Normal cerebral blood vessels under ultrasound in SD rats of different ages
Bo-Yan Luo and Jin-Xiang Liu contributed equally to this study.
Abstract
The objective of this study was to examine whether ultrasound can examine the development of cerebral vascular structure and cerebral blood flow in Sprague–Dawley (SD) rats by ultrasound in a noninvasive manner, which provides a reference for ultrasound research of SD rats. Thirty-nine SD rats (7–16 days old) were divided into seven groups according to age, and the number of SD rats in each group was, respectively, 7, 17, 1, 3, 2, 8, and 1. Ultrasound was used to detect cerebral blood vessels, cerebrovascular flow velocity, and heart rate in SD rats in the sagittal and coronal positions, and images were obtained in B-mode ultrasound. The cerebral vascular structure of 39 SD rats (7–16 days) was dynamically observed under B-ultrasound. We found that the cerebral vascular structure of the rats aged 7–10 days was clear and detectable. Rats aged 11–16 days of cerebral vascular structures became thinner and undetectable. Quantitative analysis of cerebrovascular flow rate and heart rate in rats found that there was no significant difference in cerebrovascular blood flow rate and heart rate between 7 and 8 days. Ultrasound can also be used in rat animal studies, that is, the cerebral blood flow in rats of different ages can be monitored in real-time by ultrasound in a noninvasive way.
1 INTRODUCTION
The incidence of neurodegenerative and cerebrovascular diseases enhances with age, which immensely lowers the quality of life of patients. Therefore, an increasing number of studies on the correlation between age and cerebrovascular diseases have been conducted in recent years, expecting it to hit new channels to heal cerebrovascular diseases. Hecht et al.1 evaluated the relativity of age on the restoration of hemodynamic damage and found that cerebrovascular collateral remodeling owing to age limitation ultimately makes for impairing cerebrovascular reaction, which ascends the risk of stroke in the elderly. Cerebrovascular products, including vascular endothelial growth factors, are conducive to neuronal survival; however, these cerebrovascular products exhibit age pertinence, which may ultimately result in vascular disease and neurodegenerative diseases, among others.2 Differential expression of cerebrovascular proteins in wild-type and Tg-SwDI mice with age compromises the structural integrity of cerebral blood vessels, which gives us new insights into age-related cerebrovascular dysfunction and pathological mechanisms.3 In the study, the effect of gender and age on cerebrovascular function found that compared with male rats, the middle cerebral artery (MCA) in female rats contraction reduced with age, which made the cerebrovascular response have a significant difference.4 In the cerebrovascular pathophysiology of Alzheimer's disease (AD), microvascular degeneration in 3×Tg AD mice was found to progress with age.5 In contrast, neonatal rats can automatically regulate brain pressure and vascular reactivity, which prevents damage to brain function.6
These studies have shown the relationship between brain diseases and age. To better treat brain diseases and find new treatments, it is necessary to explore the unknown application directions of existing technologies.
Medical technology, such as nuclear medicine (mainly tomography) and cerebrovascular imaging, has contributed significantly to the comprehension of cerebrovascular diseases, including stroke, transient ischemic attack, and subarachnoid hemorrhage.7, 8 Cerebrovascular imaging includes vascular cavity imaging and vascular wall imaging, among which vascular cavity imaging includes CT angiography, magnetic resonance angiography, time-resolved angiography, and transcranial Doppler (TCD) ultrasound.8 TCD ultrasound has the advantages of rapid real-time monitoring of cerebral vessels, measurement of blood flow of individual vessels, and diagnosis of focal vascular stenosis, which is a desirable option for dynamically monitoring cerebrovascular responsiveness.9 In this way, the general structures and blood flow in the brain were discerning.
Thus, this study explores whether ultrasound can noninvasively detect the general cerebrovascular system of Sprague–Dawley (SD) rats of different ages, which lays the foundation for the application of ultrasound in brain research of rats of different ages.
2 MATERIALS AND METHODS
2.1 Ethics statement
The operation was performed in the laboratory of the Department of Zoology, Kunming Medical University. Minimize animal distress in accordance with Animal Care and Research Ethics committees.
2.2 Animals
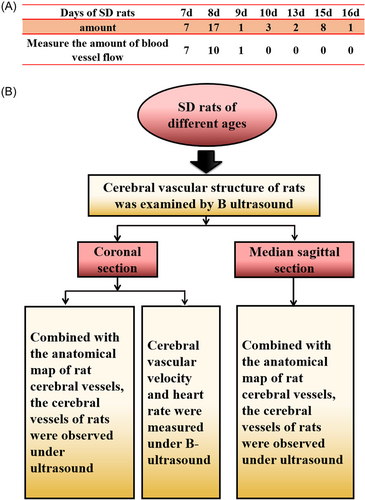
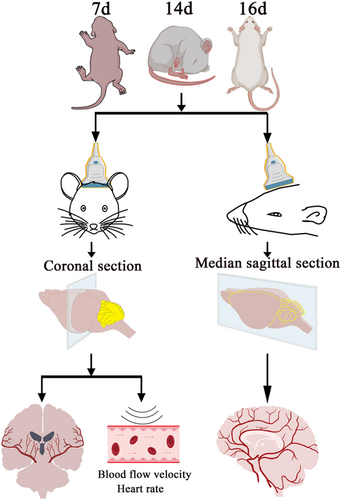
A total of 39 SD rats aged 7–16 days were used for the study. See Figure 1 for detailed grouping and experimental procedures. SD rats were collected from the specific pathogen-free room of the Experimental Animal Department of Kunming Medical University. During the imaging session, the rats were anesthetized by inhaling isoflurane.
2.3 Extracranial ultrasound
All images were acquired in B-mode ultrasound with optimization and constant adjustments in gain and depth throughout the experiments. The model of the machine is Apogee 5300, and the instrument was provided by the Shantou Institute of Ultrasonic Instrument Co., Ltd.
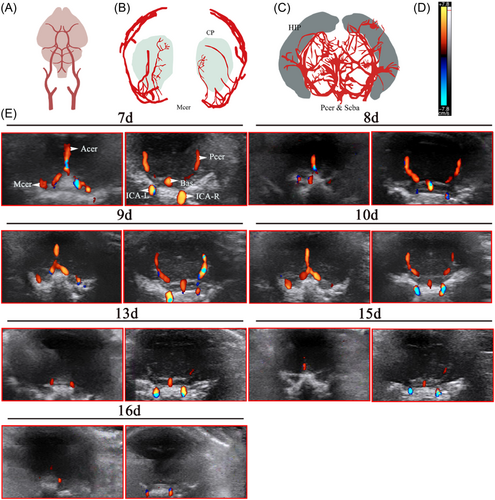
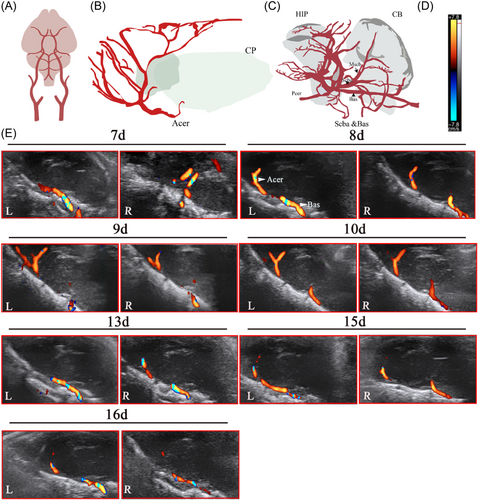
Color blood flow (CF) was used to study cerebral blood flow. In red are colored flows that move toward the transducer and blue represents flows that move away. When the CF mode was applied, an indicator bar will appear on the left or right side of the screen. The upper end of the indicator bar displays the color toward the blood flow, and the lower end displays the color away from the blood flow (Figures 2D and 3D), and the blood flow toward the probe was indicated in red and the blood flow away from the probe was indicated in blue.
To obtain the images, the animals were placed in the prone position with the sensor perpendicular to the head to obtain images from the coronal and sagittal positions. If scanning in a horizontal direction, the probe mark points toward the right side of the patient, and the screen mark points were in the default position (the left side of the screen).
The tissue structure on the side of the marker point of the probe will be displayed on the side of the marker point on the screen; the ultrasound image of the tissue on the right side of the patient will be projected on the left side of the screen, similar to the direction of CT imaging. To identify the observed cerebral blood vessels, the rats will grow hair after 7 days, and the hair in the operation area needs to be shaved, compared with the atlas of rat brain blood vessels.
This experiment explores the cerebral blood vessels of newborn rats under ultrasound and considers that the skulls of newborn rats are thin and can be probed by ultrasound. SD rats from 7 to 16 days (Figure 1A) were selected to examine cerebral blood vessels by ultrasound in the sagittal position and the coronal position. In the coronal section, the blood flow rate and heart rate were detected by ultrasound. The measurement was based on the average value of the heart rate of the left and right internal carotid arteries.
3 RESULTS
3.1 The cerebral blood flow in neonatal rats at different ages in the coronal section
Based on the anatomical diagrams of the MCA, anterior cerebral artery (Acer), posterior cerebral artery (Pcer), superior cerebral artery (Figure 2A–C), and references in literature, the cerebral vascular structure of rats from 7 to 16 days was observed dynamically under B-ultrasound. The cerebrovascular structure of rats aged 7–10 days is clear and can be explored, but it was found that the blood vessels detected with increasing age became thinner, and even undetectable (Figure 2E).
3.2 The cerebral blood flow in neonatal rats at different ages in the sagittal section
According to the anatomical diagrams of the Acer, superior cerebral artery, and basilar artery (Bas) (Figure 3A–C) and references in literature, the cerebral vascular structure of rats from 7 to 16 days was observed dynamically under B-ultrasound. The results were similar to the coronal section, the cerebrovascular structure of rats aged 7–10 days is clear and can be explored, but it was found that the blood vessels detected with increasing age became thinner, and even undetectable (Figure 3E).
3.3 Quantitative analysis of cerebrovascular velocity and heart rate in rats by coronal dynamic detection
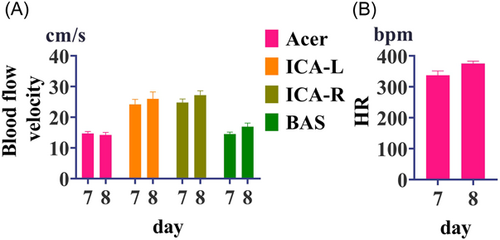
The cerebrovascular blood flow and heart rate of rats were detected by ultrasound on the 7 and 8 days, and showed no significant difference. The blood flow velocity of Acer is about 15 cm/s, the blood flow velocity of the left and right internal carotid arteries is about 25 cm/s, and the blood flow velocity of Bas is about 15 cm/s (Figure 4).
In brief, TCD ultrasound was used to explore the differences in cerebrovascular structure in SD rats of different ages, which provided the basis for the study of cerebrovascular diseases (Figure 5).
4 DISCUSSION
The sagittal and coronal normal cerebral vessels and cerebral blood flow of SD rats of different ages were detected by transcranial ultrasound for the present study. In the sagittal section, MCA, Pcer, Acer, Bas, left internal carotid artery (ICA-L), and right internal carotid artery (ICA-R) can be explored at 7–10 days. The detected cerebral vessels go thinner and undetectable with increasing age, and the detection situation in the coronal view resembles that in the sagittal view. Cerebrovascular velocity was detected in 7–9-day-old mice, and only one in 9-day-old mice, but this result may be related to the skull density becoming denser with age. Interestingly, there was no significant dissimilarity in the cerebrovascular blood flow rate and heart rate of the SD rats detected by ultrasound for 7 and 8 days, which is speculated that this may be the reason for the little age discrepancy. Therefore, the age component dissimilarity must be heeded when opting animals for cerebral vascular construction and studying the effect of cerebrovascular flow velocity on brain disease. Through this study, we learned that ultrasound can also be applied to the study of the rat brain in a noninvasive situation to realize real-time monitoring of cerebral blood flow without taking materials. The cerebrovascular building of experimental animals is one of its significant biometrics, which is a momentous indicator for the modeling and disease of course observation like cerebral hypoxic–ischemia injury, cerebral infarction, and cerebral apoplexy.10-12 Presently, there are extremely several studies on the cerebrovascular of animals of different ages. This study may afford a meaningful understructure for the observation of brain diseases or progressive brain diseases in distinct age groups.
Age makes for cerebrovascular disease. For example, the aging gene adaptor protein p66Shc mediated severe impairment of cerebral artery endothelial function with age, which caused cerebral artery vascular dysfunction.13 Migraine is related to vasodilatory stimulation of cerebral arteries, in an analysis of 248 migraine patients. Younger as associated with decreased cerebrovascular reactivity (CVR) of the Pcer, which may decipher the incremental risk why stroke in younger migraine patients.14 Arteriosclerosis is associated with age-related divergence in cerebral vascular conductance, whereas there is no palpable age pertinent dissimilarity in CVR.15 Age-induced stiffness of the vascular system such as peripheral arteriosclerosis increases pulse pressure when penetration into cerebral microvasculature give rise to endothelial dysfunction, which may contribute to neurological dysfunction and cognitive decline.16 Cerebrovascular diseases are of a great variety with complex mechanisms, such as hereditary cerebrovascular diseases and nonhereditary cerebrovascular diseases.17-19 There are 14 articles related to cerebrovascular disease merely published in the Journal of Neurology in 2012.20
All these developments demonstrate the importance of cerebral vascular structure and cerebral blood flow at different ages for the understanding of senile encephalopathy. Because this study mainly focused on exploring whether ultrasound can be used in rat research, the brain development of SD rats of different ages was not investigated in depth. With the advancement of biomedical technology, ultrasound has made many contributions to revealing the biological characteristics of the brain in-depth. This study proves that ultrasound can also detect the brain of SD rats of different ages, such as cerebral vascular structure and cerebral vascular flow velocity, which further lays the foundation for the study of brain diseases in SD rats.
5 CONCLUSION
This study shows that the cerebrovascular structure of SD rats aged 7–10 days is relatively clear and the cerebral vascular structure and cerebrovascular flow velocity can be detected. With the increase of age, the detected blood vessels become thinner and invisible. These results found that ultrasound can monitor the brain of rats in real-time in a noninvasive way. The results of this study laid the foundation for the in-depth study of rat ultrasound research and subsequent ultrasound rat brain diseases.
AUTHOR CONTRIBUTIONS
Bo-Yan Luo and Jin-Xiang Liu finished the manuscript, Liu-Lin Xiong and Chang-Le Fang helped to revise it, and Yu-Qi He conceived and reviewed it. All authors have read and ratified the final manuscript.
ACKNOWLEDGMENTS
Thanks to Shantou Ultrasound Instrument Research Institute Co., Ltd. and Kun-Zhang ([email protected]) for providing ultrasound equipment and technical support. This study was financially supported by the Department of Science and Technology of Guizhou Province (No. QKHPTRC [2019]5657), Program for Excellent Young Talents of Zunyi Medical University (15zy-004).
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICS STATEMENT
The research was conducted ethically in accordance with the Declaration of Helsinki and approved by the Animal Ethics Committee of Kunming Medical University (No. KMMU-2022-0748). [Correction added on 11 December 2023 after first online publication: This section was revised at the request of authors.]
Open Research
DATA AVAILABILITY STATEMENT
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.




