statistically significant (p < .01). Figure 5B depicts the survivin expression in the NC group. The survivin expression in the NC group decreased as compared to the C group. The mRNA levels of survivin in the C and NC groups were 1.000 ± 0.05774 and 0.8229 ± 0.1054, respectively, with no statistically significant difference. Survivin mRNA was downregulated in the miRNA-34a overexpression group when compared to the C group with an mRNA expression of 0.5088 ± 0.1206, and the difference was statistically significant (p < .05).
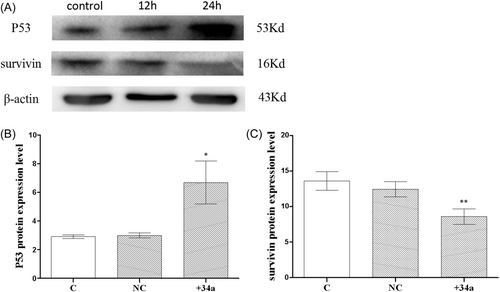
The WB results are depicted in Figure 6A,B. The protein expression of P53 was suggested to be similar across the C and NC groups, with expressions of 2.908 ± 0.07403 and 2.991 ± 0.1028, respectively, and the difference was not statistically significant. The P53 protein expression group increased compared to the C group, with a protein expression of 6.680 ± 0.8625, and the difference was statistically significant (p < .05). Figure 6C depicts the protein expressions of survivin. With protein expressions of 13.61 ± 0.7577 and 12.44 ± 0.6147, respectively, there was no statistically significant difference. Survivin protein expression was downregulated in the +34a group compared to the C group, with a protein expression of 8.585 ± 0.6227, and the difference was statistically significant (p < .01).
4 DISCUSSION
L-OHP, a third-generation platinum derivative, has shown clinical efficacy and has seen widespread use in the treatment of gastrointestinal tumors.15 Consistent with earlier research, we verified that the viability of BGC-823 gastric cancer cells was inhibited after introducing equal concentrations of L-OHP.
Cancers of the stomach, liver, and ovaries are linked to dysregulation of posttranscriptional gene expression, which is closely controlled by messenger RNA.16-18 It has been hypothesized that tumor infiltration and metastasis are connected to the downregulation of miR-34a expression in tumor cells, which in turn correlates with cell proliferation, differentiation, and apoptosis.19 The results indicated that miR-34a expression increased as a result of L-OHP treatment. This study tested the hypothesis that L-OHP could activate miR-34a by upregulating its expression, thereby preventing tumor cell proliferation and differentiation, and promoting apoptosis. Consistent with these findings, we found that the cell viability of BGC-823 gastric cancer cells in the miR-34a overexpression group decreased significantly compared to the C and NC groups.
Apoptosis, triggered by cell cycle arrest in cell injury, may be efficiently induced by the wild-type P53 protein, allowing cells to be repaired in the next cycle, and so preventing the occurrence and progression of tumor growth.20, 21 Pretreatment with L-OHP resulted in an upregulation of P53 mRNA expression, and further L-OHP treatment tended to increase P53 protein expression. The above results demonstrated that L-OHP upregulates the expression of P53, which in turn inhibits the proliferation of gastric cancer cells and reduces cell viability.
Eyal Mor et al. found that activating miR-34a, a downstream target gene of P53, might cause the upregulation of P53 expression, increase apoptosis, and suppress its occurrence and progression.22 Through this study, we have attempted to shed more light on the topic by constructing BGC-823 gastric cancer cells in the +m34a group. Both the mRNA and protein expressions of P53 were shown to be positively correlated with the miR-34a group, suggesting an upregulated trend. From these results, we were able to conclude that miR-34a, as a potential downstream target of the P53 gene, may stimulate its production when it is overexpressed. The results were consistent with the research findings by Eyal Mor et al., suggesting that L-OHP could stimulate the miR-34a/P53 pathway, with the latter perhaps acting as a suppressor of cell viability by promoting the production of P53.
L-OHP has been hypothesized to target P53 either directly or indirectly. P53, a gene that normally works as a tumor suppressor, is also involved in biological processes such as cell cycle arrest, proliferation, apoptosis, DNA repair and replication, tumor suppression, and cellular stress response.23, 24 Here, we show that overexpression of P53 is antagonistic to the mutation of the P53 gene by showing that oxaliplatin treatment reduces the viability of BGC-823 cells. P53 gene mutations account for the majority of genetic changes found in more than half of all human cancers.25 However, most P53 mutations in cancer are missense, resulting in full-length Mut P53 protein expression.26 According to our data, Oxaliplatin may be able to boost the production of wild-type P53 without causing mutations on the protein. Because of their association with P53, members of the miR-34 family are now well recognized as important tumor suppressors, and their dysregulation has been linked to many different types of human cancer.27 The miR-34a transcript is distinct from miR-34b and miR-34c, both of which are also found on chromosome 1p36.22.28 The effect of miR-34a on antitumor therapy has been demonstrated in previous research.27 Therefore, our findings provide compelling evidence for the use of miR-34a in the treatment of gastric cancer.
Malignant tumors, including those of the stomach, liver, colon, rectal, and breast, express survivin at high levels, since it is an apoptosis-inhibiting protein that is uniquely expressed by tumor cells.13 Survivin is a gene that primarily functions to prevent apoptosis by regulating the cell cycle and encouraging neovascularization.29 Both the mRNA and protein expressions of survivin in BGC-823 cells were found to be lower after L-OHP treatment. These findings demonstrated a strong association between L-OHP-induced downregulation of survivin and gastric cancer cell growth. According to another study, the P53 protein inhibited binding to survivin-related promoter regions, leading to decreased survivin expression. Therefore, P53 may be an essential negative regulator of survivin,30 which indicates that a higher positivity rate in wild-type P53 correlates with a lower positive expression of survivin, possibly playing a role in suppressing tumor cell growth. Research has shown that miR-34a can decrease tumor cell growth and increase apoptosis via downregulating expression of the survivin gene promoter.31-33 Downregulation of mRNA and protein expressions of survivin in the miR-34a overexpression group suggested that miR-34a could suppress the expression of survivin, reducing the proliferation of gastric cancer cells as well as promoting cell apoptosis.
L-OHP's toxicity has always been a drawback in the medical field. When the oxalate or chloride ions in platinum medicines are lost in a cell, they are rehydrated. Previous studies have demonstrated that platinum intrastrand crosslinks on DNA are the primary mechanism by which platinum derivatives cause cellular toxicity or damage. The nucleophilic molecules within a cell, such as RNA, DNA, and proteins, react with the positively charged molecules. Following this, purine bases of DNA can acquire four distinct lesions: interstrand crosslinks, DNA crosslinks, intrastrand crosslinks, and monoadducts.34 L-OHP was widely utilized to treat various types of cancer, including those of the stomach, ovaries, colorectal, breast, and genitourinary system.35 Instead of tumor progression, peripheral neuropathy is to blame for the end of L-OHP treatment. Recent research has proposed a novel method for L-OHP treatment, in which L-OHP induces immunogenic signaling in cancer cells before apoptosis, which then causes dendritic cells to engage with toll-like receptor-4 and produce interferon gamma.36 In more than 70% of L-OHP patients, the Food and Drug Administration found evidence of peripheral neuropathy (dysphonic syndrome and toxicity).37 L-OHP therapy is an essential treatment for cancer patients, even immunodeficient patients (HIV-positive and elderly patients), despite the aforementioned side effects.38-40 While further research is being conducted on L-OHP's potential to fight cancer, we hope that equal attention will be paid to the compound's potential dangers.
Based on these findings, it was hypothesized that L-OHP could up-regulate miR-34a, activate expression of the upstream P53 gene, and jointly promote the downregulation of survivin, all of which would lead to an increase in apoptosis and a reduction in tumor cell infiltration in BGC-823 gastric cancer cells. All of this has the potential to improve the 5-year survival rates of patients and provide new diagnostic approaches and therapeutic procedures for the treatment of advanced gastric cancer in contemporary medicine.
AUTHOR CONTRIBUTIONS
Conception and design of the research: Qiang Guo. Acquisition of data: Xin-Yuan Wang, Yan-Chang Zhai, Yong-Wei Dong. Analysis and interpretation of the data: Qiang Guo, Qing-Si He. Statistical analysis: Xin-Yuan Wang, Yan-Chang Zhai. Obtaining financing: Qiang Guo. Writing of the manuscript: Qiang Guo, Xin-Yuan Wang. Critical revision of the manuscript for intellectual content: Qiang Guo, Qing-Si He. All authors read and approved the final draft.
ACKNOWLEDGMENTS
This study was supported by Natural Science Foundation of Inner Mongolia Autonomous Region (No.: 2016MS [LH]0825).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.