Instability of Trinucleotidic Repeats During Chromatin Remodeling in Spermatids
Communicated by Andrew Wilkie
ABSTRACT
Transient DNA breaks and evidence of DNA damage response have recently been reported during the chromatin remodeling process in haploid spermatids, creating a potential window of enhanced genetic instability. We used flow cytometry to achieve separation of differentiating spermatids into four highly purified populations using transgenic mice harboring 160 CAG repeats within exon 1 of the human Huntington disease gene (HTT). Trinucleotic repeat expansion was found to occur immediately following the chromatin remodeling steps, confirming the genetic instability of the process and pointing to the origin of paternal anticipation observed in some trinucleotidic repeats diseases.
Introduction
The differentiation of spermatids into spermatozoa, termed spermiogenesis, involves one of the most striking modifications in chromatin structure. During spermiogenesis, the histone-based chromatin of round spermatids becomes modified by the removal of most of the histones that are sequentially replaced by abundant transition proteins and finally by protamines, providing a greater level of DNA compaction and mechanical stability [Kierszenbaum and Tres, 1975; Sassone-Corsi, 2002; Govin et al., 2004; Meyer-Ficca et al., 2011]. During the chromatin-remodeling steps, elimination of most of the nucleosomal DNA supercoils is associated with a transient surge of DNA double-strand breaks (DSBs). DSBs are apparently part of the normal differentiation program of spermatids since these are observed in the whole population of these cells. DSBs may be induced either enzymatically by topoisomerases or nucleases and/or mechanically, as a result of increased torsional stress [Gouraud et al., 2013]. In any case, this indicates that the process may represent a window of genetic instability that can, in part, be responsible for the now well-established male de novo mutation bias [Kong et al., 2012].
Trinucleotidic repeats (TNR) are among the most unstable DNA regions in the genome, and both expansion and contraction of TNR have been associated with a number of neurological and muscular inherited diseases [McMurray 2010]. In Huntington disease (HD), expansion of the CAG repeats within exon 1 of the Huntingtin (HTT) gene occurs almost exclusively through paternal transmission, leading to an earlier onset of symptoms in the next generation. Studies using HD transgenic mouse models showed that TNR expansion also occurs in mice and more specifically during the postmeiotic events of spermatogenesis. However, due to a lack of appropriate spermatids separation techniques, the specific steps involved and the consequences of the chromatin remodeling could not be specifically determined [Kovtun and McMurray, 2001].
In this study, we investigated TNR variation during spermiogenesis using a HD transgenic mouse model harboring 160 CAG repeats. Determining the step-specific occurrence of TNR variation however requires an efficient separation method allowing purification of differentiating spermatids to near homogeneity. Using a new flow cytometry gating strategy based on a combination of DNA staining, cells size and granulosity, we achieved separation of spermatids from HD transgenic mice into four distinct populations, allowing us to determine the step-specific occurrence of TNR expansion and investigate the genetic impact of the chromatin remodeling for the first time. Assessment of TNR variation in these homogenous cell populations confirmed that expansion arises in spermatids between steps 13 and 15, representing the final stages of the chromatin remodeling process.
Materials and Methods
Animals
For TNR expansion analysis, 8-week-old male B6CBA-Tg(HDexon1)62Gpb/1J mice were obtained from The Jackson Laboratory (Bar Harbor, ME). These mice harbor the exon 1 of the human HD gene containing 160 CAG repeats. All strains were maintained under standard housing conditions. Mice were anesthetized by isoflurane and euthanized by CO2 asphyxiation. Animal care was in accordance with the Université de Sherbrooke animal care and use committee.
Cells Sorting by Flow Cytometry
Testes were decapsulated and homogenized in sorting buffer (1x PBS, 1 mM EDTA, 25 mM HEPES pH 7.0, 1% heat-inactivated fetal bovine serum [FBS]). Homogenized cells were then filtered using a BD Falcon™ 40-μm Cell Strainer (BD Biosciences, San Jose, CA). EDTA was added to a final concentration of 0.8 mM and ice-cold absolute ethanol was slowly added to the filtrate up to a final concentration of 75%. Cells were incubated on ice for 15 min and centrifuged at 800g for 5 min. Fixed cells were resuspended in sorting buffer. SYTO® 16 Green Fluorescent Nucleic Acid Stain (Invitrogen, Carlsbad, NM) was added to a final concentration of 2 μM and cells were incubated at least 30 min on ice. Prior to sorting, cells were filtered again using CellTrics® 50-μm filters (Partec, Münster, Germany) in 5-mL polypropylene conical tube that were precoated with FBS.
Cells were sorted into four distinct populations: steps 1–9, 10–12, 13–14, and 15–16 spermatids using a BD Biosciences FACSAriaTM III cell sorter (BD Biosciences). Cells were collected in 5 mL polypropylene conical tubes that were precoated with FBS. To identify and analyze the purity of the sorted populations, 200,000 cells from each population were centrifuged at 800g for 5 min onto microscope slides using a Shandon CytoSpin® 2 Cytocentrifuge and stained with 40 ng/mL 4’,6-diamidino-2-phenylindole (DAPI) for epifluorescence microscopy (Fig. 1).
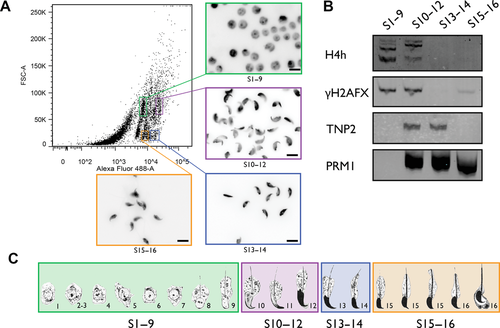
Protein Analysis by Immunoblot of Sorted Cells
For each protein to be analyzed, 350,000 cells per lane were used, based on the approximated number of events for each population sorted by flow cytometry. Proteins were extracted using an acid extraction protocol, as described by Torregrosa et al. (2006). Extracted proteins were separated on a 15% acid–urea polyacrylamide gel and detected by immunoblot, using the following primary antibodies: rabbit antihyperacetylated Histone H4 (Penta) Antibody (catalog no. #06-946; Millipore, Billerica, MA), mouse anti-H2AX-Phosphorylated (Ser139) antibody [3F2] (catalog no. #613402; Biolegend, San Diego, CA), mouse antiprotamine P1 (catalog no. #Mab-001 Hup 1N; Briar Patch Biosciences LLC, Livermore, CA), and goat anti-TNP2 Antibody (K-18) (catalog no. #sc-21106; Santa Cruz Biotechnology, Santa Cruz, CA). The following secondary antibodies were used: Alexa Fluor® 680 Goat Anti-Mouse IgG (H+L) (catalog no. #A21057; Invitrogen), IRDye 800CW Goat Anti-Rabbit IgG (H+L) (catalog no. #926-32211; Li-Cor Biosciences, Lincoln, NE), and Alexa Fluor® 680 Rabbit Anti-Goat IgG (H+L) (catalog no. #A21088; Invitrogen). Fluorescence was visualized using the Odyssey Infrared Imaging System (model 9120; Li-Cor Biosciences).
TNR Expansion Analysis
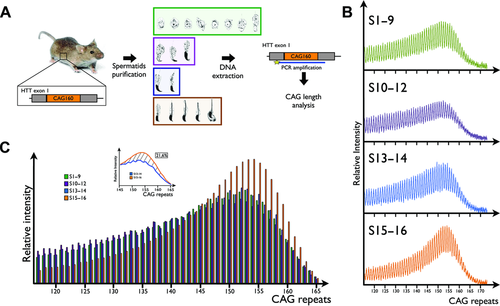
Germ cells from B6CBA-Tg(HDexon1)62Gpb/1J male mice were sorted by flow cytometry as described in the previous section. DNA from each population was extracted using QIAamp DNA Mini Kit (Qiagen, Hilden, Germany). The CAG region was amplified by PCR using the following primers: 5’-TYETM705-AAAAGCTGATGAAGGCCTTCGAG-3’ and 5’-CGGCGGTGGCGGCTGTTG-3’ and the following reagents: 0.2 mM dNTPs, 1x OneTaq® GC Reaction Buffer, 20% OneTaq® High GC Enhancer, 4% additional DMSO (rising the final DMSO concentration to 14% given that DMSO is present in both GC Reaction Buffer and High GC Enhancer), 0.16 μM of each primer, 20 ng of DNA, and 1 unit of OneTaq® Hot Start DNA Polymerase (New England Biolabs, Beverly, MA). PCR reactions were performed using the following conditions: initial denaturation at 94°C for 10 min followed by 40 cycles of denaturation at 94°C for 1 min, annealing at 58°C for 1 min and elongation at 74°C for 70 sec. Final elongation was performed at 74°C for 10 min.
The number of CAG repeats for each population was analyzed by high-resolution DNA gel electrophoresis. Samples were resolved using a 66-cm denaturing 6% acid–urea polyacrylamide gel using a 4300 DNA Analyzer (Li-Cor Biosciences). The gel was prerun at 3.3 kV for 10 min and samples were migrated at 3.3 kV for 16 hr. TNR were detected with the TYE™ 705 tag (Integrated DNA Technologies, Coralville, IA) coupled with one of the PCR primers using the 700-nm channel. The resulting images were quantified using the ImageQuant TL 5.0 Software (GE Healthcare Life Sciences, Pittsburgh, PA) and represented as graphics showing the relative intensity of each number of CAG repeats (Fig. 2B). Total intensity of the PCR product from each population is normalized to steps 15–16 spermatids. The precise number of CAG repeats was determined by comparing the bands pattern with the 50–700-bp IRDye® 700 Sizing Standard (Li-Cor Biosciences). The area under each peak, representing a specific frequency of CAG repeats, was calculated and represented for each population as a frequency histogram (Fig. 2C).
Results
Separation of Spermatids by Flow Cytometry
A systematic analysis to establish the potential for the chromatin remodeling steps to induce genetic instability and TNR expansion first required a separation method yielding pure spermatids populations. Using flow cytometry, we achieved the separation of spermatids into four distinct populations: steps 1–9, steps 10–12, steps 13–14, and steps 15–16. Separation was based on size (FSC), granulosity (SSC), and DNA staining intensity using SYTO® 16, which we found to vary according to the condensed state of the nucleus (Fig. 1A). The purity and staging of the different cell populations were determined based on nuclear shape as visualized with DAPI and immunoblots using step-specific biomarkers. Steps 1–9 spermatids population has characteristic round-shaped nuclei including few oval-shaped nuclei, typical of mouse step 9 spermatids. Steps 10–12 spermatids have a densely stained large hook-shaped nucleus. Both steps 13–14 and 15–16 have similar nuclear morphology but markedly differ in DNA staining intensity (Fig. 1A). Cell enrichment purity was also ascertained by immunoblots using steps-specific biomarkers (Fig. 1B). Both hyperacetylated histone H4 (H4h) and histone H2AFX phosphorylated at serine 139 (γH2AFX) were detected as expected between steps 8 and 12 spermatids as shown in Figure 1B [Leduc et al., 2008]. Multibanding of H4h represents other posttranslational modification as resolved by acid–urea polyacrylamide gel. The weak γH2AFX band observed in steps 15–16 spermatids reflects the low level of spermatocyte contamination. Transition protein 2 (TNP2), known to be expressed during steps 10–14 [Zhao et al., 2004], was detected accordingly. Finally, immunoblots also confirmed protamine 1 (PRM1) expression from early step 12 up to spermiation, as expected [Zhao et al., 2004]. These observations therefore confirm that the sorting procedure provides highly pure populations with minimal cross-contamination.
TNR Instability During Spermiogenesis
Using the cell separation scheme described above, spermatids were purified from transgenic mice harboring 160 CAG repeats within exon 1 of the human HTT gene. The variation in the number of CAG repeats was analyzed by PCR amplification using a fluorescent forward primer, followed by high-resolution acrylamide–urea gel electrophoresis allowing a very sensitive determination of the length of the CAG repeats. The distribution of TNR is represented as a digital scan where each peak corresponds to a specific number of CAG repeats with intensity proportional to the representation of a given repeat length within the population. Analysis of TNR variation between sorted spermatids is simplified into a histogram representing the relative intensity of each number of CAG generated from the area under every peak of the digital scan. As shown in Figure 2, no variation in the length distribution of the CAG repeats was observed up to step 14 of the spermatids’ differentiation process. However, in steps 15–16, one can observe a clear increase in the frequency of DNA repeat length ranging from 145 to 165 when compared with previous differentiation steps. Within this TNRs range, calculating the area between this population's curve and that generated from step 13–14 spermatids indicates 21.6% increase in the frequency of these TNR for this mouse (Fig. 2C). In other words, 21.6% of spermatids now harbor longer TNRs following chromatin remodeling. In addition, one can observe that the most frequent repeat lengths are shifted by two additional TNRs in the last population. When the experiment was performed using a second mice of the same strain, we observed similar results with an increased TNR frequency of 18.2% between 145 and 165 repeats and with an identical shift in the most frequent TNR length (Supp. Fig. S1).
Discussion
Chromatin remodeling in spermatids is one of the most striking changes in nuclear architecture known to the eukaryotic world. This process is associated with transient DNA strand breakage that appears at mid-spermiogenesis steps but becomes repaired during the final differentiation steps ensuring that no such DNA damage is transferred to the oocyte by the mature sperm [Laberge and Boissonneault, 2005; Leduc et al., 2008; Ward, 2011; Gouraud et al., 2013]. Chromatin remodeling and the transient surge in DNA strand breaks has been reported in human [Marcon and Boissonneault, 2004], mouse [Marcon and Boissonneault, 2004; Leduc et al., 2008], rat [Meyer-Ficca et al., 2005], and drosophila [Rathke et al., 2007], as well as in the grasshopper [Cabrero et al., 2007] and in the algae [Wojtczak et al., 2008] pointing to a highly conserved mechanism with the potential to induce de novo genetic variation through subsequent generations [Grégoire et al., 2013].
In this study, we investigated TNR expansion in a HD transgenic mouse model as a potential consequence of genetic instability of the spermatid chromatin remodeling process. We first designed a successful flow cytometry gating strategy to achieve purification of spermatids into four highly pure populations with minimal contamination and allowing to monitor molecular events through these important nuclear differentiation steps. Such degree of purity has so far not been obtained using classical techniques such as sedimentation or elutriation [Meistrich et al., 1994; Wykes and Krawetz, 2003]. The method stems from our observations of the peculiar variation of DNA staining intensity according to the condensed state of the spermatid's nucleus that has been combined with two other parameters (size and granulosity).
The spermatid chromatin remodeling process is similar in both human and mouse involving initial histone hyperacetylation, transition proteins [De Vries et al., 2012], and transient DNA strand breakage [Marcon and Boissonneault, 2004] until protamination of most of the sperm DNA. Although these events span fewer morphological differentiation steps in human than in mouse, it is likely that they share similar genetic consequences. Hence, a human transgene inserted into the mouse genome is expected to be chromatinized like an endogenous mouse sequence and impacted similarly by the chromatin remodeling process. So far, no specific genetic instability is known to arise from the transfer of a human gene into mouse [Deatly et al., 1998; Tamaoki, 2001] unless they harbor repetitive elements such as the TNRs characterized in this study. Interestingly, we show that chromatin remodeling results in approximately 20% increase in the frequency of TNR ranging specifically from 145 to 165 repeats. Once spermiation is completed, this indicates that 20% of the sperm cell population will harbor longer TNR, so transmission of TNR expansion in one out of five offspring would be expected. One cannot determine whether or not an identical proportion of TNR expansion would take place in human, but the similarity between the chromatin remodeling processes leads us to speculate that TNR expansion would be observed to the same extent in human. In HD patients, transmission of an expanded number of CAG repeats normally results in earlier onset of the symptoms in offspring.
Previous investigations suggested that postmeiotic events could drive expansion of TNR since it was observed in spermatozoa, but whether this arises as a consequence of the chromatin remodeling steps could not be determined [Kovtun and McMurray, 2001]. Our results showed that TNR expansion is detected immediately following chromatin remodeling at steps 15–16 in mice, which is equivalent to the transition between step 3 and 4 in human spermiogenesis based on histone H4 hyperacetylation and the presence of DSBs [Marcon and Boissonneault, 2004]. Hence, expansion driven by chromatin remodeling could also be implicated in other TNR diseases with paternal anticipation such as Kennedy's disease or some spinocerebellar ataxias.
It is unclear how TNR expansion arises during the chromatin remodeling. However, in vitro studies have shown that TNR are likely to form secondary structures such as hairpins when DNA breaks occur. This could lead to the recruitment of DNA mismatch repair machinery that is known to promote TNR expansion [Kovtun and McMurray, 2001]. Moreover and as outlined above, a significant number of transient DNA DSBs have been detected at chromatin remodeling steps in spermatids. The haploid character of these cells should therefore prevent the use of homologous recombination for error-free templated repair. Hence, one alternative possibility is the involvement of microhomology-mediated end joining mechanism for DSBs repair since the later has been shown to drive CAG expansion of the human HTT gene when assayed in vitro [Crespan et al., 2012].
Although this investigation establishes for the first time that expansion arises during the chromatin remodeling steps in spermatids, it certainly warrants further genome-wide assessment of sensitive loci where de novo mutations could be induced by this process. Interestingly, the recent whole-genome sequencing of parent-offspring trios confirmed that an age-dependent male mutation bias exists in the transmission of de novo mutations [Kong et al., 2012]. A male mutation bias was also reported for small insertions and deletions [Makova et al., 2004]. Given that male premeiotic germ cells can rely on HR, known to repair DNA with higher fidelity [Walter et al., 2004], the chromatin remodeling steps in haploid spermatids may therefore prove to be an important transition from which most de novo mutations are endogenously produced [Grégoire et al., 2013].
Acknowledgments
We would like to thank Josée Lamoureux and Geneviève Acteau for their skillful technical assistance.
Disclosure statement: The authors declare no conflict of interest.




