Genome-wide DNA methylation and RNA analyses enable reclassification of two variants of uncertain significance in a patient with clinical Kabuki syndrome
Abstract
Nontruncating sequence variants represent a major challenge in variant interpretation and classification. Here, we report a patient with features of Kabuki syndrome who carries two rare heterozygous variants in KMT2D: c.12935C>T, p.(Ser4312Phe) and c.15785-10T>G. The clinical significance of these variants were discordantly interpreted by different diagnostic laboratories. Parental testing showed that the missense variant was inherited from the father with a mild Kabuki phenotype and the intronic variant from the mother with mosaic status. Through genome-wide DNA methylation analysis of peripheral blood, we confirmed that the proband exhibited a previously described episignature of Kabuki syndrome. Parental samples had normal DNA methylation profiles, thus ruling out the involvement of the paternally inherited missense variant. RNA analysis revealed that the intronic change resulted in exon 49 skipping and frameshift, thereby providing a molecular diagnosis of Kabuki syndrome. This study demonstrates the utility of epigenomic and RNA analyses in resolving ambiguous clinical cases.
Disagreement over the classification of genetic variants in clinical testing is frequent. Evaluation of shared variant classification databases, for example, ClinVar, estimates there is up to a 20% discordance rate for multiple classifications of unique variants (Yang et al., 2017). The intercenter concordance of different clinical laboratories on a shared list of variants according to the American College of Medical Genetics and Genomics (ACMG) guidelines for interpretation of sequence variants (Richards et al., 2015) does not reach above 70% (Amendola et al., 2016). Such discordances mostly occur for variants with no apparent effect on the reading frame of the protein, that is, synonymous, missense, in-frame indels, and intronic variants, many of which are classified as variants of unknown clinical significance (VUS). Although discordant classifications most often do not involve a high-grade change, for example, between likely benign and likely pathogenic (Yang et al., 2017), a significant portion (~80%) of these discordances has clinical implications (Bland et al., 2018).
In clinical laboratories, a detailed review of the ACMG–AMP (Association for Molecular Pathology) guidelines and the provision of a shared framework have shown to improve the consistency in reporting the classification of variants (Amendola et al., 2016). Additional clinical information and parental testing can also resolve some of the reported discordances. Further evidence to support the classification of nontruncating variants may rely on the results of functional assays (Starita et al., 2017).
Here, we report a patient with clinical features of Kabuki syndrome, a rare genetic condition caused by heterozygous mutations in KMT2D or KDM6A (Lederer et al., 2012; Ng et al., 2010). The patient carries two rare variants in the KMT2D gene, both of which were classified discordantly by two laboratories. The molecular diagnosis was confirmed only when functional assessments, including DNA methylation and RNA analyses, were performed.
The proband was a six-year-old female presented to the medical genetics clinic for the evaluation of global developmental delay, hypotonia, and focal and generalized tonic-clonic seizures. She was born at 36 weeks’ gestational age to a healthy 29-year-old gravida one mother. Her parents were nonconsanguineous. The pregnancy was complicated by mild hypothyroidism, hypertension, and decreased fetal movements near the end of pregnancy. Her medical history included mild left-hip dysplasia, left-sided torticollis, strabismus, and premature thelarche, presumed to be secondary to an ovarian cyst. At the time of examination, her weight was 24.6 kg (92nd centile), height was 126.1 cm (99th centile), and her head circumference was 51.4 cm (65th centile). She had a tall forehead and brachydactyly. She had long palpebral fissures with eversion of her lateral lower eyelids, bilateral ptosis, and mild strabismus. Her sclerae were white. Eyelashes were long and eyebrows were high on her forehead and arched, with the sparseness of the lateral third. Her ears were prominent with a large lobe and were low-set. Her philtrum was prominent with tenting of the upper lip. She had micrognathia and a slightly high-arched palate. Teeth were small and somewhat conical. She had fetal finger pads on all fingers. Her appearance was suspicious for Kabuki syndrome, although her height was greater than most of the reported Kabuki patients. Clinical scoring for Kabuki syndrome (Adam, Hudgins, & Hannibal, 2013) resulted in a score of 6 on 10, consistent with the average score (6.1) found in patients with a pathogenic mutation in KMT2D (Adam et al., 2013).
Initial investigations, including Fragile X testing, chromosomal microarray, and basic metabolic workup, were normal. A brain MRI showed nonspecific white matter signal abnormalities, hypoplasia of the inferior cerebellar vermis, thin superior cerebellar peduncles, and optic nerve atrophy. She had a mild transient elevation in tissue transglutaminase, which can be seen in Kabuki syndrome. She was referred to clinical genetic testing for Kabuki syndrome with the sequencing of KMT2D and KDM6A. Testing identified two heterozygous variants in KMT2D (NM_003482.3): c.12935C>T, p.(Ser4312Phe), and c.15785-10T>G, neither of which were present in population genetic variant databases, including gnomAD, ESP, 1000G, and dbSNP, in disease variant databases, for example, ClinVar, or in the medical literature.
The first variant (c.12935C>T, submitted to ClinVar [https://www.ncbi.nlm.nih.gov/clinvar/] with variant ID 631483) resulted in a missense change from serine to phenylalanine at a codon conserved across all mammals and birds. In silico prediction of the amino acid change was conflicting in that two software (SIFT [score: 0.02] and PolyPhen2 [score: 0.97]) called it deleterious but two others (MutationTaster [p-value: 0.88] and AGVGD [class: C0]) called it benign. The laboratory classification for this variant was a VUS. Parental testing revealed that the variant was inherited from her father. However, the variant classification was not downgraded to likely benign as the father reported mild features of Kabuki syndrome, including speech delay in childhood, poor coordination, fetal finger tip pads, and subjectively long palpebral fissures with mild eversion of the lateral lower eyelids. Although he did not meet the criteria for a clinical diagnosis of a Kabuki syndrome, his features raised the possibility of incomplete penetrance or variable expressivity of the variant.
The second variant (c.15785-10T>G, submitted to ClinVar [https://www.ncbi.nlm.nih.gov/clinvar/] with variant ID 489237) was a noncoding change located 10-base pairs (bp) upstream of exon 49. Initial parental testing using Sanger sequencing highlighted the change as de novo. In silico analyses for splicing effects using five computational tools (SpliceSiteFinder, MaxEntScan, NNSPLICE, GeneSplicer, and Human Splicing Finder) predicted small to moderate reductions (between 4% and 36%) in the splice acceptor activity at the downstream intron–exon junction. ESEfinder predicted that the region is a binding site for three splicing enhancer factors (SRp35, SRp40, and SPr55) and that this sequence change eliminates the binding site for SRp40 and SPr55 and shifts the binding site for SRp35 to 6-bp downstream. The laboratory's interpretation of the variant was likely benign.
Without a confirmed diagnosis, clinical trio exome sequencing was offered to the patient and her parents at another laboratory. No other causative variants were identified besides those two noted above. The two variants, however, were interpreted differently at the second laboratory. This laboratory downgraded the classification of the missense variant (c.12935C>T) from VUS to benign and upgraded the intronic variant (c.15785-10T>G) from likely benign to likely pathogenic. This represented a two-level change in the classification of each variant. The only additional finding was that the intronic variant was present with a variant allele fraction of 7% in the mother, indicating possible maternal mosaicism and inheritance. Given the conflicting reports from the two laboratories, the case remained unresolved.
We have previously reported that patients with Kabuki syndrome have a highly sensitive and specific DNA methylation episignature detectable in the peripheral blood (Aref-Eshghi et al., 2017a). The DNA methylation signature of Kabuki syndrome, along with those of several other congenital conditions, has been used to generate a computational functional assay for the concurrent detection of multiple neurodevelopmental syndromes in those presenting with uncertain clinical or molecular findings (Aref-Eshghi, Rodenhiser, et al., 2018). This algorithm is a multiclass predictor, trained on the DNA methylation levels of ~900 informative CpG sites across the genome for 14 conditions with specific DNA methylation episignature in the blood (details in Supporting Information Methods; Aref-Eshghi et al., 2019). To provide a molecular diagnosis based on epigenomic assessment for this patient, we performed genome-wide DNA methylation analysis on the peripheral blood samples from the patient and her parents using infinium methylation arrays and supplied the measured methylation levels of the informative CpGs to the classification model. The algorithm confidently classified the patient as having Kabuki syndrome (score 0.75/1.0 for Kabuki category and <0.01/1.0 for all other conditions). Her parents, however, received a score <0.01/1.0 for Kabuki syndrome and the other conditions. Further assessment using 142 CpG sites fully differentiating patients with Kabuki syndrome from other conditions or controls (Aref-Eshghi et al., 2017a) led to the same observations for the proband and her parents (Figure 1). These analyses confirmed that the DNA methylation profile of the patient is consistent with Kabuki syndrome. Both parents had normal methylation profiles, indicating that the missense variant present in the father was most likely benign. However, this analysis does not examine the effect of the noncoding variant in the development of the disease.
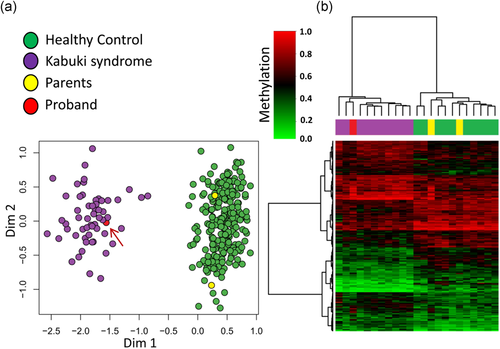
DNA methylation analysis of the proband and her parents. (a) The first two dimensions from multidimensional scaling of the DNA methylation levels at 142 CpG sites across the genome specific to Kabuki syndrome completely separate ~300 healthy controls (green) from >50 subjects diagnosed with Kabuki syndrome (purple). Addition of the proband (red—indicated with an arrow) and her parents (yellow) to this analysis, clusters the proband with other Kabuki patients and the parents with the controls. (b) The DNA methylation profile of a random set of 10 samples from healthy controls and Kabuki patients together with the proband and her parents is visualized using hierarchical clustering analysis. Rows represent 142 CpG sites and columns indicate the samples. The color scheme of the top pane is indicative of the class (purple, known kabuki patients; green, healthy controls; red, proband; and yellow, parents). The heatmap color scale from light green to light red indicates the methylation levels (0–1). The proband has a methylation profile comparable to the known Kabuki patients, whereas the parents have a profile similar to controls
To investigate the effect of the c.15785-10T>G variant on the splicing of exon 49 in KMT2D, we performed reverse transcription polymerase chain reaction (RT-PCR) using RNA extracted from the peripheral blood of the proband and two controls. To test the hypothesis that the variant induces skipping of exon 49, forward and reverse primers were designed to cover 402 bp of the coding sequence of KMT2D, comprising the 3′ end of exon 48, the entire exon 49, and exon 50 (forward primer: AATCGTCGCTGCTGCTATCGC-3′—identical to c.15667–15687; reverse primer: 5′-TTCAGGGTATGGGGCCGTTTG-3′—complementary to c.16048–16068). The PCR analysis identified a single DNA band with the expected size (402 bp) in the controls, but two DNA bands with reduced intensity in the proband (Figure 2). Among the two DNA products of the proband, the larger one corresponded to the wild-type allele, and the smaller one had a size consistent with exon 49 skipping (266 bp). To confirm this, PCR products of the patient and one control were Sanger sequenced from both forward and reverse directions. As expected, the control PCR products were homogeneously wild-type, while the patient's PCR products were a mix of two molecules, one of which corresponded to the wild-type and the other to the mutant with exon 49 skipping (Figure 2). This missplicing was predicted to result in a frameshift at codon 5263, generating a premature termination codon 36 codons downstream (p.(Val5263Alafs*36)), and likely a nonsense-mediated messenger RNA (mRNA) decay. Therefore, only one active copy of KMT2D would be present in the patient, resulting in haploinsufficiency of the protein. We, therefore, considered this intronic variant as likely causative of the proband's phenotype.

Experimental evaluation of the splicing effect of the intronic variant. The 402-bp coding region of the KMT2D mapping to 3′ end of exon 48, entire exon 49, and exon 50 from the proband, and two controls were subject to reverse-transcriptase PCR. Black arrows indicate the location of forward and reverse primers used in the reaction. The PCR product is composed of one band of the expected size, that is, 402 bp, in the controls (C1 and C2), but two bands in the proband (P). The first band has a similar size to the wild-type (402 bp), whereas the second band is ~140 bp shorter, consistent with the skipping of exon 49. The concentration of DNA for the wild-type band is estimated at 36.1 and 35.2 ng/µl in the controls and half of these in the proband (17.3 ng/µl). The concentration of the mutated band in the proband is 9.59 ng/µl. Sanger sequencing of the PCR product confirms that the proband has two overlapping sequence reads at the start and end of the exon 49, one of which matches to exon 49, whereas the other represents 3′ and 5′ of exons 48 and 50, respectively. NTC, nontemplate control; PCR, polymerase chain reaction; C1, control 1; C2, control 2
As mentioned above, this intronic variant was detected by exome sequencing to be present in the blood sample of the patient's mother with a frequency of 7%. To further investigate the origin of this variant, we performed Sanger sequencing of DNA extracted from a buccal swab sample from the mother. This variant was not identified in the mother's sample. There are two possible explanations for this result. First, the variant may be present in the buccal swab sample at a frequency below the detection limit of Sanger-sequencing technology. Second, the variant may be truly absent in the buccal swab, suggesting tissue-specific mosaicism. However, this variant is likely to be present in the germ cells of the mother.
Mutations that affect mRNA splicing account for a significant number of variants found in genetic diseases, the majority of which do not involve the canonical AG/GT dinucleotides of the intron–exon junctions (Ars et al., 2000; Lopez-Bigas, Audit, Ouzounis, Parra, & Guigo, 2005; Soukarieh et al., 2016). These variants induce a variety of effects including exon skipping, intron retention, exonification of noncoding sequences, or intronification of part of an exon. The resulting proteins are often partially or fully nonfunctional or present with a novel property, leading to disease development. High-throughput sequencing of gene panels or exome has become commonplace in the clinical setting for the detection of disease-causing variants. These assays are primarily limited to the coding sequence with limited coverage of the flanking coding DNA (often <20 bp). Variants identified within these limited intronic segments remain a challenge in clinical assessment and their interpretation is mainly performed using computational tools which evaluate the effects of genomic variants at the mRNA and protein levels. These algorithms have been shown to incorrectly predict the effects of 2.6–76.1% of the splicing events in the genome (Jian, Boerwinkle, & Liu, 2014). Therefore, the majority of the splice-impacting variants are not identified or remain unexplored in routine clinical testing. In addition to noncoding variants, molecular testing for clinical genetic conditions is complicated by the identification of rare missense, synonymous, and in-frame indels, which can impact both protein structure/function and splicing. Lack of standard approaches for assessment of such variants makes them susceptible to judgmental interpretations. As such, they account for the majority of the discordantly classified variants in disease variant databases (Yang et al., 2017).
Here, we have shown that functional assessment can be a valuable tool when molecular interpretation is unable to fully clarify the pathogenicity. Using a novel episignature-based approach, we have demonstrated the ability, independently from sequence variant assessment, to assign a diagnosis to those presenting with ambiguous clinical or molecular findings, or at instances where a variant cannot be identified in the patients (Aref-Eshghi, Bend, et al., 2018). In Kabuki syndrome, up to 30% of patients are not found to have a causative variant (Adam et al., 2013). While some of these patients may have noncoding variants or a VUS in known disease-causing genes, others may have variants in genes yet-to-be identified in Kabuki syndrome. In cases where a nucleotide change with a potential splicing effect is found, RNA analysis can be an ideal follow-up assay. We propose that combined epigenomic analysis and transcriptome sequencing has superior performance to DNA sequencing alone in the molecular diagnosis of genetic conditions. In addition to the coding variants that will be mutually detectable in both of the exome and transcriptome, RNA analysis allows for the evaluation of aberrant posttranscriptional processing of the mRNA. The addition of epigenomic analysis allows for the detection of a genetic condition without any prior knowledge of sequence variants and will be particularly important when sequencing analysis results in a VUS or no candidate variant. We have successfully developed a similar technology for more than 20 constitutional disorders and cancer, routine application of which in the clinical setting will enable drastic improvements in variant interpretation and diagnosis (Aref-Eshghi et al., 2017b; Aref-Eshghi, Schenkel, et al., 2018; Aref-Eshghi, Schenkel, Carere, Rodenhiser, & Sadikovic, 2018; Bend et al., 2019; Schenkel et al., 2018).
ACKNOWLEDGMENTS
The authors would thank the patient and her family for their active participation in this study.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.