Targeted sequencing with expanded gene profile enables high diagnostic yield in non-5q-spinal muscular atrophies
Communicated by Johan T. den Dunnen
Abstract
Spinal muscular atrophies (SMAs) are a heterogeneous group of disorders characterized by muscular atrophy, weakness, and hypotonia due to suspected lower motor neuron degeneration (LMND). In a large cohort of 3,465 individuals suspected with SMA submitted for SMN1 testing to our routine diagnostic laboratory, 48.8% carried a homozygous SMN1 deletion, 2.8% a subtle mutation, and an SMN1 deletion, whereas 48.4% remained undiagnosed. Recently, several other genes implicated in SMA/LMND have been reported. Despite several efforts to establish a diagnostic algorithm for non-5q-SMA (SMA without deletion or point mutations in SMN1 [5q13.2]), data from large-scale studies are not available. We tested the clinical utility of targeted sequencing in non-5q-SMA by developing two different gene panels. We first analyzed 30 individuals with a small panel including 62 genes associated with LMND using IonTorrent-AmpliSeq target enrichment. Then, additional 65 individuals were tested with a broader panel encompassing up to 479 genes implicated in neuromuscular diseases (NMDs) with Agilent-SureSelect target enrichment. The NMD panel provided a higher diagnostic yield (33%) than the restricted LMND panel (13%). Nondiagnosed cases were further subjected to exome or genome sequencing. Our experience supports the use of gene panels covering a broad disease spectrum for diseases that are highly heterogeneous and clinically difficult to differentiate.
1 INTRODUCTION
Spinal muscular atrophies (SMAs) without deletions or point mutations in SMN1 (5q13.2) (non-5q-SMA) are a clinically and genetically heterogeneous group of disorders characterized by muscular atrophy, weakness, and hypotonia due to suspected lower motor neuron degeneration (LMND) (Peeters, Chamova, & Jordanova, 2014). Depending on the age of onset, muscle involvement, and inheritance pattern, non-5q-SMAs are divided into SMA with proximal predominant muscle weakness, and SMA with distal predominant muscle weakness or distal hereditary motor neuropathies (dHMN) (Wee, Kong, & Sumner, 2010). In both disease groups, SMA and HMN, autosomal recessive (AR), autosomal dominant (AD), and X-linked inheritance have been described (Wee, Kong, & Sumner, 2010). However, while the majority of SMA patients show an AR mode of inheritance, individuals with HMN are predominantly presenting an AD mode of inheritance (Farrar & Kiernan, 2015). In the era of novel therapeutic options for 5q-SMA patients (Finkel, et al., 2017; Mendell et al., 2017), a correct genetic diagnosis is important. Around 94% of individuals with a strong clinical diagnosis of SMA show biallelic mutations causing functional loss of SMN1 (Lefebvre et al., 1995; Wirth, 2000). These individuals with 5q-SMA carry mainly deletions of SMN1, including exons 7 and 8, or only exon 7, or gene conversion of SMN1 into SMN2, which are identified by MLPA, as the gold standard method for gene testing (Mercuri et al., 2018; Wirth, 2000). Rarely other subtle mutations in SMN1 are found in combination with an SMN1 deletion that can be identified by various methods including specific long-range PCR of genomic SMN1 followed by resequencing of the SMN1 coding region or by reverse transcription PCR (RT-PCR) of cloned SMN1 cDNA transcripts (Kubo, Nishio, & Saito, 2015; Wirth et al., 1999). According to newly established international guidelines for SMA, non-5q-SMA individuals, who usually carry two SMN1 copies should be subjected to gene panel testing (Mercuri et al., 2018). However, there is no study yet that shows which genes should be included and how powerful such a strategy is. Since the implementation of high-throughput screening (HTS) methods in clinical practice, the identification of other SMA-causing genes has exponentially increased (Gene Table of Neuromuscular Disorders, including 492 different genes and 71 mapped loci awaiting gene identification, https://musclegenetable.fr, latest accession on April 2018). While the success rate of genetic testing in individuals who have been thoroughly phenotyped by neuromuscular specialists as proximal SMA is very high (94%) (Wirth et al., 1999), in routine diagnosis of individuals with suspected SMA, SMN1 deletions or mutations are found in only about 50% of cases (see our results). These affected individuals may be affected by one of several overlapping neuromuscular disorders such as HMN, Charcot-Marie-Tooth disease type 2 (CMT2), amyotrophic lateral sclerosis (ALS), distal myopathies, or even hereditary spastic paraplegia (HSP) (Rossor, Kalmar, Greensmith, & Reilly, 2012). Furthermore, very early stages or milder/late-onset motor neuropathies may lead to misclassification with other types of neuromuscular disorders. For example, differentiation of dHMNs from distal myopathies can be difficult due to similar muscular involvement and disease course (Komlosi et al., 2014). In some families, an HMN can be the main clinical feature among several clinical manifestations, such as ataxia, spasticity, growth retardation, and dysmorphic features (Bansagi et al., 2017). The overlap between neuropathies and myopathies is also shown by myopathic individuals carrying nonsense mutations in NEFL gene, formerly linked to neurogenic disorders (Agrawal et al., 2014). Mutations in BICD2, traditionally resulting in a congenital SMA (Neveling et al., 2013; Oates, et al., 2013; Peeters et al., 2013), were recently linked to myopathic features or arthrogryposis multiplex congenita (AMC) (Ravenscroft et al., 2016; Storbeck et al., 2017; Unger et al., 2016). Moreover, myasthenic syndromes due to SYT2 (Herrmann et al., 2014), SLC5A7 (Barwick et al., 2012; Bauche et al., 2016), and VAMP1 (Salpietro et al., 2017) mutations may also resemble HMNs, and should be included in the differential diagnosis on an individual basis.
In terms of cost and time effectiveness, and several quality control parameters, targeted sequencing has been accepted by several large-scale studies as the method-of-choice in the neuromuscular disease (NMD) diagnostics (Ankala et al., 2015; Biancalana & Laporte, 2015; Gorokhova et al., 2015; Savarese et al., 2014; Vasli et al., 2012). However, defining the optimal size of the gene panel remains the biggest challenge in targeted sequencing (Volk & Kubisch, 2017). In this study, we provide a first comprehensive genetic testing of 69 individuals with non-5q-SMA in two different gene panels, a relatively small LMND panel with 62 genes and a large NMD panel with 479 genes. Moreover, in order to test the efficacy of the NMD panel for another disease group that shows a similar clinical and genetic heterogeneity as non-5q-SMA, we included 26 additional individuals with axonal CMT. Axonal CMT occurs in all age groups, has overlapping features with non-5q-SMA like muscle weakness/atrophy, hypotonia, and musculoskeletal abnormalities, and is caused by mutations in many different genes (Rossor, Evans, & Reilly, 2015). Furthermore, undiagnosed cases were subjected to whole exome or whole genome sequencing (WES and WGS). We compare the diagnostic performance of two panels, provide a full overview of genes included, and suggest the most meaningful strategy to test non-5q-SMA patients.
2 MATERIAL AND METHODS
2.1 Patient selection
Sixty-nine individuals with a suspected diagnosis of non-5q-SMA and 26 patients with axonal CMT, for whom extensive clinical data had been collected and 5q-SMA had been excluded, were included in the NeurOmics-LMND study (https://rd-neuromics.eu/disease/spinal-muscular-atrophy-lower-motor-neuron-disease/). We phenotypically characterized the patients based on a questionnaire developed by NeurOmics disease coordinators. This information includes the age of onset, inheritance pattern, parental consanguinity, family history, muscle weakness pattern, musculoskeletal anomalies, cognition and respiration status, creatine kinase levels, electromyography, and nerve conduction studies (NCSs). All phenotype data were stored in PhenoTips (https://phenotips.org) in the NeurOmics database (Lochmuller et al., 2018), a platform which is based on Human Phenotype Ontology (Kohler et al., 2017), and available to each researcher upon request and approval. The study protocol was approved by the institutional review board and ethics committee of the University of Cologne. Written informed consent for targeted HTS, for public access to HTS data and for publication purposes was obtained from all participants during recruitment using standardized form sheets. Only individuals who provided full consent were included in this study. The ethnicities of the probands were from Western Europe (N = 43), Turkey (N = 25), Middle East, Arabic Peninsula and North Africa (N = 11), Iran (N = 10), Russia (N = 3), and Balkans (N = 3).
2.2 LMND panel—design, enrichment, and sequencing
For the small LMND gene panel, we selected 62 genes implicated in SMA, LMND, CMT, and ALS by using public databases ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/), OMIM (https://www.omim.org/), and Muscle Gene Table (https://www.musclegenetable.fr/) (Supp. Table S1). Target regions (coding sequence ± 50 base-pair flanking region) were enriched using the multiplex PCR-based Ion AmpliSeq 2.0 enrichment system (Thermo Fisher, Waltham, MA). Semiconductor-sequencing was carried out on an Ion Torrent Personal Genome Machine (PGM) using single-end sequencing and a 200 bp sequencing chemistry (Ion PGM Hi-Q Sequencing Kit; Life Technologies, Carlsbad, CA, USA). Life Technologies Torrent Browser with latest available software was used to facilitate the analysis workflow from raw data analysis to variant calling and simple variant annotation. A full description with technical details can be found in the online supplementary information.
2.3 NMD panel—design, enrichment, and sequencing
In order to cover the genes implicated in overlapping phenotypes with motor neuron disorders (i.e., HSP, hereditary ataxias, myopathies, muscular dystrophies, myasthenic syndromes, muscle channelopathies, and fetal akinesia deformation sequence), we designed a large NMD gene panel and selected 443 genes using public databases, such as Muscle Gene Table, OMIM, Washington University NMD Database (https://neuromuscular.wustl.edu/) and PubMed (https://www.ncbi.nlm.nih.gov/pubmed). We excluded the disease genes described in pure ataxia phenotypes and the mitochondrial myopathies with severe encephalopathy, hepatorenal disease, lactic acidosis, and cardiomyopathy. Newly identified genes were added in each update process, resulting in 446 genes in version 2 (NMD.v2), 463 genes in NMD.v3, 474 genes in NMD.v4, and 479 genes in NMD.v5 (Supp. Table S1). The panels included between 7,462 and 8,234 exons and covered between 1,156 and 1,246 kb (data corresponding to the first and last version). RefSeq identification numbers of each gene with pathogenic/likely pathogenic variants or variants of uncertain significance (VUS) are provided in the footnote of the corresponding table (Table 1 and Supp. Table S3). The reported variants in this article are submitted to Clinvar, and can be reached with Clinvar submission ID: SUB4031888.
| Pat. | Suspected diagnosis | Final diagnosis (OMIM entry) | Age of onset (years) | Ethnc. | Method | Mutated gene (OMIM entry) | Zyg. | Novelty | cDNAa | Protein | CADD score | ACMG2015 class (evidence of pathogenicity) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LMND gene panel | ||||||||||||
| 1 | SMA with AMC |
|
Birth | GER | LMND |
|
het | Known | c.[806G>A];[806=] | p.(Arg269His) | 31 |
|
| 2 | dSMA |
|
3 | RUM/HUN | LMND |
|
CHZ | Known; known | c.[715C>T];[769C>T] | p.(Leu239Phe)(;) (Arg257Ter) |
|
|
| 3 | dSMA |
|
43 | GER | LMND |
|
het | Known | c.[572G>A];[572=] | p.(Arg191Gln) | 35 |
|
| 4 | dHMN |
SMAX3 (300489) |
30 | ITA | LMND |
|
hemi | Known | c.[2972C>A];[0] | p.(Ala991Asp) | 34 |
|
| NMD gene panel (v1–v5) | ||||||||||||
| (A) Non-5q-SMA and HMN | ||||||||||||
| 5 | SMARD1 |
|
Birth | TUR | NMD.v4 |
|
hmz | Known | c.[1738G>A];[1738G>A] | p.(Val580Ile)(;) (Val580Ile) | 34 |
|
| 6 | SMARD1 |
|
Birth | TUR | NMD.v1 |
|
CHZ | Novel; Novel | c.[-110-1G>A];[c.297G>C] | p.(?)(;) (Trp99Cys) |
13,97 24.1 |
|
| 7 | SMA-infantile onset with cognitive delay |
|
1 | TUR | NMD.v3 |
|
hmz | Known | c.[2185G>A];[2185G>A] | p.(Gly729Arg)(;) (Gly729Arg) | 25,1 |
|
| 8 | SMA-adult onset |
|
35 | GER | NMD.v4 |
|
het | Novel | c.[770G>A];[770=] | p.(Arg257His) | 24 |
|
| 9 | dSMA |
|
2 | TUR | NMD.v5 |
|
het | Known | c.[320C>T];[320=] | p.(Ser107Leu) | 32 |
|
| 10 | dSMA |
|
14 | GER | NMD.v2 |
|
het | Known | c.[1415A>G];[1415=] | p.(His472Arg) | 27.7 |
|
| 11 | dHMN |
|
Childhood | GER | NMD.v3 |
|
het | Novel | c.[328T>C];[328=] | p.(Phe110Leu) | 29.4 |
|
| 12 | dHMN |
|
51 | ITA | NMD.v1 |
|
het | Novel | c.[415A>G];[415=] | p.(Thr139Ala) | 24.2 |
|
| 13 | dHMN+spasticity |
|
13 | ITA | NMD.v1 |
|
CHZ | Known; novel | c.[2254C>T]; [2196_2198del] | p.(Gln752*)(;) (Val733del) |
38 22 |
|
| 14 | HMN+epilepsy |
|
Birth | YEM | NMD.v4 |
|
hmz | Novel | c.[2873T>C];[2873T>C] | p.(Phe958Ser)(;) (Phe958Ser) | 33 |
|
| 15 | HMN+ataxia |
|
8 | TUR | NMD.v3 |
|
het | Known | c.[438C>G];[?] | p.(Asn146Lys) | 31 |
|
| 16 | Anterior horn cell disease |
|
22 | GER | NMD.v1 |
|
het | Known | c.[112G>C];[112=] | p.(Gly38Arg) | 24.3 |
|
| 17 | Anterior horn cell disease |
|
39 | IRA | NMD.v5 |
|
hmz | Known | c.[272A>C];[272A>C] | p.(Asp91Ala)(;) (Asp91Ala) | 0.26 |
|
| 18 | Anterior horn cell disease |
|
36 | HUN | NMD.v2 |
|
het | Known | c.[1552A>G];[1552=] | p.(Arg518Gly) | 26.2 |
|
| 19 | SMARD1/AMC |
|
Birth | TUR | NMD.v1 |
|
hmz | Known | c.[7001T>C];[7001T>C] | p.(Ile2334Thr)(;) (Ile2334Thr) | 24.5 |
|
| 20 | SMA/FADS |
|
Fetal | GER | NMD.v4 |
|
CHZ | Novel; novel | c.[1153C>T];[c.200T>G] | p.(Gly385Ter)(;) (Leu67Arg) |
|
|
| (B) Axonal Charcot-Marie-Tooth neuropathy | ||||||||||||
| 21 | AD-CMT |
|
1 | TUR | NMD.v4 |
|
het | Known | c.[245A>G];[245=] | p.(Tyr82Cys) | 26.4 |
|
| 22 | AD-CMT |
|
5 | YEM | NMD.v4 |
|
het | Known | c.[868G>A];[868=] | p.(Gly290Arg) | 26.2 |
|
| 23 | AR-CMT |
|
1 | IRA | NMD.v4 |
|
hmz | Novel | c.[1055del]; [1055del] | p.(Gly352AlafsTer56)(;) (Gly352AlafsTer56) | 23.4 |
|
| 24 | AR-CMT |
|
1 | TUR | NMD.v2 |
|
hmz | Known | c.[1502+1G>T]; [1502+1G>T] | p.(?)(;)(?) | 25.6 |
|
| 25 | AR-CMT |
|
12 | TUR | NMD.v5 |
|
hmz | Known | c.[110G>C];[110G>C] | p.(Arg37Pro)(;) (Arg37Pro) | 23.3 |
|
| 26 | AR-CMT |
|
6 | TUR | NMD.v3 |
|
hmz | Known | c.[638A>G];[638A>G] | p.(His213Arg)(;) (His213Arg) | 23.6 |
|
| 27 | AR-CMT |
|
3 | TUR | NMD.v4 |
|
hmz | Novel | c.[1523C>T];[1523C>T] | p.(Ser508Leu)(;) (Ser508Leu) | 31 |
|
| 28 | AR-CMT |
|
4 | TUR | NMD.v1 |
|
hmz | Novel | c.[404G>A];[404G>A] | p.(Arg135Gln)(;) (Arg135Gln) | 23.6 |
|
| 29 | AR-CMT |
|
7 | TUR | NMD.v4 |
|
hmz | Known | c.[1085C>T];[1085C>T] | p.(Thr362Met)(;) (Thr362Met) | 29.7 |
|
| 30 | AR-CMT |
|
3 | IRA | NMD.v5 |
|
hmz | Novel | c.[154G>A];[154G>A] | p.(Glu52Lys)(;) (Glu52Lys) | 33 |
|
| 31 | AR-CMT |
|
1 | IRA | NMD.v5 |
|
hmz | Novel | c.[1164G>A];[1164G>A] | p.(Trp388Ter)(;) (Trp388Ter) | 47 |
|
| 32 | AR-CMT |
|
2 | IRA | NMD.v4 |
|
hmz | Novel | c.[3703G>T];[3703G>T] | p.(Glu1235Ter)(;) (Glu1235Ter) | 36 |
|
| 33 | AR-CMT |
|
1 | TUR | NMD.v2 |
|
hmz | Novel | c.[2903_2906del]; [2903_2906del] | p.(Asp968ValfsTer13)(;) (Asp968ValfsTer13) | 35 |
|
| 34 | AR-CMT |
|
7 | TUR | NMD.v3 |
|
hmz | Novel | c.[20970del]; [20970del] | p.(Asp6991IlefsTer9)(;) (Asp6991IlefsTer9) | 35 |
|
| 35 | CMT-ataxia |
|
1 | KAS | NMD.v5 |
|
het | Known | c.[310C>T];[310=] | p.(Arg104Trp) | 34 |
|
| 36 | CMT-ataxia |
|
15 | IRA | NMD.v5 |
|
hmz | Known | c.[5308_5311del];[5308_5311del] | p.(Glu1770IlefsTer15)(;) (Glu1770IlefsTer15) | 35 |
|
| 37 | AR-CMT |
|
1 | TUR | NMD.v1 |
|
hmz | Known | c.(695_1077)del; (695_1077)del | p.(Glu232GlyfsTer32)(;) (Glu232GlyfsTer32) | N/A |
|
| WES/WGS | ||||||||||||
| 38 | SMA-childhood onset |
|
Childhood | GER | WGS |
|
het | Known | c.[493G>C];[493=] | p.(Val165Leu) | 29.9 |
|
| 39 | Facioscapular SMA |
|
2 | TUR | WES |
|
hmz | Novel | c.[265del];[265del] | p.(Arg89GlyfsTer38)(;) (Arg89GlyfsTer38) | 32 |
|
| 40 | Distal arthrogryposis |
|
Birth | LIB | WES |
|
hmz | Novel | c.[1550_1552delinsCGAA];[1550_1552delinsCGAA] | p.(Ser517ThrfsTer48)(;) (Ser517ThrfsTer48) | 34 |
|
| 41 | HMN | novel | 8 | TUR | WES | Novel candidate gene | N/A | Novel | N/A | N/A | ||
| 42 | SMA-infantile Onset |
|
Birth | ISR | WGS |
|
hmz | Novel | c.[146G>C];[146G>C] | p.(Arg49Pro)(;) (Arg49Pro) | 27.1 |
|
| 43 | SMA-scapulohumeral |
|
11 | SYR | WGS |
|
hmz | Novel | c.[1645A>C];[1645A>C] | p.(Met549Leu)(;) (Met549Leu) | 23.3 |
|
| 44 | HMN | novel | 10 | TUR | WGS | Novel candidate gene | N/A | Novel | N/A (N/A) | N/A | ||
| 45 | dHMN | dHMN | 56 | GER | WGS | Novel candidate gene | N/A | Novel | N/A (N/A) | N/A | ||
- AMC, arthrogryposis multiplex congenita; AD, autosomal dominant; AR, autosomal recessive; CHZ, compound heteozygote; CMT, Charcot-Marie-Tooth neuropathy; dSMA, distal spinal muscular atrophy; dHMN, distal hereditary motor neuropathy; FADS, Fetal akinesia deformation sequence; hemi, hemizygote; het, heterozygote; hmz, homozygote; LMND, lower motor neuron disease; SMARD, spinal muscular atrophy with respiratory distress; CADD, combined annotation dependent depletion.
- ACMG: American College of Medical Geneticists. AARS: NM_001605.2, ACTA1: NM_001100.3, ATP7A: NM_000052.6, BICD2: NM_001003800.1, COL6A1: NM_001848.2, COL6A2: NM_001849.3, COL12A1: NM_004370.5, DHTKD1: NM_018706.6, FUS: NM_001170937.1, FXN: NM_000144.4, GAN: NM_022041.3, GARS: NM_002047.3, GDAP1: NM_018972.2, HINT1: NM_005340.6, HSPB1: NM_001540.4, IGHMBP2: NM_002180.2, LGI4: NM_139284.2, MFN2: NM_001127660.1, MORC2: NM_014941.3, MPZ: NM_000530.7, MTMR2: NM_016156.5, PIEZO2: NM_022068.3, PLEKHG5: NM_001265593.1, PRX: NM_181882.2, SACS: NM_014363.5, SETX: NM_015046.6, SLC52A2: NM_024531.4, SOD1: NM_000454.4, SYNE1: NM_182961.3, TRPV4: NM_021625.4, VAMP1: NM_014231.4, VCP: NM_007126.4, ZFYVE26: NM_015346.3.
- a GRCh37/hg19 genomic reference sequence was used.
- b Condition ID was given according to Orphanet due to no available entry in OMIM.
DNA samples of 200 ng each were prepared individually at Cologne Center for Genomics (CCG). Target regions consisting of the coding exons and 25 bp flanking regions were enriched using the Agilent's solution-based post-capture SureSelectXT automated system for Illumina paired-end multiplex sequencing, and the Agilent Bravo automated liquid handling platform. Targeted sequencing using an Agilent's solution-based post-capture SureSelectXT enrichment system on an Illumina HiSeq 4000 machine was carried out. We used the CCG's VARBANK online pipeline (v.2.26) (https://varbank.ccg.uni-koeln.de/) and the filter interface for data analysis. A full description with technical details and references can be found in the online Supplementary Information.
2.4 Quality management and data quality measurements of both panels
In order to measure the analytical sensitivity and specificity of variant calling, we sequenced the benchmark control DNA NA12878 (Zook et al., 2014) and one patient sample with a known disease mutation. NA12878 DNA contains 141 variants within the target region of the LMND panel. LMND panel sequencing generated 139 of 141 variants (98.6%, true positive) by using routine analysis parameters. Additional 75 (35%) were false positive of which 18 were indels and 57 were SNV. NA12878 DNA contains 1,646 variants within the target region of the NMD panel, by which 1,618 (98.2%) of the total variants were successfully called. Additional 249 variants (13.3%) were false positive, consisting of 66 indels and 183 SNVs. In order to monitor and detect sample mix-up, 10 regions with polymorphic SNPs were sequenced for each sample. To ensure uniformity and accuracy, we set a threshold of more than 20× coverage for more than 97.5% of the target region and mean depth of coverage of more than 100×. As a second validation, each potentially pathogenic variant was sequenced by the Sanger method (Sanger F, 1977) before further testing or reporting.
2.5 Optimization methods for NMD panel
In the NMD panel, we sequenced the samples in 16-reaction batches. This allowed us to monitor and optimize the regions with low coverage, as well as to update the gene panel with new genes. Regions with less than 20× average read depth and less than 100% uniformity were regarded as poorly covered regions. We used Agilent's eArray online tool for optimization of poorly covered regions. The full description of optimization strategy can be found in the online Supplementary Material.
To ensure the clinical utility of the NMD panel, we checked the sensitivity of detection of known disease-causing mutations in these genes. By using the HGMD® Professional database Mutation Mart advanced tool, up to 28,000 known disease-causing mutations, frameshift or truncating variants, functional polymorphisms, and disease-associated polymorphisms annotated by HGMD were screened in each sequence output (Stenson et al., 2017). Missed mutations were then included in the boosting process if located in the coding region.
2.6 WES and WGS
The individuals without any candidate variant from both panels were subjected to WES or WGS if the parental material and informed consent were available for trio WES/WGS. In total, WES was performed for six probands and WGS was performed for 18 probands. DNA samples for WES were prepared using the NEXTERA Rapid Capture Exome Kit (Illumina, San Diego, CA), followed by 150-nucleotide paired-end sequencing on the Illumina NextSeq500. Alignment and variation calling was carried out as described previously (Delle Vedove et al., 2016). For WGS, samples were prepared with Illumina's TruSeq PCR-Free sample preparation kit (Illumina). Details of the WGS method are described in the Supplementary Information.
2.7 Variant interpretation and classification
For an unbiased variant interpretation, we utilized meta-scores that are calculated by integration of different prediction algorithms. We used Saphetor's online tool, Varsome (https://varsome.com/, latest accession in April 2018) and combined annotation dependent depletion (CADD v1.3) scores (Kircher et al., 2014) (https://cadd.gs.washington.edu/, latest accession in April 2018) to assess the deleteriousness of the variants. The PHRED-like (−10 × log(rank/total)) scaled CADD-scores more than 15 were taken as cut-off value for deleterious variants. Splice site change analysis was carried out by using Human Splice Finder 3.0 (https://www.umd.be/HSF3/) (Desmet et al., 2009), in which consensus values more than 65 (±10%) were taken as threshold for donor/acceptor splice site changes. For variant classification, we used ACMG 2015 guidelines (Richards et al., 2015), and annotated the variants as pathogenic, likely pathogenic or uncertain significance.
2.8 Copy number variation analysis from NMD panel data
We used publicly available copy number variation (CNV) analysis tools XHMM (Fromer & Purcell, 2014), CoNIFER (Krumm et al., 2012), and ExomeDepth (Plagnol et al., 2012) in order to uncover possible structural variants in the unsolved individuals and in the individuals with a pathogenic monoallelic SNV in a recessive inheritance.
3 RESULTS
3.1 Identification of non-5q-SMA affected individuals and selection of appropriate cohort
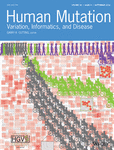
Since the identification of SMN1 in 1995 (Lefebvre et al., 1995), we performed genetic testing of SMN1 deletion/point mutation as a routine diagnostic laboratory procedure in 3,465 individuals with suspected SMA. In 1,692 individuals (48.8%) homozygous deletions in SMN1 exon 7 and 8 or only 7 were identified. In 97 individuals (2.8%), subtle mutations in combination with one deletion in SMN1 were found. Among the 5q-SMA individuals (N = 1,789), 94.6% carried a homozygous deletion in SMN1 and 5.4% a subtle mutation in combination with an SMN1 deletion. The remaining 1,676 individuals (48.4%) failed to show a deletion/mutation in SMN1 (Figure 1A).
In the NeurOmics study, extensive clinical data were collected from 69 non-5q-SMA individuals and 26 axonal CMT individuals with suspected features of non-5q-SMA and data stored in PhenoTips. Informed consent for participation in the NeurOmics study including consent for making clinical and sequencing data publicly available was a prerequisite.
Of the 95 cases, 50 had an inheritance pattern suggestive of autosomal-recessive, 13 of autosomal-dominant, one of X-linked inheritance, and 21 were sporadic cases. The inheritance pattern in ten families was ambiguous. Parental consanguinity was present in 36% of families. In 40 non-5q-SMA individuals (58%) and 24 axonal CMT individuals (92%), disease onset was in pediatric or adolescent age (<18 years).
3.2 Gene panel development and targeted sequencing of 69 non-5q-SMA individuals
We tested a total of 69 unrelated non-5q-SMA probands using targeted sequencing. Two different gene panels (small LMND and large NMD) were developed and analyzed. In addition, 24 cases unsolved by both panels were analyzed by WES/WGS (Figure 1B). Advantages and disadvantages will be presented and discussed. The clinical and genetic data for these individuals are referred to by their respective number identifiers in the text, Table 1 and Suppl. Table S2.
3.2.1 LMND gene panel
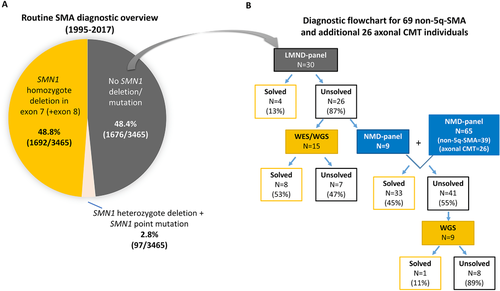
We first developed a small LMND gene panel including 62 known LMND genes causing SMA, HMN, axonal CMT, and ALS (Supp. Table S1). A total of 985 exons covering 384.57 kb of genomic DNA were included. Targeted sequencing using a multiplex PCR-based Ion AmpliSeq 2.0 enrichment system on an Ion Torrent PGM was carried out (see Material and Methods). The data quality of the total target region was adequate with a mean coverage of 450× and 98.5% of the target regions covered at 20× (Figure 2A).
Thirty individuals with non-5q-SMA were analyzed on the small LMND panel of which four (13%) were diagnosed with known pathogenic mutations in GDAP1, ATP7A, TRPV4, and VCP (Table 1 and Supp. Table S2). Based on the rather low outcome, we decided to send 15 cases for WES or WGS. In parallel, we developed the larger NMD panel and included nine unsolved cases, where no consent for WES could be obtained or no trios were available.
3.2.2 WES/WGS of unsolved cases upon LMND gene panel analysis
Fifteen of the unsolved cases were sent to WES or WGS at DeCODE (Reykjavik, Iceland). WES/WGS revealed the disease causing variant in eight (53%) cases, among which only PLEKHG5 has been previously defined as LMND gene (Figure 1B). The PLEKHG5 variant had been annotated as insignificant variant due to insufficient proof in the literature at the time of the dedicated LMND analysis. Moreover, one known dominant missense mutation in ACTA1 was identified in a patient with suspected SMA type III with respiratory insufficiency (#38). Among the unsolved families, we identified two novel genes, PIEZO2 in a case with distal arthrogryposis with impaired proprioception and touch (DAIPT, OMIM 617146) (Delle Vedove et al., 2016) (#40), and VAMP1 in a case (#42) with severe congenital muscle atrophy and respiratory insufficiency, later termed as CMS (Salpietro et al., 2017). Three further novel candidate genes are under functional investigation (data not shown).
The above-described phenotypes that overlap with non-5q-SMA in different disease stages, together with diagnostic entities due to the mutations in several NMD genes demonstrated the necessity of a more comprehensive approach to panel diagnosis. For this reason, we changed our approach applying a broader NMD panel, and discontinued the LMND panel.
3.2.3 NMD gene panel
Nine unsolved non-5q-SMA cases from the LMND panel cohort and additional 65 (39 non-5q-SMA and 26 axonal CMT) individuals were tested on a large NMD panel.
The NMD panel showed a very good coverage performance: on average, 99.3% of the target regions (about 1.2 Mb) were covered at least 20×, and 94% at least 100×. Although the unique mapped reads differ at each version, the lowest mean coverage reached 320× (Figure 2A). The coverage of poorly covered regions was improved after each panel update, with the exception of highly GC-rich regions, or highly repetitive regions.
The analysis of 48 non-5q-SMA individuals yielded a definite diagnosis in 16 individuals (33%) (Figure 2B). According to ACMG2015 criteria (Richards et al., 2015), the identified mutations included 12 pathogenic and seven likely pathogenic variants, including both CHZ variants (Table 1). In 18 (38%) non-5q-SMA cases, candidate variants did not fulfill the criteria of pathogenicity, and therefore had to be annotated as VUS (Supp. Tables S3 and S4). Among the 16 disease causing mutations identified by using the NMD panel, we found only six mutations in five genes that were implicated in non-5q-SMA (IGHMBP2, GARS, AARS, HSPB1, BICD2). The remaining cases with a suspected non-5q-SMA were diagnosed with mutations in two genes involved in ALS (SOD1, FUS), and in genes involved in CMT (MORC2), ataxia (FXN), HSP (ZFYVE26), AMC (LGI4), Brown-Vialetto-Van-Laere Syndrome type 2 (BVVLS2) (SLC52A2), and 2-aminoadipic 2-oxoadipic aciduria (AMOXAD) (DHTKD1), respectively. In addition, we found the causative mutation in one muscle/extracellular matrix-related gene (COL12A1) in a patient with a suspected SMA with respiratory distress (SMARD1) and congenital arthrogryposis (#19) (Table 1 and Supp. Table S2).
In order to verify the diagnostic power of the NMD panel beyond the SMA phenotype, we included 26 individuals with axonal CMT for a SMA-related disease group (Supp. Table S2). In 17 out of 26 (65%) individuals, pathogenic mutation(s) were found in COL6A1, COL6A2, GAN, GDAP1, HINT1, IGHMBP2 (2×), MFN (4×), MPZ, MTMR2, PRX, SACS, SYNE1, and SETX (Table 1 and Figure 2C), respectively, thus exceeding the diagnostic yield of non-5q-SMA individuals. Of note, the CNV analysis of the NMD panel data through the ExomeDepth programme revealed an additional diagnosis: the homozygous deletion of exon 6 in GDAP1 in a patient with autosomal-recessive CMT (AR-CMT) (#37) (Table 1 and Supp. Figure S1), which underlines the diagnostic value of the CNV assessment from the targeted sequencing data.
3.2.4 WGS of unsolved cases upon NMD gene panel analysis
Among the unsolved NMD panel individuals, nine of them had the consent for WES/WGS as further analysis, therefore they were subjected to the WGS. In contrast to the high diagnostic yield for patients whose diagnoses had not been solved by the LMND panel (53%), WGS provided a genetic diagnose of only one individual among the nine unsolved cases of the NMD panel (11%) (Figure 1B). Interestingly, the single mutation found by WGS was in DHTKD1, which was already included in the NMD panel. However, the variant was missed in the first analysis due to the strict in-house database filter including 511 exomes of individuals with epilepsy, which was solved by disabling the filter. Afterwards, the variant appeared in NMD panel with a sufficient quality.
In summary, both panels and additional WES/WGS analysis provided the genetic diagnosis in 28 of 69 (41%) non-5q-SMA individuals, and 17 of 26 (65%) axonal CMT individuals (Figure 2C). Among the diagnosed cases, 22 novel allelic variants and 26 known allelic mutations were described (Table 1). Moreover, the diagnostic yield in the pediatric and adolescent group (<18 years) of non-5q-SMA patients was with 50% (20 out of 40) far higher than in the adult group with about 27% (eight out of 29) (Figure 2D). In 20 individuals, the selected variants did not have enough evidence to be pathogenic, therefore annotated as VUS (Supp. Tables S3 and S4).
3.3 Mutations in genes that are not directly associated with non-5q-SMA or AR-CMT
Some individuals were diagnosed with known mutations in the genes for which no previous clear association with non-5q-SMA or axonal CMT had been described.
In individual (#7) with an infantile-onset SMA-like phenotype and an onset of a psychomotor delay at 5 years of age, we found a known homozygous mutation in the DHTKD1 gene, in which mutations cause AMOXAD (OMIM 204750) (Table 1 and Supp. Table S2). Infant (#6) with SMARD1 phenotype was included in the study after the exclusion of SMN1 deletion and negative IGHMBP2 mutational screening. We found a compound-heterozygote mutation in SLC52A2 (Table 1 and Supp. Table S2), in which biallelic mutations were described in patients with BVVLS2 (OMIM 614707). Hearing status of the patient was unknown. However, riboflavin supplementation alleviated the symptoms, and the patient was disconnected from mechanical ventilation after the therapy.
Individual (#14) was an 8-year-old boy from a consanguineous family from Yemen, who had severe contractures since birth, motor neuropathy, cognitive delay, seizure history, and progressive secondary microcephaly. We identified a likely pathogenic homozygous missense variant in AARS (Table 1 and Supp. Table S2). Previously, missense variants in AARS causing a significant reduction in protein function were shown to be causative for AR epileptic encephalopathy, early infantile, 29 (EIEE29) (OMIM 616339) (Simons et al., 2015), which is partially recapitulated by the phenotype of our patient. The functional effect of the homozygous mutation in our patient remains elusive. Individual (#15) with suspected HMN had previously been tested for GAA repeats in the FXN gene due to severe and rapid progression compatible with Friedreich's Ataxia (Supp. Table S2). Having shown normal repeats in both alleles by Southern blotting, and profound motor neuropathy features, this individual was included in our NMD panel and showed a rare known heterozygous missense mutation in the FXN gene (Table 1) (Zuhlke et al., 2004). The search for a potential second pathogenic allele is still in progress.
Among the novel variants, we found two loss-of-function (LoF) mutations in two genes, which are classified as ataxia-related genes. In a 17-year-old male (#33) with axonal CMT and gait problems, we identified a truncating variant in the SACS gene, which usually causes spastic ataxia Charlevoix-Saguenay type (OMIM 270550). Patients with atypical features, such as later onset or initial presentation of peripheral neuropathy have been described (Baets et al., 2010). A later follow-up showed that the gait pattern had changed to an ataxic gait and detailed cerebellar tests unveiled the cerebellar phenotype (Table 1 and Supp. Table S2). Furthermore, a homozygous truncating variant in SYNE1 was found in a 17-year-old female (#34) with axonal CMT and intellectual disability (Table 1 and Supp. Table S2). Recently, ataxia has been described as a main phenotypical manifestation of recessive LoF SYNE1 mutations. However, ataxia may develop at later stages, whereas motor neuron signs are the initial features of this disease (Synofzik et al., 2016).
Interestingly, we identified mutations in collagen-6 (COL6A1, COL6A2) and collagen-12 (COL12A1) genes in three individuals with suspected non-5q-SMA or axonal CMT. In a floppy infant with congenital contractures, global muscular weakness and respiratory distress (#19) (Table 1 and Supp. Table S2), a known mutation in COL12A1 was identified, which has been shown to cause congenital myopathy with similar early symptoms (Zou et al., 2014). In another family, the proband (#22) presented with hand/foot muscle atrophy, pes cavus deformity, paraspinal muscle atrophy, kyphoscoliosis, hyperkeratotic skin changes, obstructive sleep apnea, and axonal involvement on NCSs. Our NMD panel revealed a known dominant mutation in COL6A1, which was also confirmed in the similarly affected son. Our NMD panel also uncovered a Bethlem myopathy through a homozygous LoF splicing variant in COL6A2 in a consanguineous family with an affected individual (#23) and his sister, who had initially been diagnosed with suspected AR-CMT with distal muscle atrophy (Table 1 and Supp. Table S2).
3.4 Confirmation of diagnostic gain with expanding gene profile
In order to compare the diagnostic power of the two gene panels in non-5q-SMA individuals and to eliminate the patient selection bias in two cohorts, a virtual cross-analysis of the two cohorts was carried out (Figure 2B). Compared with only four out of 30 (13%) diagnosed individuals by LMND panel, the virtual analysis of the same individuals on the NMD panel would have solved 11 out of 30 (37%) individuals. In contrast to the 16 out of 48 (33%) of cases solved by the NMD panel, the virtual analysis of the same cohort on the LMND panel would have solved only nine out of 48 (19%) individuals (Figure 2B). This comparison proves that the larger NMD panel provides a higher yield compared with the restricted LMND panel. Moreover, expanding the gene profile about sevenfold did not cause any significant variant burden during the variant analysis. Depending on the inheritance pattern, variant output of each version of NMD panel stayed between 6 and 13 variants on average. This specificity also restrained the number of incidental findings, which is still a recurrent concern for WES/WGS. In terms of consumable items and sequencing investment, the cost to sequence each sample was almost the same between the two panels. This might be due to the substantial reduction of the HTS costs per sample in the last years considering the start of LMND panel was in 2014 and NMD panel was in 2016.
3.5 Known mutation detection rate and optimization of low-covered regions
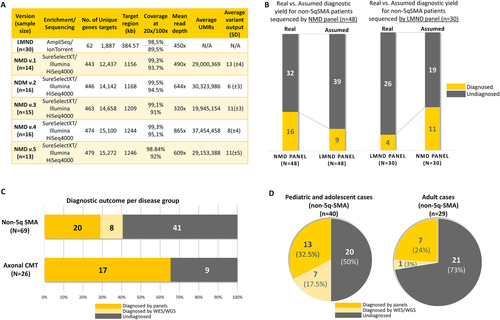
For the NMD panel, we also tested the sensitivity of detecting known disease causing mutations. From the data output of each panel version, we retrieved up to 28,000 HGMD-annotated known disease mutations or disease associated polymorphisms, including deep intronic mutations and structural variations. Screening of known mutation spots with a low coverage (<20×) resulted in an average of 89 low-covered mutation regions (0.3%) among 28,000 reported mutations. However, only 20% of these “possibly missed” mutations were located in our target of interest, the remaining were deep intronic or structural rearrangements (Figure 3A). During the optimization process between panels, we tried to boost the regions of known mutations with suboptimal coverage. Most of those regions were located in the first exons of the genes (e.g., SEPN1, DOK7), where the GC-content is extremely high. Thus the boosting did not yield any coverage gain (Figure 3B). However, the regions outside of the first exon and with lower GC-content (e.g., CHRND) could be enriched sufficiently after the boosting (Figure 3B).
3.6 Comparison of coverage performance between WES and NMD panel
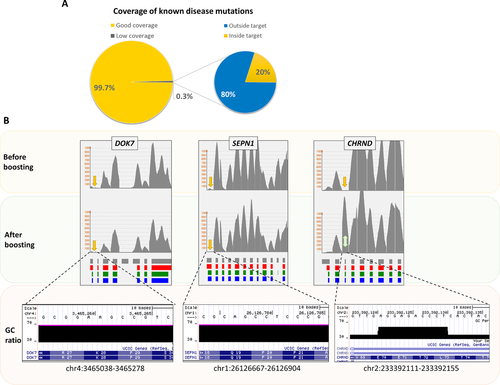
In order to find out if WES has any coverage gaps for the 479 genes that were included in the NMD panel, we investigated the WES coverage data of the coding exons of the 479 genes. The analysis revealed that 85 (0.97%) coding exons had zero coverage, and 202 (2.3%) less than 20× coverage by WES. None of the detected variants described in this article was inside any of the low covered regions by WES. In contrast, the NMD panel showed only 11 coding exons with null coverage, and 22 exons with a coverage of less than 20× (Supp. Table 5).
4 DISCUSSION
Since 1995, we have been performing the SMN1 genetic testing in individuals referred to us with suspected SMA, leading to an overall diagnostic yield of about 50%. In order to identify the genetic cause of the other half of our cohort, we developed two HTS-based gene panels: a relatively small LMND panel including 62 LMND genes and a large NMD panel including 479 NMD genes (including the 62 LMND genes), and analyzed 69 non-5q-SMA individuals. We used two different HTS platforms (multiplex-PCR-based AmpliSeq for the LMND panel and SureSelect Agilent for the NMD panel) and different machines (Ion Torrent PGM for the LMND panel and Illumina HiSeq 4000 for the NMD panel). Due to low diagnostic yield from the LMND panel (four out of 30; 13%), we changed our approach by continuing with a larger NMD panel where a significant higher diagnostic yield (16 out of 48, 33% for non-5q-SMA; 33 out of 74, 45% for non-5q-SMA and axonal CMT) was achieved. Expanding the gene profile with the NMD panel turned out to be far superior at similar sequencing quality. Moreover, the choice of the NMD panel offered several advantages. First, the chance to update the panel allowed the inclusion of novel genes in the next batch. Thus, it was possible to identify pathogenic mutations in the recently identified LGI4 gene (Xue et al., 2017) in a fetal sample (#20), a valuable genetic diagnosis for a future pregnancy in this family. We identified a heterozygous variant in the recently identified MORC2 gene (Sevilla et al., 2016) in a patient with adult-onset non-5q-SMA (#8). Secondly, including other motor neuron-associated genes unveiled pathogenic variants in three ataxia genes (FXN, SACS, and SYNE1) in patients with a prominent or initial motor neuropathy phenotype (#15, #33, #34,) and pathogenic variants in ZFYVE26, an HSP-related gene in an individual with dHMN and only mild spasticity (#13). Thirdly, we identified pathogenic variants not only in motor neuron-associated disease genes, but also the genes encoding muscular/extracellular matrix proteins (COL12A1, COL6A1, and COL6A2,) in three individuals (#19, #22, #23).
In the literature there are several targeted sequencing cohorts for NMDs, focusing on cases with motor neuron disorders; the number of the genes in the panels vary between 35 and 93, and the diagnostic yield between 20% and 35% (Antoniadi et al., 2015; Dohrn et al., 2017; Hoyer et al., 2014; Kruger et al., 2016; Lassuthova, et al., 2016; Lupo, et al., 2016). In an ALS cohort of 80 individuals, first tier analysis with ALS core genes (n = 39) provided a genetic diagnosis in 20 probands (25%). An additional search for variants in other 238 neurodegenerative disease-associated genes identified deleterious variants in 12 more individuals (Kruger et al., 2016). In a CMT cohort of 59 families, 51 genes were included in the panel with a diagnostic yield of 25% (excluding previously diagnosed cases). In this cohort, mutations in “non-CMT” genes were detected, and therefore a more comprehensive approach was suggested (Hoyer et al., 2014). Of note, in another study with a total of 33 HMN and CMT families, the importance of broader panels was underlined in case of families referred from non-specialist centers (Lupo et al., 2016).
WES is used as an alternative approach in HTS-based diagnostics for motor neuron disorders. Thus, in a cohort with inherited peripheral neuropathy, 102 out of 354 (29%) individuals were diagnosed by WES (Hartley et al., 2017). When we resequenced our unsolved cases by WES or WGS, the diagnostic contribution of WES/WGS was much lower for cases unsolved by NMD panel analysis (11%) than by LMND panel (53%), which most likely reflects the larger known disease genes included in the NMD panel (Figures 1B and 2A). Moreover, we regularly updated the NMD panel with newly identified genes. Since the discovery of novel disease genes is constantly decreasing (Boycott et al., 2017), the use of comprehensive NMD panels including all known NMD genes will provide a consistently increasing diagnostic performance in routine diagnostic testing.
The NMD panel with a larger gene profile showed a robust performance in accuracy, analytical precision, analytical sensitivity, and specificity, reportable and reference range of test results, hence assured the validity requirements for an HTS-based diagnostics (Gargis et al., 2012). Furthermore, the expectations from a targeted sequencing (Savarese et al., 2014) were met by the NMD panel, such as shorter turnaround time (about 3 weeks), more robustness in target reproducibility, increase in sensitivity and specificity, to be upgradeable with newly discovered genes, and thus comprehensiveness regarding all relevant genes.
The diagnostic yield of NMD panel in pediatric and adolescent cases (32.5%) was higher than in adult cases (24%). This is compatible with two previous HMN cohorts providing data on the age of onset (Hartley et al., 2017; Schabhuttl et al., 2014), and emphasizes the importance of HTS diagnostics for non-5q-SMA in childhood. An early diagnosis for those cases may improve decision-making for symptomatic interventions, especially for such conditions as SMA with lower extremity predominance (SMALED2) in which no severe progression of weakness is expected (Neveling et al., 2013; Peeters et al., 2014). In order to assess the efficacy of the NMD panel not only in non-5q-SMA, we also included 26 individuals with axonal CMT. Our results show that even a higher diagnostic performance (65%) was obtained in the axonal CMT group.
As the first causal therapeutic option for 5q-SMA patients, Nusinersen, has recently been approved by U.S. Food and Drug Administration and European Medicines Agency (Hoy, 2017), early molecular genetic diagnosis of SMA is of prime importance. A definite genetic diagnosis for patients with non-5q-SMA will help to counsel the affected patients in terms of prognosis and heredity of their illness, even if for most cases there is no specific treatment available at present.
5 CONCLUSIONS
We developed two gene panels for non-5q-SMA with different gene profiles. The larger NMD panel provided us with a higher diagnostic yield, and confirmed the necessity of a comprehensive approach in the diagnosis of NMDs, especially as there may be phenotypic overlap between some forms of anterior horn cell diseases, myopathies, and congenital myasthenic syndromes. Moreover, additional WES/WGS did not provide a significant diagnostic gain for individuals with negative results from the NMD panel. Therefore, we recommend the use of gene panels covering a broad disease spectrum for diseases that are highly heterogeneous and clinically difficult to differentiate.
ACKNOWLEDGMENTS
We would like to thank the family members described here in for participating in this study. We thank all our clinical collaborators for the support at clinical follow-up, diagnostics and the genetic analysis. We furthermore thank the Regional Computing Center of the University of Cologne (RZZK) for providing computing time and storage on the CHEOPS high performance computing cluster.