RETRACTED: MicroRNA-425-3p inhibits myocardial inflammation and cardiomyocyte apoptosis in mice with viral myocarditis through targeting TGF-β1
Abstract
Objective
Emerging articles have profiled the relations between microRNAs and viral myocarditis. This research was unearthed to explore the capacity of miR-425-3p on cardiomyocyte apoptosis in mice with viral myocarditis and its mechanism.
Methods
A total of 120 mice were classified into 4 groups in a random fashion (n = 30). The mice were intraperitoneally injected with coxsackievirus type B3 (CVB3) to induce myocarditis. On the 7th day after CVB3 infection, 10 mice in each group were euthanized to assess the heart function indices of mice, observe the pathological conditions, detect myocardial tissue apoptosis, and measure the inflammatory factor levels in myocardial tissues. Expression of miR-425-3p, transforming growth factor (TGF-β1), and apoptosis-associated proteins in myocardial tissues was determined. The remaining 20 mice in each group were used for survival observation. The luciferase activity assay was implemented to validate the relationship between miR-425-3p and TGF-β1. miR-425-3p mimic was transfected into mouse cardiomyocytes HL-1 and then infected with CVB3 to further verify the regulatory effect of miR-425-3p on the cardiomyocyte apoptosis in viral myocarditis.
Results
miR-425-3p was lowly expressed in myocardial tissues of mice with viral myocarditis. Overexpressed miR-425-3p improved the cardiac function, alleviated pathological conditions, reduced cardiomyocyte apoptosis, decreased Bax and cleaved Caspase-3 expression, elevated Bcl-2 expression, decreased levels of inflammatory factors and improved survival rate of mice with viral myocarditis. Luciferase activity assay verified that miR-425-3p could bind to TGF-β1, and overexpressed miR-425-3p suppressed TGF-β1, p-smad2/smad2 and p-smad3/smad3 expression. In vitro experiments further verified that overexpression of miR-425-3p inhibited the apoptosis of CVB3-HL-1 cells, and the addition of TGF-β1 would reverse this effect.
Conclusion
Our research indicates that miR-425-3p is poorly expressed in myocardial tissues of mice with viral myocarditis. Overexpressed miR-425-3p inhibits cardiomyocyte apoptosis and myocardial inflammation in mice with viral myocarditis as well as improves their survival rates through suppressing the TGF-β1/smad axis.
1 INTRODUCTION
Myocarditis, resulted from myocardial infiltration of immunocompetent cells after any type of cardiac injury, is defined as an inflammatory disease that happens in cardiac muscle tissues.1 Myocarditis, as a potential threat, is a major reason for sudden unforeseen death amongst children and young adults.2, 3 Various infectious agents contribute to myocarditis, including bacteria, viruses, and parasites to allergens or toxins.4 Coxsackievirus type B3 (CVB3) is regarded an important cause of viral myocarditis.5 Achievements in cardiac magnetic resonance imaging and molecular detection of viruses have been made to better understand the mechanisms of viral myocarditis. Nevertheless, therapeutic regimens are currently restricted for both the acute/chronic phases of myocarditis.6 Therefore, understanding the molecular mechanisms of viral myocarditis is urgent for successfully treating cardiomyopathy.
microRNA (miRNA) negatively modulates target gene expression via messenger RNA (mRNA) degradation and translation suppression.7 miRNAs are essential regulators in many cellular processes, involving in angiogenesis, cardiovascular diseases, and cancers.8, 9 miRNAs can modulate the pathogenesis of CVB3-induced viral myocarditis.10 Recently, emerging articles have profiled the relations between miRNAs and viral myocarditis. Xu et al.11 have stated that miR-20b decreased ZFP-148 expression12 and miR-1 suppressed Cx43 in viral myocarditis. Another study has revealed that miR-155 alleviated cardiac injury and dysfunction in viral myocarditis.13 Based on these observations, we could hypothesize that miRNAs participate in the regulation of viral myocarditis. As a member of miRNA family, miR-425-3p deregulation has been explored in human cancer tissues, suggesting downregulated miR-425-3p levels in colorectal cancer patients14 and upregulated miR-425-3p in patients with breast cancer.15 However, no comprehensive overview of miR-425-3p in viral myocarditis is currently available. In the heart, transforming growth factor (TGF)-β1 has been revealed to control the procollagen gene expression and induce the synthesis of extracellular matrix components, further contributing to myocardial fibrosis in varying kinds of cardiomyopathy.16 An article has indicated that TGF-β is upregulated in CVB3-infected hearts in a murine model of viral myocarditis.17 The relationship between miR-425-3p and TGF-β1 has not been explored, but a study has demonstrated that downregulated miR-425 promoted collagen expression and promoted fibroblast proliferation after TGF-β1 treatment.18 Based on these findings, we unearthed this research to probe into the mechanism of miR-425-3p in viral myocarditis. Collectively, this current study disclosed that miR-425-3p inhibited myocardial inflammation and cardiomyocyte apoptosis in mice with viral myocarditis through targeting TGF-β1, which could provide a new basis for the mechanism of miR-425-3p in viral myocarditis.
2 MATERIALS AND METHODS
2.1 Ethics statement
This study was ratified by the Animal Ethics Committee of our hospital and was conducted as per the recommendations of the Care and Use of Laboratory Animals by the National Institutes of Health.
2.2 Establishment of CVB3 mouse models
A total of 120 male BALB/c mice (specific pathogen-free [SPF] grade, 4–6 weeks, 18–22 g) were purchased from Shanghai Experimental Animal Center (Shanghai, China) and raised in the SPF animal facility of the Medical University of our hospital. The CVB3 Nancy strain was provided by Institute of Virology, Wuhan University (Wuhan, China). It was maintained by HeLa cells (ATCC code: CCL-2), and the virus titer was determined by the 50% tissue culture infectious dose (TCID50) method. Mice were injected intraperitoneally with 0.1 ml of phosphate-buffered saline (PBS) to dilute CVB3 (105 TCID50) to induce myocarditis, and controlled mice were intraperitoneally injected with the same dose of PBS.19 The day of virus inoculation was set as Day 0. The mice were randomly divided into four groups (n = 30): Control group, CVB3 group, CVB3 + negative control (NC) agomiR group and CVB3 + miR-425-3p agomiR group. For in vivo miR-425-3p treatment, BALB/c mice were anesthetized, intubated, and then mechanically ventilated. Next, 3 days before CVB3 infection, 30 ml of Lipofectamine 2000 was blended with 170 μl PBS-dissolved miR-425-3p agomiR or NC agomiR (10 nmol/site; Ribbio), and 30 G needle was injected into the apex of the left ventricle at six locations in each mouse.13, 20 After the cardiac function test on the 7th day, the animals were anesthetized (10 mice randomly selected from each group), and the hearts were quickly frozen and kept at −80°C for subsequent experiments.
2.3 Echocardiography
On the 7th day after CVB3 infection, the mice were anesthetized by inhalation of isoflurane and put on a heating pad at 37°C. Transthoracic echocardiography was performed to measure the left ventricular end-systolic diameter (LVESd), left ventricular end-diastolic diameter (LVEDd), left ventricular fraction shortening (LVFS), and left ventricular ejection fraction (LVEF) at the papillary muscle level by using Sonos 5500 system (12 MHz phased array transducer; Philips). The short-axis view of the M type from front to back was recorded later. All measurements were executed by an experienced technician in a blind way.19
2.4 Hematoxylin-eosin staining
Subsequently, the heart was fixed with 4% paraformaldehyde, embedded in a paraffin block, and sliced into 5-μm thickness sections for hematoxylin-eosin (HE) staining. According to the previous study,21 the pathology scores were evaluated as follows: 0 point, no disease; 1 point, 25% of myocardium affected by the disease; 2 points, 25%–50% of myocardium affected by the disease; 3 points, 50%–75% of myocardium affected by the disease; 4 points, 75%–100% of myocardium affected by the disease. The pathological change was observed under a microscope in five randomly-selected visual fields, and an experienced technician calculated the pathology score in a blind way.
2.5 Transferase-mediated deoxyuridine triphosphate-biotin nick end labeling staining
Transferase-mediated deoxyuridine triphosphate-biotin nick end labeling (TUNEL) staining was implemented to detect apoptosis in myocardial tissue slices as per the instructions of the TUNEL fluorescent FITC kit (Roche Diagnostics Corp). Myocardial tissue sections were incubated with TUNEL reaction mixture (50 µl) embodying terminal deoxynucleotidyl transferase at 37°C for 60 min and then stained with 4',6-diamidino-2-phenylindole 2hci. The fluorescence microscope system (DP72; Olympus) was adopted to observe the fluorescence of FITC. Positive apoptotic cells were marked in green, and all nuclei, in blue. Five fields of view (×400) were selected in a random fashion, and the positive apoptotic cells were counted.22
2.6 Real-time polymerase chain reaction
Referring to the manufacturer's requirements (A33252; Invitrogen), the extraction of total RNA from heart tissue was implemented with the TRIzol reagent. The reverse transcription kit (K1691; Thermo Fisher Scientific) was implemented to convert RNA into complementary DNA (cDNA). The sequences are displayed in Table 1. For miRNA real-time polymerase chain reaction (PCR), the Hairpin-it miRNAs quantitative PCR (qPCR) quantitative kit (Shanghai GenePharma Co, Ltd) was adopted for quantitative real-time reverse transcription PCR. In brief, with 2 μg of RNA as a template, the reverse transcription was performed with miR-425-3p specific reverse transcription primers. The specific forward primer and universal reverse primer were adopted to further amplify cDNA. The 2−ΔΔCt method was conducted to analyzed mRNA or miRNA expression and normalized to glyceraldehyde phosphate dehydrogenase (GAPDH) or U6 small nuclear RNA expression. All reactions were in triplicate.
| Gene | Sequence (5′ → 3′) |
|---|---|
| miR-425-3p | F: TGCGGAATGACACGATCACTCC |
| R: CCAGTGCAGGGTCCGAGGT | |
| U6 | F: GCTTCGGCAGCACATATACTAAAAT |
| R: CGCTTCACGAATTTGCGTGTCAT | |
| TGF-β1 | F: GACTCTCCACCTGCAAGACCA |
| R: GGGACTGGCGAGCCTTAGTT | |
| GAPDH | F: TGCGACTTCAACAGCAACTC |
| R: ATGTAGGCCATGAGGTCCAC |
- Abbreviations: F, forward; GAPDH, glyceraldehyde phosphate dehydrogenase; miR, microRNA; R, reverse; TGF, transforming growth factor.
2.7 Western blotting
The mouse heart tissues and the transfected cells were dissolved with radioimmunoprecipitation assay lysis buffer, and the protein extracts were separated with 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis, and next, transferred onto polyvinylidene difluoride membrane (Millipore). After that, the membrane was blocked for 1 h with 5% milk in Tris-buffered saline solution containing 0.1% Tween-20 and then incubated at 4°C overnight with rabbit anti-TGF-β1, Bax, Bcl-2, cleaved Caspase-3 antibodies (1:100 dilution; Cell Signaling Technology) and the GAPDH antibody (1:1000; Cell Signaling Technology, as a load control). Afterwards, the membrane was incubated for 2 h with horseradish peroxidase-conjugated secondary antibody (1:10,000; Zhongshan Golden Bridge Biotechnology). The enhanced chemiluminescence system (Chemicoscope) was utilized to detect the protein. Western blots were quantified with Quantity One analysis software (Bio-Rad), and all experiments were run in triplicate.12
2.8 Enzyme-linked immunosorbent assay
The measurement of cytokine levels of tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-12 in the heart homogenate was complemented using respective cytokine enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems) se per the manufacturer's instructions.
2.9 Survival rate
The remaining 20 mice were taken from each group and the survival rate was observed. The survival time of mice in each group was 14 days.
2.10 Dual-luciferase reporter gene assay
Targetscan (http://www.targetscan.org/vert_71/) website was searched for predicting whether miR-425-3p could target TGF-β1. The TGF-β1 fragment containing miR-425-3p binding site was amplified by PCR and subsequently, cloned into the pmirGLO dual luciferase expression vector (Promega Corporation) for the formation of TGF-β1-wild type (WT). Subsequently, HEK-293T cells (Shanghai Cell Research Institute) transfected with the TGF-β1 mutant type (MUT) reporter gene vector was introduced with miR-425-3p mimic or mimic NC with Lipofectamine 2000. Forty-eight hours posttransfection, firefly luciferase and renilla luciferase activities were evaluated with the dual luciferase reporter system (Promega). Each experiment was performed in triplicate.
2.11 Cell culture and grouping
Mouse cardiomyocyte-like cell line HL-1 (Shanghai Yaji Biotechnology Co, Ltd) was cultured in complete Claycomb medium (Sigma-Aldrich Chemie GmbH) containing 10% fetal bovine serum (Biochrom), 1% penicillin/streptomycin (Life Technologies), 0.1 mmol/L norepinephrine (Sigma-Aldrich Chemie GmbH) and 2 mmol/L glutamine (Biochrom). The medium was renewed approximately every 24 h. Cells grew in an environment of 37°C with 5% CO2 and 95% air, and the relative humidity was about 95%. miR-425-3p mimic and its control were transfected into HL-1 cells by lipofectamine 2000 (Life-Technologies). Then the cells were treated with CVB3 alone at a dose of 100 MOI. Three days after CVB3 infection, the cells were collected for TUNEL staining, and dissolved with standard radioimmunoprecipitation assay solution. Standard sodium dodecyl sulphate-polyacrylamide gel electrophoresis technology was used to resolve 20 g of protein and then analyzed by Western blotting.
2.12 Statistical analysis
The quantitative data were depicted as mean ± SE, and Graphpad Prism 6.0 was adopted for statistical processing. The two-group data were compared by the Student t test, and the multiple-group data were compared by one-way analysis of variance and Bonferroni post hoc test. Kaplan–Meier analysis was implemented for the survival analysis. "N" indicated the number of independent experiments, and the significance was presented as follows: **p < .05; **p < .001; ***p < .001.
3 RESULTS
3.1 miR-425-3p is poorly expressed in mice with viral myocarditis
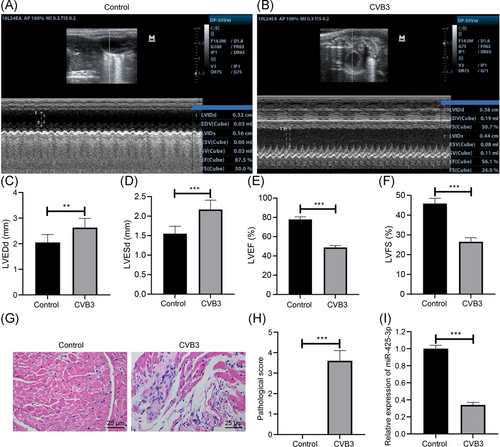
The left ventricular function in mice was assessed by the observation of transthoracic echocardiography (Figure 1A–E): CVB3-induced viral myocarditis presented elevated LVEDd and LVESd and reduced LVEF and LVFS. HE staining observation showed that on the 7th day after CVB3 infection, increased number of inflammatory cell infiltration and necrosis of cardiomyocytes were found in mice with CVB3-induced viral myocarditis, but almost no inflammatory cells and necrosis were observed in normal mice (Figure 1F). The pathological score of myocardial tissues in mice with CVB3-induced viral myocarditis was higher than the normal mice (Figure 1G, p < .001). Then we detected miR-425-3p expression in mice with viral myocarditis by qPCR, and miR-425-3p was downregulated in mice with CVB3-induced viral myocarditis (Figure 1H, p < .001). The results indicate the low expression of miR-425-3p in mice with viral myocarditis.
3.2 The overexpression of miR-425-3p improves cardiac function and pathological status in mice with viral myocarditis
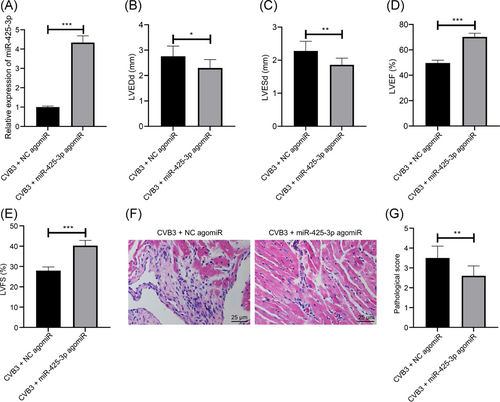
To observe the therapeutic effect of miR-425-3p in mice with viral myocarditis, we injected miR-425-3p agomiR into mice 3 days before CVB3 infection, and qPCR results confirmed that miR-425-3p was enhanced in the myocardial tissues of mice after the miR-425-3p overexpression (Figure 2A, p < .001). The results of the cardiac function test indicated that compared with the CVB3 + NC agomiR group, reduced LVEDd and LVESd, along with elevated LVEF and LVFS were found in mice of the CVB3 + miR-425-3p agomiR group (Figure 2B–E, all p < .05). In the CVB3 + NC agomiR group, a large number of inflammatory cell infiltration and necrosis occurred in cardiomyocytes, while the inflammation and pathological scores of cardiomyocytes in the CVB3 + miR-425-3p agomiR group were reduced versus those in the CVB3 + NC agomir group (Figure 2F,G, p < .001). As a result, our results show that overexpression of miR-425-3p can improve the heart function and pathological state of mice with viral myocarditis.
3.3 Overexpressed miR-425-3p suppresses myocardial tissue apoptosis in mice with viral myocarditis
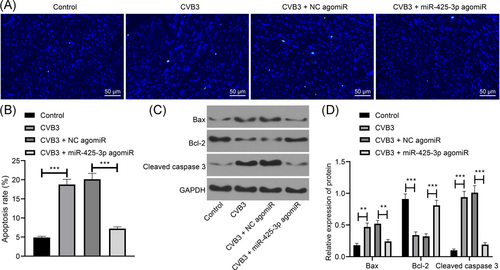
The capability of overexpressing miR-425-3p on myocardial tissue apoptosis in mice with viral myocarditis was further observed by TUNEL staining (Figure 3A,B) and Western blotting (Figure 3C,D). The results showed that the apoptotic index increased, Bax and cleaved caspase 3 were elevated while Bcl-2 was decreased in the myocardial tissues of CVB3 mice (all p < .01). miR-425-3p upregulation resulted in inhibited myocardial tissue apoptosis and decreased the Bax and cleaved caspase 3, as well as enhanced the Bcl-2 expression in CVB3 mice (all p < .01). The results suggest that the upregulated miR-425-3p inhibits myocardial tissue apoptosis in mice with viral myocarditis.
3.4 Upregulated miR-425-3p alleviates myocardial inflammation and improves survival rate in mice with viral myocarditis
Subsequently, we analyzed the capacity of overexpressed miR-425-3p on myocardial inflammation in viral myocarditis mice by ELISA, and the results exhibited that the myocardial inflammatory factors TNF-α, IL-6, and IL-12 in CVB3 mice increased significantly, but overexpressed miR-425-3p degraded the expression of these myocardial inflammatory factors in CVB3 mice (Figure 4A–C, all p < .01). Kaplan–Meier findings suggested that overexpressed miR-425-3p increased the survival rate in mice with viral myocarditis (Figure 4D, p < .01). Therefore, the overexpression of miR-425-3p suppresses myocardial inflammation and improves survival rate in mice with viral myocarditis.
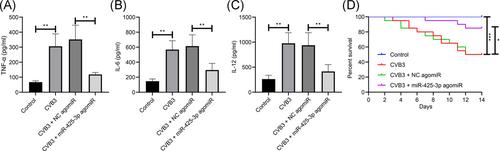
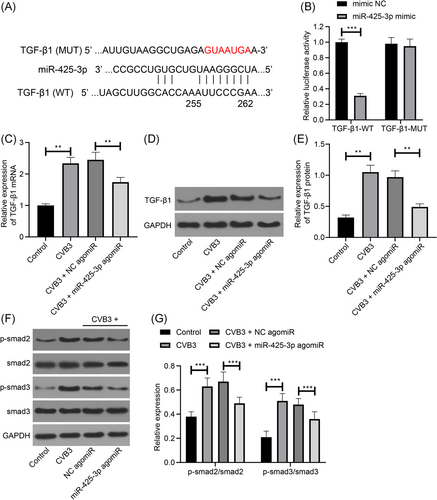
3.5 miR-425-3p suppresses the activation of the TGF-β1/smad pathway
Targetscan website predicted a binding site of TGF-β1 and miR-425-3p (Figure 5A). To further determine whether TGF-β1 acted as a downstream target gene of miR-425-3p in cardiomyocytes, we prepared a luciferase reporter containing TGF-β1 (WT or MUT miR-425-3p binding site), and cotransfected HEK-293T cells with miR-425-3p mimic. The result of the luciferase assay showed that miR-425-3p restricted the luciferase activity of TGF-β1-WT, while not TGF-β1-MUT (Figure 5B). Finally, we observed the TGF-β1 expression in the myocardial tissues of mice with viral myocarditis after the overexpression of miR-425-3p, and the findings demonstrated that upregulated miR-425-3p inhibited TGF-β1 expression (Figure 5C–E). Next, Western blotting was conducted to detect the expression of smad2/3 in the downstream pathway of TGF-β1. The findings suggested that p-smad2/smad2 and p-smad3/smad3 were significantly upregulated in the myocardial tissues of mice in the CVB3 group compared with the Control group. Overexpression of miR-425-3p could significantly inhibit p-smad2/smad2 and p-smad3/smad3 expression (Figure 5F,G). It shows that miR-425-3p can inhibit the activation of smad2/3 pathway. It indicates that miR-425-3p may downregulate the expression of TGF-β1 and inhibit the activation of smad2/3 pathway, which has a therapeutic effect on mice with viral myocarditis.
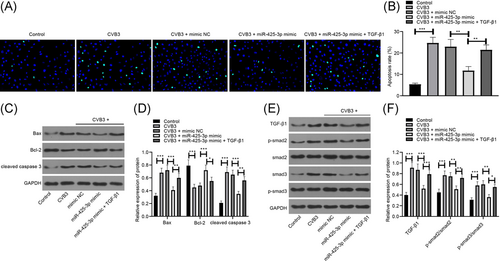
3.6 miR-425-3p participates in the antiapoptotic effect in vitro in viral myocarditis by inhibiting the expression of TGF-β1
Finally, to verify the regulatory mechanism of miR-425-3p/TGF-β1/smad axis in viral myocarditis, we transfected miR-425-3p mimic and its control into HL-1 cells, and then treated the cells with CVB3. The results of TUNEL staining and Western blotting showed that 3 days after CVB3 treatment, HL-1 cell apoptosis and proapoptotic factors Bax and cleaved-caspase 3 were significantly increased, while antiapoptotic factor Bcl-2 was significantly decreased. The apoptosis of HL-1 cells transfected with miR-425-3p mimic was significantly reduced, and the apoptosis effect was reversed after TGF-β1 treatment (Figure 6A–D). Furthermore, Western blotting was utilized for the detection of TGF-β1/smad axis-related protein expression, and the results indicated that miR-425-3p mimic-treated CVB3-HL-1 cells were consistent with in vivo results, while TGF-β1/smad axis-related protein was significantly upregulated after TGF-β1 treatment (Figure 6E,F). The results further confirmed that miR-425-3p inhibited the TGF-β1/smad pathway activation by inhibiting TGF-β1, thereby inhibiting cell apoptosis in viral myocarditis.
4 DISCUSSION
Nowadays, viral myocarditis has no effective treatment options in clinical practice, which is considered as a challenging disease in the diagnosis and therapy of the cardiovascular field.23 Although considerable progress has been achieved in the pathogenesis and molecular basis of viral myocarditis, the advances are lacking behind in diagnosis, monitoring as well as treatment.24 In view of this, we established a mouse model of viral myocarditis by CVB3 injection to elucidate the impact of miR-425-3p and TGF-β1 in this disease.
First, mice with CVB3-induced viral myocarditis exhibited elevated LVEDd and LVESd and reduced LVEF and LVFS. On the 7th day after CVB3 infection, elevated number of inflammatory cell infiltration and necrosis of cardiomyocytes were observed, indicating successful modeling of viral myocarditis. Incremental evidence has revealed that miRNAs are of great importance during the process of heart failure and cardiac fibrosis.25, 26 Also, many articles have focused on the functions of miRNA in viral myocarditis.13, 27 For example, miR-223 has been implied to protect against CVB3-induced viral myocarditis, which could attribute to the modulation of macrophage polarization by binding to Pknox1.28 Zhang et al.29 have stated that miR-133b suppresses the cardiomyocyte proliferation and the TNF-α and IL-6 release, and relieves CVB3-induced myocardial injuries through the modulation of Rab27B. All these evidences imply that miRNAs play a protective role in have viral myocarditis. In our study, the miR-425-3p expression and its function in viral myocarditis was determined, and the obtained findings implied that miR-425-3p expression was suppressed in mice with CVB3-induced viral myocarditis; overexpressed miR-425-3p improved the cardiac function, alleviated pathological conditions, reduced cardiomyocyte apoptosis, and decreased levels of inflammatory factors in mice with viral myocarditis on the 7th day of CVB3 infection. In line with our findings, Guo et al.30 have stated that reduced miR-425 level is found in the samples from patients with heart failure, and miR-425 has the capability to predict heart failure and cardiac fibrosis, indicating that miR-425 may be regarded as a novel biomarker for heart failure development. Meanwhile, the suppressive function of miR-425-3p has also been discussed in other diseases. For example, it is suggested that miR-425 is capable of inhibiting melanoma metastasis by targeting the IGF-1, suggesting miR-425 may act as a tumor inhibitor in melanoma.31 Another article has demonstrated that elevated miR-425-3p expression is related to longer time to progression and progression free disease, and assessment of miR-425-3p expression in liver biopsies is helpful for stratifying patients with advanced hepatocellular carcinoma.32 However, the mechanisms of miR-425-3p are still in its infancy.
To find out the downstream target gene of miR-425-3p in cardiomyocytes, we searched the Targetscan website and found a binding site of TGF-β1 and miR-425-3p. Additionally, we performed the luciferase activity assay and found that TGF-β1 was a target gene of miR-425-3p. TGF-β1 has been revealed to strongly result in fibrotic disorders of myocarditis and suppressed TGF-β1 is a significant future drug for treating fibrotic diseases resulting from overproduction of TGF-β1.33 Meanwhile, TGF-β has been indicated to be able to activate NF-κB (a main controller of the immune response) in different types of cardiac diseases, including myocarditis.34 In agreement with our research, Guo et al.30 in their article have demonstrated that TGF-β1 is the direct target of miR-425 via luciferase assay and immunoblotting, and miR-425 acts as a negative controller of cardiac fibrosis through inhibiting TGF-β1 expression. Another study has indicated that SMAD4, a TGF-β pathway-associated protein, is able to control miR-425 expression by binding to its promoter region.35 miR-425 targets to TGF-β1. Furthermore, Liu et al.31 have found that miR-425 could retard melanoma metastasis via inhibiting the IGF-1-mediated PI3K-Akt pathway. All these data suggest the combined action of miR-425-3p and TGF-β1 in human diseases. Nevertheless, the combined functions of miR-425-3p and TGF-β1 in virus myocarditis should be confirmed in future research.
Furthermore, Western blotting was conducted to detect the expression of smad2/3 in the downstream pathway of TGF-β1. The findings indicated that miR-425-3p could inhibit the activation of smad2/3 pathway. Smad proteins, the intracellular effectors of TGF-β1 pathway, could translocate into the nucleus in which they regulate transcription.36 Liu et al.37 have stated that miR-425 inhibited smad2 expression through targeting the second binding site in the 3′-untranslated region, which was in line with our findings. Except that, miR-425-3p mimic was transfected into mouse cardiomyocytes HL-1 and then infected with CVB3 to further verify the regulatory effect of miR-425-3p on the cardiomyocyte apoptosis in viral myocarditis. In vitro experiments further verified that overexpression of miR-425-3p inhibited the apoptosis of CVB3-HL-1 cells, and the addition of TGF-β1 would reverse this effect.
In summary, this article suggests that miR-425-3p downregulates TGF-β1 expression and plays a therapeutic role in mice with viral myocarditis. Our findings showed that the miR-425-3p/TGF-β1 axis could be a new potential therapeutic biomarker of virus myocarditis. In addition, these findings should prompt further research aimed at identifying genes affected by miR-425-3p in viral myocarditis. In spite of this, the miR-425-3p-modulated mechanisms of TGF-β1 in affecting virus myocarditis remain unclear and further studies are still in need.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.