FAS Gene Copy Numbers are Associated with Susceptibility to Behçet Disease and VKH Syndrome in Han Chinese
Contract grant sponsor(s): Natural Science Foundation Major International (Regional) Joint Research Project (81320108009); National Basic Research Program of China (973 Program) (2011CB510200); Key Project of Natural Science Foundation 81130019); National Natural Science Foundation Project (31370893, 81200678); Basic Research program of Chongqing (cstc2013jcyjC10001); Chongqing Key Laboratory of Ophthalmology (CSTC, 2008CA5003); National Key Clinical Specialties Construction Program of China; Key Project of Health Bureau of Chongqing (2012-1-003) and Fund for PAR-EU Scholars Program.
Communicated by Michael Dean
ABSTRACT
Previous studies have identified that disturbed apoptosis was involved in the pathogenesis of Behçet disease (BD) and Vogt–Koyanagi–Harada (VKH) syndrome. This study aims to investigate whether copy number variations of apoptosis-related genes, including FAS, CASPASE8, CASPASE3, and BCL2, are associated with BD and VKH syndrome in Han Chinese. A two-stage association study was performed in 1,014 BD patients, 1,051 VKH syndrome patients, and 2,076 healthy controls. TaqMan® Copy Number Assays and real-time PCR were performed. The first-stage study showed that increased frequency of high FAS copy number (>2) was found in BD (P = 1.05 × 10−3) and VKH syndrome (P = 2.56 × 10−3). Replication and combined study confirmed the association of high copy number (>2) of FAS with BD (P = 3.35 × 10−8) and VKH syndrome (P = 9.77 × 10−8). A significant upregulated mRNA expression of FAS was observed in anti-CD3/CD28 antibodies-stimulated CD4+ T cells from individuals carrying a high gene copy number (>2) as compared to normal diploid 2 copy number carriers (P = 0.004). Moreover, the mRNA expression of FAS both in active patients with BD and VKH syndrome was significantly higher than that in controls (P = 0.001 and P = 0.007, respectively). Our findings suggest that a high copy number of FAS gene confers risk for BD and VKH syndrome.
Introduction
Uveitis, known as inflammation of the uvea consisting of iris, ciliary body, and choroid, is one of the leading causes of blindness in the world [Suttorp-Schulten and Rothova, 1996]. Behçet disease (BD) and Vogt–Koyanagi–Harada (VKH) syndrome are two commonly seen uveitis entities in China [Yang et al., 2005]. BD is an autoinflammatory disease accompanied with diverse clinical manifestations, such as recurrent uveitis, oral aphthae, genital ulcerations, typical skin lesions, and hypopyon [Yang et al., 2008]. It is more prevalent in Asia and Middle East rather than in the United Kingdom and United States [Khairallah et al., 2012]. VKH syndrome is a rare multisystemic autoimmune disease characterized by bilateral granulomatous panuveitis accompanied with tinnitus, headache, alopecia, poliosis, and vitiligo [Yang et al., 2007a]. There is a higher incidence of VKH syndrome in Asians and Native Americans. Up to now, the exact aetiology and pathogenesis of both diseases remain unclear.
Copy number variations (CNVs), known as a source of genetic diversity being as significant as single nucleotide polymorphisms (SNPs), have been reported to confer susceptibility to several complex disease phenotypes and autoimmune diseases, such as glomerulonephritis, lung cancer, nasopharyngeal carcinoma, rheumatoid arthritis, systemic lupus erythematosus, and psoriasis [Aitman et al., 2006; Yang et al., 2007b; Tse et al., 2011; Yu et al., 2011; Liu et al., 2012; Robinson et al., 2012; Yang et al., 2013; Wu et al., 2014]. CNVs include gene duplication and gene deletion, which may result in phenotypic variation by changing gene expression or biological function [Redon et al., 2006; Stranger et al., 2007; Cantsilieris and White, 2013; Hollox and Hoh, 2014]. Recently, CNVs of complement component C4 were identified to be involved in the pathogenesis of BD and VKH syndrome [Hou et al., 2013, 2014b].
A variety of apoptosis-related genes, including FAS (MIM# 134637), FASL (MIM# 134638), CASPASE8 (MIM# 601763), CASPASE3 (MIM# 600636), and BCL2 (MIM# 151430), have been reported to play a pivotal role in the pathogenesis of various cancers and autoimmune diseases [Wajant, 2002; Sun et al., 2007; Fan et al., 2010; Zhu et al., 2010; Zhang et al., 2011; Yildir et al., 2013; Trevino-Talavera et al., 2014]. Previous studies also showed that disturbed expression of FAS, FASL, and BCL2 was involved in the perpetuation and recurrence of BD and VKH syndrome [Yang et al., 2001; Wakisaka et al., 2002; Baris et al., 2005]. As yet, there is still no report concerning the association between CNVs of apoptosis-related genes and various uveitis entities.
In the current study, we conducted a case–control study to investigate whether CNVs of FAS, CASPASE8, CASPASE3, and BCL2 contributed to susceptibility to BD and VKH syndrome. Our findings suggest that a high copy number of FAS gene is associated with an increased risk of BD and VKH syndrome in Han Chinese.
Materials and Methods
Case–Control Cohorts
One thousand fourteen patients with BD and 1,051 VKH syndrome patients were recruited from the Zhongshan Ophthalmic Center of the Sun Yat-sen University (Guangzhou, China) and the Department of Ophthalmology in the First Affiliated Hospital of Chongqing Medical University (Chongqing, China) between October 2005 and July 2014. Among them, only 136 BD patients and 90 VKH syndrome patients were recruited from Guangzhou city. The diagnoses of BD and VKH syndrome were stringently based on the respective International Workshop criteria [Disease, 1990; Read et al., 2001]. In parallel, a total of 2,076 unselected and consecutive control subjects, 253 of which were recruited from Guangzhou city, were recruited from the same communities, thus representing the same ethnical population and geographic area with the patients. A two-stage case–control study was carried out. In the first stage, 300 BD patients, 300 VKH patients, and 600 normal subjects were enrolled. All the samples recruited from Guangzhou city (136 BD patients, 90 VKH syndrome patients, and 253 healthy controls) were included in the first-stage study. The second stage consisted of another 714 BD patients, 751 VKH patients, and 1,476 healthy controls. The study was approved by the Local Ethics Research Committee (Permit Number: 2009–201008) and followed the tenets of the Declaration of Helsinki. Written informed consent was obtained from all patients and controls before blood collection.
Genomic DNA Extraction
Genomic DNA was extracted from venous blood of BD patients, VKH syndrome patients and healthy controls using the QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA).
CNV Selection and Genotyping
By searching the Database of Genomic Variants (DGV) (http://dgv.tcag.ca/dgv/app/home), we found that there were CNVs located in four apoptosis-related genes (FAS, CASPASE8, CASPASE3, and BCL2), whereas no CNVs were detected in the FASL gene, which was therefore excluded from this study. The gene CNVs were quantified by TaqMan® Copy Number Assays (Applied Biosystems, Foster City, CA) and performed in 96-well plates on the 7500 System (Applied Biosystems) based on the manufacturer's protocols. The probes of FAS (Hs00679718_cn), CASPASE8 (Hs00561070_cn), CASPASE3 (Hs02217623_cn), and BCL2 (Hs02007358_cn) labeled with FAM were selected from the predesigned TaqMan® Copy Number Assays (Applied Biosystems). TaqMan probe of the RNase P gene labeled with VIC was used as an internal reference to represent two copies per diploid genome (Applied Biosystems). Each target assay was performed in same PCR as RNase P and replicated three times according to the manufacturer's instructions (Applied Biosystems).
Cell Isolation and Culture
Peripheral blood mononuclear cells (PBMCs) were isolated from venous blood of active BD patients, active VKH syndrome patients, and healthy controls by Ficoll-Hypaque density gradient centrifugation as described earlier [Yu et al., 2014]. All active patients were not receiving immunosuppressive treatment at the time of sampling. The CD4+ T cells were isolated from PBMCs of healthy controls using Magnetic beads (Miltenyi Biotec, Palo Alto, CA) according to the manufacturer's instructions, and then the purified CD4+ T cells were treated with a combination of anti-CD3 and anti-CD28 antibodies (5:1) (Miltenyi Biotec, Palo Alto, CA) at 37℃ for 72 h, as described previously [Yu et al., 2014].
Real-Time PCR
Total RNA was extracted from PBMCs, CD4+ T cells, and anti-CD3/CD28 antibodies stimulated CD4+ T cells using TRIzol Reagent (Invitrogen, Carlsbad, CA), and the reverse transcription was performed using a transcriptase kit (Applied Biosystems). Real-time PCR was carried out using the Applied Biosystems 7500 System based on the SYBR-Green method. The expression of FAS and β-actin (the internal reference) was detected using the primers as described in earlier studies [Das et al., 2000; Yu et al., 2014]. All tests were conducted in triplicate, and the 2−ΔΔCt method was applied to calculate and quantify the relative expression levels of the target gene.
Flow Cytometric Analysis
The extent of apoptosis was evaluated by flow cytometry utilizing annexin V-fluorescein isothiocyanate /propidium iodide kit (BD Biosciences, San Diego, CA) according to the manufacturer's instructions. Flow cytometry was conducted on FACS Aria (BD Bioscience, San Diego, CA) and the data were analyzed with FACSDiva Software (BD Bioscience, San Diego, CA).
Statistical Analysis
The threshold cycle (Ct) number of Real-time PCR data was collected using 7500 software v2.0.6 (Applied Biosystems), and then the copy numbers were calculated using CopyCaller v2.0 software (Applied Biosystems). Pearson's χ2 test and Fisher's exact test were used to estimate the significance of the difference between the frequencies of the copy numbers of the target genes in cases and controls using SPSS version 17.0 (SPSS, Inc., Chicago, IL). Calculating the odds ratio (OR) and 95% confidence intervals (95% CI) was performed by one copy number group versus the other two groups pooled together. P values were corrected for multiple comparisons with the Bonferroni correction method by multiplying with the number (12) of analyses performed. Thus, a P value less than 4.17 × 10−3 (0.05/12) was considered to be statistically significant. A binary logistic regression analysis was used to evaluate the influence of gender on the association of CNVs with BD. The expression of FAS in carriers with low copy number (<2), normal diploid 2 copy number, and high copy number (>2) was analyzed by the nonparametric Mann–Whitney U test or independent samples T-test. A two-tailed P value (<0.05) was considered to be statistically significant.
Results
Clinical Manifestations of the Enrolled BD and VKH Syndrome Patients
The detailed demographic characteristics and clinical manifestations of the enrolled patients are shown in Table 1.
| Extraocular findings | Total | Percentage (%) |
|---|---|---|
| Patients with BD | 1,014 | |
| Mean age ± SD | 32.6 ± 10.1 | |
| Male | 858 | 84.6 |
| Female | 156 | 15.4 |
| Uveitis | 1,014 | 100 |
| Oral ulcer | 1,014 | 100 |
| Genital ulcer | 559 | 55.1 |
| Skin lesions | 712 | 70.2 |
| Arthritis | 153 | 15.1 |
| Positive pathergy test | 235 | 23.2 |
| Hypopyon | 269 | 26.5 |
| Patients with VKH syndrome | 1,051 | |
| Mean age ± SD | 39.7 ± 13.4 | |
| Male | 555 | 52.8 |
| Female | 496 | 47.2 |
| Uveitis | 1,051 | 100 |
| Headache | 418 | 39.8 |
| Tinnitus | 443 | 42.2 |
| Vitiligo | 205 | 19.5 |
| Alopecia | 386 | 36.7 |
| Poliosis | 376 | 35.8 |
| Controls | 2,076 | |
| Mean age ± SD | 38.4 ± 10.6 | |
| Male | 1,084 | 52.2 |
| Female | 992 | 47.8 |
- BD, Behçet disease; VKH, Vogt–Koyanagi–Harada.
Comparison of Copy Number Frequency of Test Genes between Cases and Controls in the First-Stage Study
Copy number polymorphisms of FAS, CASPASE3, CASPASE8, and BCL2 genes were investigated in 300 patients with BD, 300 VKH syndrome patients, and 600 normal controls for the first-stage study. A significantly increased frequency of high FAS gene copy number (>2) was found in BD (P = 1.05×10−3, OR = 2.27) and VKH syndrome (P = 2.56×10−3, OR = 2.03) (Tables 2 and 3). No significant association between CNVs of the other three genes and BD or VKH syndrome was observed (Supp. Tables S1 and S2).
| BD | Controls | |||||
|---|---|---|---|---|---|---|
| Copy number | N | % | N | % | P valuea | OR (95% CI)a |
| Stage 1 | N = 300 | N = 600 | ||||
| <2 | 22 | 7.3 | 28 | 4.7 | 0.06 | 1.81 (0.98–3.35) |
| =2 | 237 | 79.0 | 532 | 88.7 | 1.01 × 10−4 | 0.45 (0.30–0.67) |
| >2 | 41 | 13.7 | 40 | 6.6 | 1.05 × 10−3 | 2.27 (1.39–3.72) |
| Stage 2 | N = 714 | N = 1,476 | ||||
| <2 | 31 | 4.3 | 41 | 2.8 | 0.09 | 1.55 (0.94–2.57) |
| =2 | 593 | 83.1 | 1,349 | 91.4 | 2.43 × 10−6 | 0.51 (0.38–0.68) |
| >2 | 90 | 12.6 | 86 | 5.8 | 9.50 × 10−6 | 2.12 (1.52–2.96) |
| Combined | N = 1,014 | N = 2,076 | ||||
| <2 | 53 | 5.2 | 69 | 3.3 | 0.02 | 1.60 (1.09–2.37) |
| =2 | 830 | 81.9 | 1,881 | 90.6 | 1.17 × 10−9 | 0.49 (0.39–0.62) |
| >2 | 131 | 12.9 | 126 | 6.1 | 3.35 × 10−8 | 2.16 (1.65–2.85) |
- a The value was gender adjusted.
- P value with Bonferroni correction less than 4.17 × 10−3 was considered to be significant (shown in bold).
- OR, odds ratio; CI, confidence interval.
| VKH syndrome | Controls | |||||
|---|---|---|---|---|---|---|
| Copy number | N | % | N | % | P value | OR (95% CI) |
| Stage 1 | N = 300 | N = 600 | ||||
| <2 | 20 | 6.7 | 28 | 4.7 | 0.21 | 1.46 (0.81–2.64) |
| =2 | 242 | 80.6 | 532 | 88.7 | 1.11 × 10−3 | 0.53 (0.36–0.78) |
| >2 | 38 | 12.7 | 40 | 6.6 | 2.56 × 10−3 | 2.03 (1.27–3.24) |
| Stage 2 | N = 751 | N = 1,476 | ||||
| <2 | 37 | 4.9 | 41 | 2.8 | 9.11 × 10−3 | 1.81 (1.15–2.85) |
| =2 | 631 | 84.0 | 1,349 | 91.4 | 1.61 × 10−7 | 0.50 (0.38–0.65) |
| >2 | 83 | 11.1 | 86 | 5.8 | 1.07 × 10−5 | 2.01 (1.47–2.75) |
| Combined | N = 1,051 | N = 2,076 | ||||
| <2 | 57 | 5.4 | 69 | 3.3 | 4.80 × 10−3 | 1.67 (1.17–2.39) |
| =2 | 873 | 83.1 | 1,881 | 90.6 | 7.87 × 10−10 | 0.51 (0.41–0.63) |
| >2 | 121 | 11.5 | 126 | 6.1 | 9.77 × 10−8 | 2.01 (1.55–2.62) |
- P value with Bonferroni correction less than 4.17 × 10−3 was considered to be significant (shown in bold).
- OR, odds ratio; CI, confidence interval.
The Distribution of FAS Gene CNV in the Second-Stage and Combined Studies
In order to validate the significant association between a high copy number (>2) of the FAS gene and BD or VKH syndrome found in the first stage, another set of 714 BD patients, 751 VKH syndrome patients, and 1,476 controls were enrolled for the second-stage study. The results again revealed significantly increased frequencies of the high copy number (>2) of the FAS gene in BD (P = 9.50 × 10−6, OR = 2.12) and VKH syndrome (P = 1.07 × 10−5, OR = 2.01) (Tables 2 and 3). The combined data also confirmed the association between the high copy number (>2) of the FAS gene in BD (P = 3.35 × 10−8, OR = 2.16) and VKH syndrome (P = 9.77 × 10−8, OR = 2.01) (Tables 2 and 3).
Stratified Analysis for FAS Gene CNVs with Main Clinical Features of BD and VKH Syndrome
A stratified analysis was conducted to test the association of FAS gene copy numbers with the main clinical features of BD and VKH syndrome. The main clinical features of BD consisted of genital ulcer, arthritis, skin lesions, hypopyon, and positive pathergy reaction, and the main clinical manifestations of VKH syndrome included headache, vitiligo, tinnitus, alopecia, and poliosis. We could not demonstrate a significant association between FAS gene copy numbers and any clinical manifestation of BD or VKH (Supp. Tables S3 and S4).
The Influence of CNVs on FAS Expression and Apoptosis Rate
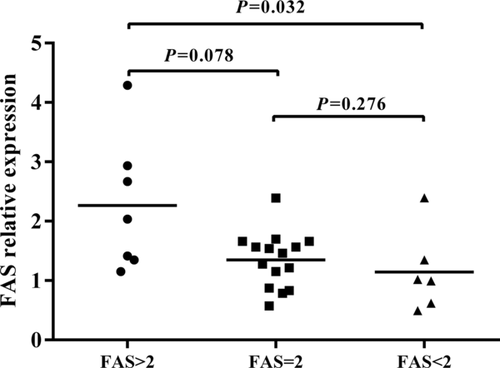
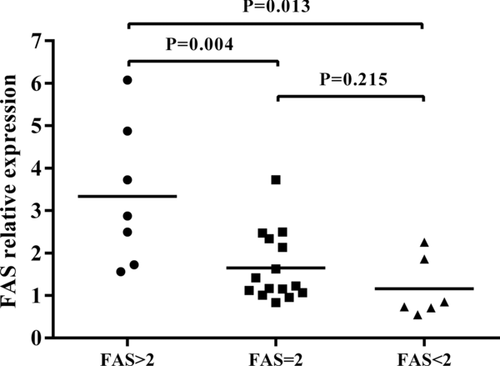
The aforementioned result showed a significant association between having a high copy number (>2) of the FAS gene and developing BD or VKH syndrome. To investigate a possible function associated with CNVs of the FAS gene, we performed real-time PCR analysis to evaluate its effect on the mRNA expression of this gene using CD4+ T cells derived from healthy individuals with a known gene copy number. The result showed that there was a significantly upregulated mRNA expression of FAS in healthy individuals carrying a high copy number (>2) versus those with low copy number (<2) (Fig. 1, P = 0.032). Although the result showed a higher FAS expression in cells with a high copy number (>2) as compared to those with a normal diploid 2 copy number, the difference was not statistically different (Fig. 1, P > 0.05). We subsequently examined whether the mRNA expression of FAS was affected by the various copy numbers in anti-CD3/CD28 antibodies-stimulated CD4+ T cells. The results showed that a significant upregulated mRNA expression of FAS was observed in healthy individuals carrying a high copy number (>2) versus those with a normal diploid 2 copy number (Fig. 2, P = 0.004) or low copy number (<2) (Fig. 2, P = 0.013), suggesting that CNVs of the FAS gene may affect FAS expression. We also investigated the influence of FAS CNVs on apoptosis rate. Although the results showed a lower apoptosis rate in the samples with a low FAS gene copy number (<2) and samples with diploid ( = 2) than individuals with a high FAS gene copy number (>2) both in purified CD4+ T cells and anti-CD3/CD28 antibodies-stimulated CD4+ T cells, there were no statistically significant differences among the three groups (Supp. Fig. S1, P > 0.05).
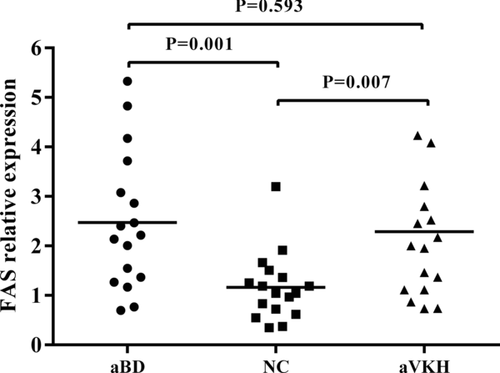
Increased FAS Expression in Active Patients with BD and VKH Syndrome
Previous studies have shown that the protein expression of the FAS gene in CD4+ T cells and CD8+ T cells from patients with BD and VKH syndrome was significantly higher than that seen in controls [Yang et al., 2001]. We extended these studies and investigated whether the mRNA expression of the FAS gene in PBMCs obtained from active BD patients and VKH syndrome patients was different from that seen in healthy controls. The results showed that FAS gene expression in PBMCs from active patients with BD as well as VKH syndrome was significantly higher than that in healthy controls (Fig. 3, P = 0.001 and P = 0.007, respectively).
Discussion
In the present study, we showed that a high copy number of the FAS gene was associated with an increased risk of BD and VKH syndrome in Han Chinese. Furthermore, we found a positive correlation between CNVs of FAS and its expression. To the best of our knowledge, this is the first study to show that variation in the copy number of the FAS gene is associated with BD and VKH syndrome.
Apoptosis, also known as programmed cell death, occurs widely in multicellular organisms. Accumulating evidence has shown that disturbed regulation of apoptosis contributes to many types of cancers and autoimmune diseases [Sun et al., 2007; Munoz et al., 2008; Fan et al., 2010; Zhu et al., 2010; Favaloro et al., 2012]. It has been shown that FAS, FASL, CASPASE8, CASPASE3, and BCL2 play an important role in modulating cell apoptosis and maintaining cellular homeostasis [Nagata, 2010; Favaloro et al., 2012]. An aberrant expression of FAS, FASL, and BCL2 has been shown in BD and VKH syndrome patients and suggests that abnormal cell apoptosis may be involved in their pathogenesis [Yang et al., 2001; Wakisaka et al., 2002; Baris et al., 2005]. Earlier genome-wide association studies (GWAS) in BD and VKH syndrome in Han Chinese [Hou et al., 2012, 2014a] did not show evidence of an association with SNPs of apoptosis-related genes, which may be due to the strict GWAS P value threshold levels.
CNVs are another prevalent genetic variants covering more than 1 kb deletion or duplication, which may cause phenotypic variation by directly modifying gene expression or biological function [Feuk et al., 2006; McCarroll and Altshuler, 2007; Stranger et al., 2007; Ionita-Laza et al., 2009; Hollox and Hoh, 2014]. Previous studies have reported that germline CNVs may have an important impact on conferring risk to the later development of complex disease phenotypes and autoimmune diseases [Aitman et al., 2006; Yang et al., 2007b; Tse et al., 2011; Yu et al., 2011; Liu et al., 2012; Robinson et al., 2012; Yang et al., 2013; Wu et al., 2014]. In the current study, we investigated the association between CNVs of FAS signaling pathway genes and BD or VKH syndrome. The result showed that a high copy number of FAS gene was associated with an increased risk of BD and VKH syndrome in Han Chinese, whereas no significant association between CNVs of the other three genes (CASPASE8, CASPASE3, and BCL2) and BD or VKH syndrome was observed. It should be noted that we excluded the FASL gene from our study, since no CNVs could be found for this gene following a search in the DGV. Besides, low FAS gene copy number also showed a trend of association with a higher risk of BD (OR = 1.60) and VKH syndrome (OR = 1.67), similar to FCGR3A [Chen et al., 2014] and FCGR3B [Wu et al., 2014], which may be due to low frequency of FAS gene copy number (<2) in healthy controls.
Further functional assays were performed to detect whether CNVs of the FAS gene would influence its expression. The result suggested that an increased copy number of this gene was positively correlated with elevated FAS mRNA levels, which was in agreement with earlier findings on other CNVs showing a gene dosage effect [Liu et al., 2012; Hou et al., 2013; Yang et al., 2013; Hollox and Hoh, 2014]. Our gene expression analysis was performed in healthy control subjects, because the patients are individuals with varying degrees of inflammation and are often treated with immunosuppressive drugs which may alter the response of the lymphocytes. Although the results showed a lower apoptosis rate in the samples with a low FAS gene copy number (<2) and samples with diploid ( = 2) than individuals with a high FAS gene copy number (>2) both in purified CD4+ T cells and anti-CD3/CD28 antibodies-stimulated CD4+ T cells, no significant association was observed. Further studies are needed to investigate whether CNVs of FAS can influence the degree of cell apoptosis.
Previous studies reported an overexpression of FAS protein in CD4+ T cells and CD8+ T cells from patients with BD and VKH syndrome [Yang et al., 2001]. Our findings confirmed these earlier findings by showing that the mRNA expression of the FAS gene in PBMCs from active patients with BD and VKH syndrome was significantly higher than that in healthy controls. It has been shown that FAS/FASL interaction can regulate inflammation and prevent damage to delicate ocular structure, which is one of the important mechanisms of “Immune privilege” in the eye [Roychoudhury et al., 2010; Zhao et al., 2013]. Theoretically, a high FAS expression on T cells would lead to their apoptotic death once they would enter the eye and come into contact with FASL expressing ocular resident cells such as the retinal pigment epithelial cell. An explanation for the apparent discrepancy is not readily available and further research is needed to clarify this issue.
Our study has several limitations. Although we tried to match the controls for gender, it was not achieved in the BD group, which predominantly includes male patients. Further studies should be validated in a gender-matched population. Because this survey was performed only in Han Chinese, it is not certain whether our findings can be generalized to other ethnic populations. Since our patients were recruited from ophthalmic centers, a selection bias in our patient population may be present. Only two uveitis entities were studied and a similar study should be performed in patients with other uveitis entities before generalizing our findings. Furthermore, only the FAS signaling pathway genes were investigated in this survey, and it is possible that other apoptosis-related genes might also be involved.
In conclusion, our results suggest that a high FAS gene copy number is associated with an increased risk of BD and VKH syndrome in Han Chinese, which is likely related to an increased expression of FAS in patients with BD and VKH syndrome.
Acknowledgment
The authors would like to thank all donors enrolled in the present study.
Disclosure statement
The authors declare no conflict of interest. This study was conducted with the approval of the Ethical Committee of Chongqing Medical University. The consent of patients was also obtained.




