Novel missense mutation in VPS33B is associated with isolated low gamma-glutamyltransferase cholestasis: Attenuated, incomplete phenotype of arthrogryposis, renal dysfunction, and cholestasis syndrome
Abstract
The typical phenotype of arthrogryposis, renal dysfunction, and cholestasis (ARC) syndrome involves three cardinal symptoms as the name describes, harboring biallelic mutations on VPS33B or VIPAS39. Except for ARC syndrome, low gamma-glutamyltransferase (GGT) cholestasis often implies hereditary hepatopathy of different severity; however, some remain undiagnosed. Several monogenic defects typically with multiorgan manifestations may only present liver dysfunction at times, such as DGUOK defect and AGL defect. Previously, four VPS33B mutated cases were reported without arthrogryposis, or with less severe symptoms and longer lifespan, indicating the possibility of incomplete ARC phenotype of isolated hepatopathy. So we retrospectively reviewed all patients with confirmed VPS33B/VIPARS39 defect in our center and identified three presenting isolated low-GGT cholestasis with intractable pruritus. Distinguished from others with typical ARC phenotype, these patients did not suffer the other two typical characteristics, survived much longer, and shared a novel missense VPS33B variation c.1726T>C, p.Cys576Arg, causing declined protein expression and abolished interaction with VIPAS39 in-vitro. Serum bile acid profiles of our VPS33B/VIPAS39 mutated patients revealed similar changes to primary defect of bile salt export pump, among which those with isolated cholestasis phenotype had a higher level of total secondary bile acids than that with typical ARC phenotype, indicating the partial residual function of VPS33B.
1 INTRODUCTION
Arthrogryposis, renal dysfunction, and cholestasis (ARC) syndrome is a rare autosomal recessive disorder caused by biallelic pathogenic variations on VPS33B or VIPAS39, which product forms complex together for intracellular protein trafficking (Cullinane et al., 2010; Gissen et al., 2004). Typically, patients with ARC syndrome suffer three major characteristic symptoms involving congenital arthrogryposis, renal dysfunction mainly for distal renal tubular dysfunction, and low gamma-glutamyltransferase (GGT, <100 IU/L) cholestasis; severity of each characteristic may vary in patients (Arhan, Yusufoğlu, & Şayli, 2009; Cullinane et al., 2009a; Gissen et al., 2006). Additional features that affected other organs include ichthyosis and hyperkeratosis, hearing loss, platelet dysfunction, bleeding tendency, recurrent infection, hypothyroidism and so forth (Gissen et al., 2006; Gruber et al., 2017).
Infantile low/normal GGT cholestasis comprises a series of monogenic defects. Except for ARC syndrome, other defects usually manifest a spectrum of familial cholestasis of different severity, or varied phenotypes from intractable watery diarrhea to isolated cholestasis (Bull & Thompson, 2018; Chen et al., 2018). In addition, hereditary diseases that typically result in multiorgan disorders may manifest isolated hepatopathy at times. For example, DGUOK deficient patients of mitochondrial disorder typically suffer early-onset rapid-progressive liver failure and neurological developmental problems, but some of them will just present hepatomegaly and elevated liver enzymes (Dimmock et al., 2008); AGL deficient patients of type III glycogen storage disease (GSD) mostly manifest diffuse muscle weakness and hepatomegaly with fibrosis, whereas 15% of them only show symptoms and enzyme defect in liver (Lucchiari, Santoro, Pagliarani, & Comi, 2007; Shen et al., 1996). Those subtypes/incomplete phenotypes make it difficult to differentiate through routine clinical investigations without genetic analysis. Some of low-GGT cholestatic patients still remain undiagnosed, indicating the existence of undiscovered pathogenic genes or unknown phenotypes of known defects (Qiu et al., 2017).
For ARC syndrome, so far four cases are reported with incomplete/attenuated ARC phenotype: One VPS33B mutant had two major characteristics of cholestasis and renal tubular dysfunction without arthrogryposis or any other musculoskeletal involvement (Bull et al., 2006); the other three VPS33B (NM_018668.4 and NP_061138.3) mutants presented with less severe symptoms and had better prognosis, sharing a splicing variation c.1225+5G>C (Bem et al., 2015; Smith et al., 2012). This suggested the possibility of incomplete ARC phenotype of isolated cholestasis like other multiorgan disorders.
To further investigate the genotype-phenotype correlation in ARC syndrome, we reviewed all the VPS33B/VIPAS39 mutated patients in our center (2011–2017) with confirmed biallelic pathogenic variations, and identified three from previously undiagnosed patients with isolated low-GGT cholestasis; all shared a novel missense variation c.1726T>C, (p.Cys576Arg) on VPS33B. In vitro study demonstrated that VPS33BCys576Arg cannot colocalize with its interacting protein VIPAS39, and caused sharply reduced expression of both proteins. The other four patients with typical characteristics of ARC syndrome carried known and/or biological pathogenic variations, resulting in no protein expression.
2 SUBJECTS AND METHODS
2.1 Subjects and genomic analysis
Subjects were enrolled from 2011 to 2017, with informed consent, under a clinical-diagnosis protocol approved by Children's Hospital and Jinshan Hospital of Fudan University (Shanghai, China) and according to the ethical guidelines of the 1975 Declaration of Helsinki. They underwent routine tests for liver diseases (Qiu et al., 2017). Genomic DNA for sequencing was extracted from ethylenediaminetetraacetic acid (EDTA)-treated peripheral blood cells (QIAamp DNA Blood Mini Kit, Cat. 51106; Qiagen, Germany). Before 2014, two ARC patients (P4, P5 in this study) were diagnosed by the three major symptoms, and then were confirmed biallelic pathogenic variations through Sanger sequencing of all VPS33B exons (Gissen et al., 2006; Li, Zhao, Chen, & Wang, 2014; J. S. Wang, Zhao, & Li, 2014). Since 2014, patients with suspected hereditary cholestasis receive targeted panel sequencing or whole exome sequencing to screen underlying pathogenic gene (Qiu et al., 2017); three more VPS33B mutated patients (P1, P2, and P6) and one VIPAS39 mutated patient (P7) were identified. P1 and P2 presented isolated cholestasis; P6 and P7 presented typical ARC phenotype. P3 was identified by Sanger sequencing within family members of P2 and presented isolated cholestasis.
Twenty patients with primary defect of bile salt export pump (BSEP) and 37 healthy controls in bile acid profiling investigations were described in former studies (Liu et al., 2018; Qiu et al., 2017).
2.2 In-silico variation prediction and genotype-phenotype correlation study
All the VPS33B/VIPAS39 variations in these patients were searched for frequency in public databases as well as in-house databases. Next, online tools were applied to predict their pathogenicity (Schwarz, Cooper, Schuelke, & Seelow, 2014). And then, we compared types of mutations between patients with typical ARC phenotype and isolated cholestasis. Websites of these online tools were presented as below.
Websites for public variant databases:
dbSNP. https://www.ncbi.nlm.nih.gov/SNP/
1000 genomes. http://www.1000genomes.org/
ExAC Browser. http://exac.broadinstitute.org/
Exome Variant Server (ESP6500). http://gvs-1.gs.washington.edu/EVS/
gnomAD. http://gnomad.broadinstitute.org/
ClinVar. http://www.clinvar.com
HGMD. http://www.hgmd.compareac.uk/ac/index.php
Websites for online variation prediction:
SIFT. http://sift.jcvi.org
PolyPhen2. http://genetics.bwh.harvard.edu/pph2/
MutationTaster. http://www.mutationtaster.org
PROVEAN. http://provean.jcvi.org/genome_submit_2.php
BDGP. http://fruitfly.org/seq_tools/splice.html
Swiss-model. https://www.swissmodel.expasy.org/
2.3 Construct of mutated VPS33Bc.1726T>C, p.Cys576Arg plasmid
To study the impact of the novel missense variation c.1726T>C, (p.Cys576Arg) on VPS33B, we bought wild-type human ORF cloned plasmids of VPS33B (NM_018668.4, pCMV3-C-Flag, Cat. HG16334CF, Sino Biological, Beijing, China) and VIPAS39 (NM_001193315.1 pCMV3-N-HA tag, Cat.HG22032-NY, Sino Biological, Beijing, China) that encoding its interacting protein. Mutated VPS33B plasmid was generated through point mutation by KOD -Plus- Mutagenesis Kit (Cat. 0811, TOYOBO, Osaka, Japan). Forward primer for point mutation was GTGGTCGTACATTCTCTGAGATCTC, and the reverse primer CCAAGAACACCACCAAGATGAGGCG.
2.4 Cell culture and transfection
For western blot study, HEK-293T cells were bred in 6-well plates. In each well, a total of 2 μg plasmids were transferred into when the cells grew near 90% (PolyJet In Vitro DNA Transfection Reagent, Cat. SL100688, SignaGen, Gaithersburg, MD). Plasmid pcDNA3.1 was set as the control. One microgram wild-type and mutated VPS33B plasmid were cotransfected with 1 μg pcDNA3.1, or with 1 μg Wild-type VIPAS39 plasmid, respectively. For the immunofluorescent study, HEK-293T and MDCK cells were bred in 24-well plates with glass coverslips respectively, with similar procedure but transfected with a total of 1 μg plasmids. Cells were harvested 48 hrs after transfection for following experiments.
2.5 Immunofluorescence microscopy
Cells were fixed with 4% paraformaldehyde for 15–30 min and gently washed by phosphate-buffered saline (PBS) 10 min for three times. And then, cells were incubated at 4°C overnight with mouse anti-Flag antibody (1:500, dilute with PBS containing 1% goat serum & 0.3% Triton, Cat. 8146S, CST, Danvers, MA) and rabbit anti-HA antibody (1:800, dilute with PBS containing 1% goat serum and 0.3% Triton, Cat. 3724S, CST, Danvers, MA). After gently washed by PBS containing 0.2% tween20 10 min for three times, cells were stained with fluorescent secondary antibodies for 1 hr (Goat antimouse Secondary Antibody, Alexa Fluor 594 conjugated, Cat. A-11032, Invitrogen, [Shanghai], Shanghai, China; Goat anti Rabbit Secondary Antibody, Alexa Fluor 488 conjugated, Cat.A0423, Beyotime, Shanghai, China), washed with 0.2% tween-20 PBS and stained with DAPI for 5 min (5 μg/ml, dilute with ddH2O, Cat. 1155GR001, NeoFROXX, Einhausen, Germany). Coverslips were put up-side-down on the microslide with Eukitt® Quick-hardening mounting medium for microscopy (Cat. 03989, Sigma-Aldrich, Osterode am Haz, Germany) and photographed with a fluorescent microscope and confocal microscope (Leica SP8).
2.6 Western blot
Five hundred microliters radioimmunoprecipitation assay (Cat. P0013B, Beyotime, Shanghai, China) with protease inhibitor were added to 6-well plates on ice. After 15-30 min, cell lysis was transferred to centrifuge tubes, centrifuged at the speed of 12000r for 5 min. After concentration measurements (Cat. 23227, Pierce™ BCA Protein Assay Kit - Reducing Agent Compatible, Thermo Fisher Scientific, [Shanghai], Shanghai, China), the supernatant was added with SDS loading and boiled for 10 min for sample preparation. The routine procedure of western blot study was performed, using the same primary antibodies in the immunofluorescent study (1:2,000), and the secondary antibodies from Beyotime (Cat. A0208 & A0216 respectively).
2.7 Bile acid analysis in serum
Serum sample was separated from blood by routine centrifugation, ali-quoted (50 μl), freeze-dried and stored at −80°C until bile acid analysis was performed by reversed-phase ultrahigh-performance liquid chromatography/multiple-reaction monitoring-mass spectrometry (UPLC/MRM-MS) with negative ion detection as described previously (Han et al., 2015; Liu et al., 2018; Qiu et al., 2017). The quantitation of bile acids utilized standard substances of 60 bile acids included all of the major bile acids and some minor species with 14 deuterium-labeled analogs of bile acids as internal standards. Serum bile acid profiling was carried out in all available VPS33B/VIPAS39 deficient patients, compared with healthy donors and patients with primary BSEP deficiency as controls.
3 RESULTS
3.1 Novel missense VPS33B variation c.1726T>C, p.Cys576Arg was shared in patients with isolated cholestatic phenotype
From 2011–2017, 6 VPS33B mutated patients (P1–P6) and one VIPAS39 mutated patient (P7) were identified in our center. They were all term babies from healthy unrelated Han parents (Figure 1). Of interest, P1–P3 presented only low-GGT cholestasis, but lack proof of other two typical manifestations of arthrogryposis and renal dysfunction. Moreover, P1–P3 shared a novel missense variation c.1726T>C, p.Cys576Arg in VPS33B (NM_018668.4 and NP_061138.3): P1 was homozygous, whereas P2 & P3 from another family were compound heterozygous. This novel variation has never been reported in public databases such as dbSNP, 1,000 genomes, ExAC, ESP6500, and gnomAD, nor does in our in-house database or other patients with typical ARC phenotype, predicted pathogenic by online software PROVEAN, SIFT, Polyphen2 and MutationTaster (Schwarz et al., 2014). Besides, no other disease-causing mutation was detected in genes associated with low GGT cholestasis.
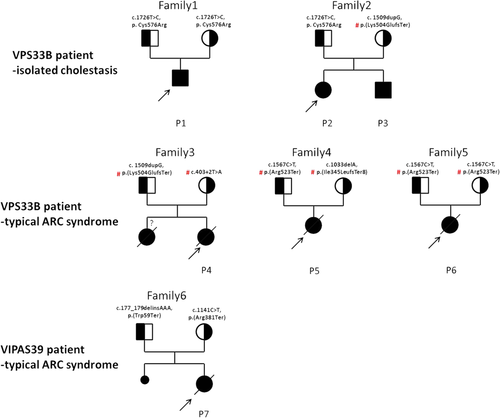
Family trees and mutations of patients with confirmed VPS33B/VIPAS39 defects. three patients with isolated cholestasis from two unrelated families were identified harboring biallelic VPS33B mutants, all sharing novel, predicted pathogenic missense variation c.1726T>C, (p.C576R). Three patients with VPS33B variations and one with VIPAS39 variations were found to present typical characteristics of ARC syndrome, all carrying biological pathogenic mutations that predicted with absent protein expression. # Reported pathogenic variations in ARC patients. ARC, arthrogryposis, renal dysfunction, and cholestasis
For the rest patients (P4–P7) with complete ARC phenotype, they all carried biallelic mutations that were biologically pathogenic, including frameshift mutation, nonsense mutation, and classical splicing mutation (Figure 1). VPS33B mutations in P4-P6 were seen in reported patients, whereas VIPAS39 mutations in P7 were novel.
3.2 Clinical features of VPS33B mutant patients with isolated low-GGT cholestasis
Major clinical features of P1–P7 were shown in Table 1. Clinical course of P1–P3 were detailed below. P4–P7 had typical, complete phenotype of ARC syndrome and all died before the age of 2.
| Patient | P1 | P2 | P3 | P4 | P5 | P6 | P7 |
|---|---|---|---|---|---|---|---|
| VPS33B defect | VIPAS39 defect | ||||||
| Gender | male | male | female | female | female | female | female |
| Parental consanguinity | + | − | − | − | − | − | |
| Maternal spontaneous abortion | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Siblings | none | 1 affected sister (P3) | 1 affected brother (P2) | 1 affected sister without genetic diagnosis | none | none | none |
| Paternal variation* | c.1726T>C, p. Cys576Arg | c.1726T>C, p. Cys576Arg | c.1726T>C, p. Cys576Arg | *c. 1509dupG, p.(Lys504GlufsTer) | *c.1567C>T, p.(Arg523Ter) | *c.1567C>T, p.(Arg523Ter) | c.177_179delinsAAA, p.(Trp59Ter) |
| Maternal variation* | c.1726T>C, p. Cys576Arg | *c. 1509dupG, p.(Lys504GlufsTer) | *c. 1509dupG, p.(Lys504GlufsTer) | *c.403+2T>A | *c.1033delA, p.(Ile345LeufsTer8) | *c.1567C>T, p.(Arg523Ter) | c.1141C>T, p.(Arg381Ter) |
| Presenting age | 1mo | 1mo | 1mo | 3d | 3d | 3d | 1d |
| Outcomes | cholestasis & pruritus | cholestasis and intractable pruritus | mild cholestasis and intractable pruritus | died at the age of 6 months | died at the age of 7 months | died younger than the age of 2 yr | died at the age of 11 months |
| Typical cardinal triad | |||||||
| Cholestasis | + | + | + | + | + | + | + |
| GGT (6–57 IU/ml) | 25–66 | 21–65 | 16.5–19 | 150–216 | 28–33 | NA | 30–39 |
| Pruritus | + | + | + | + | + | + | + |
| Hepatomegaly | + | − | + | + | − | + | + |
| Splenomegaly | + | − | + | − | − | − | − |
| Typical kidney dysfunction | − | − | − | + | + | + | + |
| Urinary glucose | − | − | − | + | + | + | ++ |
| Urinary amino-acid | ND | Slight elevated phenylalanine | Slight elevated cystine | ND | Poly-aminoaciduria | ND | Poly-aminoaciduria |
| Serum lactic acid | normal | normal | normal | slightly elevated | ND | ND | normal |
| Metabolic acidosis | − | ND | − | ND | ND | ND | + |
| Urinary protein | + (once) | ++ (once) | + | + | + | − | +++ |
| BUN | normal | normal | normal | normal | NA | ND | normal |
| Cr | normal | normal | normal | normal | NA | ND | normal |
| Urinary microprotein | ND | slightly elevated | slightly elevated | normal | ND | ND | remarkably elevated |
| Kidney structure via ultrasound | normal | normal | normal | polysitic kidney | unclear kidney structure | ND | ND |
| Arthrogryposis | − | − | − | + | + | + | + |
| Affected joints | none | none | none | most of the joints | both knees and ankles | joints on both lower limbs | both ankles |
| Accompanied symptoms | |||||||
| muscle skeletal problems | − | − | dextroscoliosis | − | dislocation of both hip joints | hypotonia | − |
| skin involvment | − | − | desquamation on joints | ichthyosis | mild ichthyosis | desquamation on abdominal skin | NA |
| Hematologic disorder | slightly prolonged APTT | − | slightly prolonged APTT | moderate anemia | − | moderate anemia | mild anemia |
| Infections in infancy | − | pneumonia | urinary tract infections | pneumonia | pneumonia | urinary tract infections | pneumonia |
| Characteristics | Isolated cholestasis | Isolated cholestasis | Isolated cholestasis | Typical ARC phenotype | Typical ARC phenotype | Typical ARC phenotype | Typical ARC phenotype |
- Abbreviations: ARC, arthrogryposis, renal dysfunction, and cholestasis; APTT, activated partial thromboplastic time; GGT, gamma-glutamyltransferase; BUN, blood urea nitrogen; Cr, creatinine; NA, not available; ND, not done.
- * GenBank reference sequences of mentioned variations: VPS33B: NM_018668.4 and NP_061138.3; VIPAS39: NM_001193315.1 and NP_001180244.1.
- # Reported pathogenic variations in ARC patients.
3.2.1 Patient 1 (P1)
P1 is a boy from Hubei Province, who presented like recurrent cholestasis. The first three episodes of cholestasis occurred at the age of 1 month, 21 months, and 33 months, respectively, and alleviated by ursodeoxycholic acid (UDCA). Body examination and routine tests at 33 months revealed short stature (84 cm, 11 kg; less than 3% percentile), enlarged liver and spleen (5 cm and 3 cm below costal arc, respectively), mild cholestasis, with normal GGT level, elevated transaminases and slightly prolonged activated partial thromboplastic time (APTT: 47.1 seconds; normal range 28–44.5 seconds), but without muscular skeletal abnormalities or signs of renal dysfunction (urine protein positive for once; Li, 2009). Sanger sequencing of major pathogenic genes for low-GGT cholestasis (ATP8B1 and ABCB11) were negative; others were unremarkable.
When the fourth episode attacked at 38 months, UDCA therapy seemed of no response. Jaundice and severe pruritus finally alleviated by an internal biliary diversion in another hospital. He received a liver biopsy during operation and was diagnosed cholestasis with giant cell formation (slides not available). Considered hereditary unexplained cholestasis, the boy received whole exome sequencing and novel homozygous VPS33B missense mutation was identified. Till the latest follow-up, the boy is still alive (7-yr old).
3.2.2 Patient 2 (P2)
P2 is also a boy from Shandong Province, who presented like persistent cholestasis. Jaundice occurred at the age of 1 month following pneumonia, with slightly elevated aspartate aminotransferase (AST) and without joint problems. Therapy of UDCA and some so-called liver protective drugs seemed of little use; mild conjugated hyperbilirubinemia sustained with intractable pruritus since 4 months old. Cholestyramine was then added, with total and direct bilirubin declined to the normal range but pruritus did not improve. He was then transferred to a bigger hospital in Beijing. Eliminating other possible etiologies for jaundice, the boy received needle liver biopsy and targeted panel sequencing (aged 10 months). Pathological diagnosis was “G1S2-3, early bile duct vanishing syndrome” (slides not available), whereas panel sequencing revealed suspected biallelic mutations in VPS33B (details in the following results).
To make a definitive diagnosis, he visited our center at 13 months. Body examination demonstrated very dry skin with scratches and ceaseless scratching (even in asleep), but without jaundice, hepatosplenomegaly, edema or muscular skeletal abnormalities. Liver function tests showed mild elevated transaminases and total bile acids; bilirubin, GGT, alpha-fetoprotein and others were within the normal range. Kidney function tests revealed normal blood urea nitrogen (BUN), serum creatinine (Cr) and urine pH and osmolality, positive urine protein, slightly elevated α1 and β2 microglobulin, transferrin and white blood cells, whereas urine glucose was not detected. Other tests were unremarkable. Sanger sequencing confirmed compound heterozygosity of him and his sister (P3). Pruritus could not alleviate through routine therapy of UDCA, cholestyramine, and moisturized cream. He was discharged with further medication refused by his parents. But through recent telephone contact, the boy is still alive with pruritus (4 yrs).
3.2.3 Patient3 (P3)
P3 is the elder sister of P2. She presented nearly the same symptoms and biochemical features to her younger brother, suffering hyperbilirubinemia since 1-month old and intractable pruritus since 4 months. She had no response to routine medication of UDCA, cholestyramine, soluble vitamins, and moisturized cream either. Slight different from P2, the girl had dextroscoliosis when she visited us at the age of seven, which was not observed several years ago. The further medication was refused by parents either. She is now 9 years old, alive with pruritus.
3.3 VPS33Bc.1726T>C, p.Cys576Arg caused remarkably declined VPS33B expression and hampered interaction with VIPAS39 in vitro
To further investigate the pathogenicity of the novel VPS33B mutation c.1726T>C, p.Cys576Arg in isolated cholestatic patients, we constructed Flag-tagged wild type VPS33B and mutant plasmids, and HA-tagged VIPAS39 plasmid. Compared with the wild-type, VPS33Bc.1726T>C, p.Cys576Arg caused significantly decreased protein expression, no matter overexpressed individually or co-overexpressed with VIPAS39 in HEK-293T cell lines (Figure 2a). Surprisingly, VPS33Bc.1726T>C, p.Cys576Arg also resulted in declined VIPAS39 expression when co-overexpressed (Figure 2b). Confocal microscopy repeated the observations above in both HEK-293T and MDCK cell lines and revealed that VPS33BCys576Arg cannot co-localize with VIPAS39, indicating hampered interaction (Figure 3).
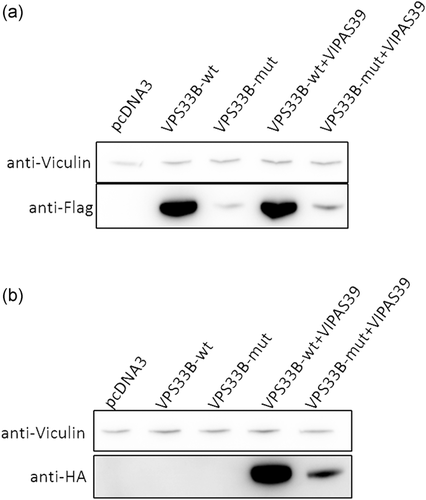
Novel missense VPS33B variation c.1726T>C caused declined protein expression in HEK-293T cell lines. (a) Compared with Flag-tagged wild type VPS33B plasmids, Flag-tagged VPS33Bc.1726T>C, p.Cys576Arg plasmid resulted in a significant decrease of VPS33B expression in HEK-293T cell lines, no matter overexpressed itself or co-overexpressed with HA-tagged wild type VIPAS39. (b) Of note, when co-overexpressed with wild-type HA-tagged VIPAS39, Flag-tagged VPS33B c.1726T>C, p.Cys576Arg also cause decline expression of its interacting protein VIPAS39
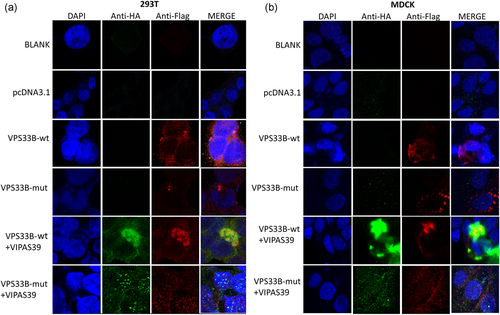
Confocal microscopy revealed abolished the interaction between VPS33Bp.Cys576Arg and VIPAS39 in-vitro (original magnification, all images, ×630). HA-tagged wild-type VIPAS39 was co-overexpressed with Flag-tagged wild type VSP33B and Flag-tagged VPS33Bc.1726T>C, p.Cys576Arg respectively. Under the same condition of confocal microscopy, wild-type VPS33B colocalized with wild-type VIPAS39, whereas VPS33B p.Cys576Arg could not co-localize with VIPAS39, indicating hampered interaction. The observations repeated in both HEK-293T and MDCK cell lines
3.4 Bile acid profiling suggested secondary BSEP dysfunction in VPS33B/VIPARS39 mutants
Previously studies have suggested that the defect of VPS33B/VIPAS39 complex leads to the dislocation of BSEP (or ABCB11) (Ackermann et al., 2014; Bull et al., 2006; Hanley et al., 2017), the main transporter for bile acid exocytosis on the canalicular membrane. In this study, the bile acid profiling in both ARC-isolated cholestasis group and ARC-typical phenotype group (ARC-IC and ARC-TPY, respectively, Figure 4) revealed “typically” cholestatic bile acid profiles with significantly increased total bile acids, total primary bile acids, and total conjugated bile acids(Figure 4a,b,d). The total secondary bile acids, which reflect the bile acid secretion through the canalicular membrane into the lumen via BSEP (Hylemon & Harder, 1998), were decreased in ARC group, resemble to those with primary BSEP defects. Of note, patients with isolated cholestasis had a much higher concentration of secondary bile acids than those with typical ARC symptoms.
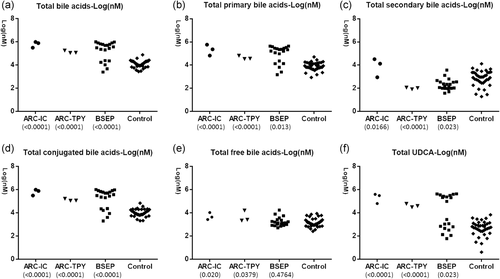
Plasma bile acids profiling of patients with confirmed VPS33B/VIPAS39 defects. Bile acid (BA) profiles obtained by UPLC-ESI/MRM-MS (μM) of plasma from six patients carrying VPS33B/VIPAS39 defects (P1-P3 & P5-P7), compared to 20 patients with confirmed primary BSEP defect and 37 healthy controls.ARC-IC: ARC patients with isolated cholestasis; ARC-TPY: ARC patients with typical phenotype. (a) Total bile acids covered all major bile acids and many rare bile acids, while (b) total primary bile acids, (c) total secondary bile acids, (d) total conjugated bile acids, (e) total free bile acids, and (f) total UDCA were calculated as previous description (Han et al., 2015; Qiu et al., 2017). The number below each of the ARC and BSEP groups is the P value of the group versus controls. The bile acid profiling in both ARC groups revealed “typically” cholestatic bile acid profiles with significantly increased total bile acids, total primary bile acids, and total conjugated bile acids (Figure 4a,b,d). The total secondary bile acids reflecting the bile acid secretion through the canalicular membrane into the lumen via BSEP (Hylemon & Harder, 1998) were decreased in both ARC group and those with primary BSEP defects. Of note, patients within the ARC-IC group had a much higher concentration of secondary bile acids than those in ARC-TPY group. ARC, arthrogryposis, renal dysfunction, and cholestasis; BSEP, bile salt export pump; UPLC-ESI/MRM-MS, ultrahigh-performance liquid chromatography/multiple-reaction monitoring-mass spectrometry
4 DISCUSSION
4.1 Existence of incomplete phenotype of ARC syndrome with isolated low GGT cholestasis
In this study, we identified three VPS33B mutated patients (P1–P3) presenting isolated cholestasis. Different from previously reported ARC patients and P4–P7 with the typical, complete phenotype (Gissen et al., 2006), P1–P3 manifested only low-GGT cholestasis after birth, without other two major characteristics of arthrogryposis or kidney dysfunction. Besides, renal tubular function test of urine amino-acids profiling (Matsumoto Institute of Life Sciences, Beijing, China) revealed only one slight elevated amino-acid in P2 and P3 with isolated cholestasis, whilst multiple elevated amino-acids in P5 and P7 with typical ARC phenotype (10-fold over the normal controls, Table 1). Moreover, P1-P3 live much longer than that with typical ARC phenotype (Gissen et al., 2006; Smith et al., 2012), and have less accompanied symptoms such as platelet dysfunction or recurrent infection, indicating an attenuated phenotype.
4.2 VPS33B variation c.1726T>C, p.Cys576Arg is associated with the attenuated ARC phenotype of isolated cholestasis
As described, a missense VPS33B variation c.1726T>C, p.Cys576Arg was shared in all patients with isolated cholestasis without other explainable mutations: P1 was homozygous and P2 and P3 were compound heterozygous. This mutation was never reported before in public/in-house databases or patients with complete ARC phenotype proved pathogenic by online predicted software and in-vitro study. Like genotype-phenotype correlation between VPS33B mutation c.1225+5G>C and attenuated ARC phenotype, a strong association was suggested between VPS33B c.1726T>C, p.Cys576Arg and isolated cholestasis.
The previous study suggested that mutations in patients with severe, complete ARC phenotype caused absent protein expression or complete abolished interaction with VIPAS39, whereas mutations in patients with attenuated phenotype would be less severe with partially preserved protein expression and function (Cullinane et al., 2009b; Smith et al., 2012). The observation in our patients with complete ARC phenotype was consistent with the inference, all carrying biallelic recurrent mutations that predicted with absent protein expression. But strange for the shared mutation c.1726T>C, p.Cys576Arg in isolated cholestatic phenotype; it did result in partial protein expression but with completely abolished interaction with VIPAS39, which means loss of VPS33B function. Thus, further investigations were required.
4.3 Bile acid profile suggested secondary BSEP defect and more residual function of VPS33Bp.Cys576Arg mutant
Physically, VPS33B colocalizes and interacts with VIPAR to form the VPS33B/VIPAR complex, interacting with endosome marker RAB11A and playing a vital role in the intracellular trafficking and the hepatocyte polarity maintenance (Golachowska, Hoekstra, & van IJzendoorn, 2010; Wakabayashi, Dutt, Lippincott-Schwartz, & Arias, 2005; C. Wang et al., 2018). For VPS33B/VIPAR complex defect, aberrant location of canalicular membrane proteins of BSEP, MDR3, and CEA were observed in VPS33B/VIPAR deficient patients and Vps33b knockout mice (Ackermann et al., 2014; Bull et al., 2006; Hanley et al., 2017). In our study, bile acid profiling in six patients with ARC syndrome showed decreased total secondary bile acids, resemble to those with primary BSEP defects and secondary BSEP dysfunction due to MYO5B defect and consist with former studies. Another notable phenomenon is that patients with isolated cholestasis and longer lifetime had much higher secondary serum bile acids than those with typical ARC phenotype, reflecting more residue function of VPS33Bp.Cys576Arg and require further investigations.
4.4 Follow-up of kidneys and other possible involved organs were required for VPS33B mutated patients with isolated cholestasis
So far, we have not observed typical kidney and joint involvement in P1–P3 with isolate cholestasis. However, the slightly elevated urine microglobulin in P2 and P3 and progressive dextroscoliosis in the oldest patient P3 reminded us of the possibility that joint and renal damage might progress silently. Progressive kidney problems were indeed described in elder patients with ARC syndromes (Rosales et al., 2018), and VPS33B/VIPAS39 complex was reported essential for collagen homeostasis that is required for normal joint structure (Gruber et al., 2017; Rogerson & Gissen, 2018). With the extended life expectancy in patients with attenuated ARC phenotype of isolated cholestasis, follow-ups for kidney and other possible involved organs were indispensable.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China through grant numbers 81361128006, 81570468, and 81741056.




