Spectrum of the Mutations in Bernard–Soulier Syndrome
Communicated by John McVey
Contract grant sponsors: IRCCS Burlo Garofolo (16/12); INSERM [GIS Maladies Rares (GISMR2010)]; Etablissement Français du Sang (EFS) (APR2010, APR2013-14).
ABSTRACT
Bernard–Soulier syndrome (BSS) is a rare autosomal recessive bleeding disorder characterized by defects of the GPIb-IX-V complex, a platelet receptor for von Willebrand factor (VWF). Most of the mutations identified in the genes encoding for the GP1BA (GPIbα), GP1BB (GPIbβ), and GP9 (GPIX) subunits prevent expression of the complex at the platelet membrane or more rarely its interaction with VWF. As a consequence, platelets are unable to adhere to the vascular subendothelium and agglutinate in response to ristocetin. In order to collect information on BSS patients, we established an International Consortium for the study of BSS, allowing us to enrol and genotype 132 families (56 previously unreported). With 79 additional families for which molecular data were gleaned from the literature, the 211 families characterized so far have mutations in the GP1BA (28%), GP1BB (28%), or GP9 (44%) genes. There is a wide spectrum of mutations with 112 different variants, including 22 novel alterations. Consistent with the rarity of the disease, 85% of the probands carry homozygous mutations with evidence of founder effects in some geographical areas. This overview provides the first global picture of the molecular basis of BSS and will lead to improve patient diagnosis and management.
Background
Bernard–Soulier syndrome (BSS; MIM #231200) is a rare and often severe inherited disorder characterized by macrothrombocytopenia with some platelets larger than red blood cells (giant platelets), spontaneous muco-cutaneous bleeding, and posttraumatic hemorrhages. BSS is caused by mutations in GP1BA (MIM #606672), GP1BB (MIM #138720), and GP9 (MIM #173515), three of the four genes encoding for the subunits of the glycoprotein (GP) Ib-IX-V complex. This is a key platelet receptor constituted of four subunits, GP1BA (GPIbα), GP1BB (GPIbβ), GP9 (GPIX), and GP5 (GPV), which associate in the ratio 2:4:2:1 in the endoplasmic reticulum and mature in the Golgi apparatus before localizing at the cell surface [Lopez et al., 1998; Ulsemer et al., 2001; Luo et al., 2007]. Indeed, there is a requirement for the concurrent expression of all the subunits to enable efficient expression of the GPIb-IX complex on the cell surface [Li and Emsley, 2013].
After removal of the signal peptide, the mature GPs consist of an N-terminal extracellular domain containing leucine-rich repeats (LRRs), a transmembrane helix, and a relatively short cytoplasmic tail. The GPIbα N-terminal domain is the major binding site for adhesive proteins, counter receptors, and coagulation factors. In particular, its 282 N-terminal residues mediate the initial capture of platelets by the von Willebrand factor (VWF) exposed at the vascular injury site. This event, defective in BSS, is detected in vitro by inducing platelet agglutination with ristocetin (RIPA), an antibiotic that triggers binding of VWF to GPIbα and consequent platelet clumping in the aggregometer [Lopez et al., 1998].
The incidence of BSS has been estimated at 1 per 1 million live births, but is likely to be higher as it is often misdiagnosed [Savoia et al., 2011; Balduini et al., 2013b; Moiz and Rashid, 2013]. In its typical form, BSS is an autosomal recessive disease (biallelic BSS) characterized by severe bleeding tendency despite a moderate thrombocytopenia (60 × 109/L platelets on average) [Savoia et al., 2011]. Family members with only one mutant allele (BSS carriers) are usually asymptomatic with normal platelet counts. Nonetheless, BSS carriers might have slightly enlarged platelets and decreased GPIb-IX-V complex expression, as well as a moderately reduced RIPA response [Noris et al., 2012]. In addition to the classical recessive forms of BSS, some rare mutations of GP1BA or GP1BB correlate with a mild, usually asymptomatic, macrothrombocytopenia, which is transmitted as a completely penetrant autosomal dominant trait (monoallelic BSS) [Miller et al., 1992; Kunishima et al., 2006a; Vettore et al., 2008a; Noris et al., 2012].
Although BSS has been known since the mid 20th century and the BSS genes have been identified more than 20 years ago, no studies have been carried out on large cohorts. In most cases, only single patients have been reported, making comparison of their clinical presentation and the search for genotype/phenotype correlations very speculative. As the scientific community still lacks a comprehensive genetic and biological database on the disease, we established an International Consortium for the study of BSS with the aim of providing a more complete overview of the molecular defects. The project was approved by the Bioethics Committee of IRCCS Burlo Garofolo (Record number CE/V-127). At present, the BSS Consortium has collected data from 132 families affected with the “biallelic” form of BSS. In this database (BSS Consortium database), 56 are novel families, whereas the remaining 76 have previously been reported in the literature. The molecular data from these families have been integrated with those of another 79 families from the literature, accounting for a total of 211 unrelated families. To make the spectrum of mutations in GP1BA, GP1BB, and GP9 as comprehensive as possible, we also include the few mutations identified in the monoallelic form of BSS and in the platelet-type von Willebrand disease (VWDP).
Variants in Biallelic BSS
Among the 211 families with the biallelic form of BSS, mutations were identified in the GP1BA, GP1BB, and GP9 genes in 60 (28%), 59 (28%), and 92 (44%) cases, respectively (Supp. Table S1). In three of these families, more than one variant per allele was identified (Supp. Table S1): in the first family (BSS3), the affected individuals were homozygous for two GP1BA mutations; in the second (BSS103), the patient was heterozygous for three different GP1BB mutations [Hadjkacem et al., 2010]; and in the third (BSS99), the patient was homozygous for two GP1BB and heterozygous for one GP1BA mutation [Sumitha et al., 2011]. Consistent with the rarity of the disease, 179 (85%) were homozygous and 28 (13%) were compound heterozygous for mutations in one of the three genes. Indeed, more than 50% of the families enrolled in the BSS database derived from marriages with different degrees of consanguinity (mostly first cousins). Finally, in four (BSS1, BSS26, BSS61, and BSS120; Supp. Table S1) out of 211 probands (2%) identified as BSS by defective RIPA, molecular screening detected only one mutated allele. Although in compound heterozygote BSS26, the absence of the wild-type GPIbα subunit suggested that the second mutant allele was missed [Ware et al., 1990], information on the other probands are incomplete for any hypothesis (see below in Clinical Relevance).
In this cohort, 112 different variants, including 22 novel, were identified in the three BSS genes (Tables 1, 2 and 3; Fig. 1). Mutational screening was carried out using a Sanger sequencing approach as previously described [Savoia et al., 2011]. Mutations are distributed over the entire genes without evidence for mutational hotspots. They are missense, nonsense, or frameshift alterations. Because of the compact structure of the genes, which are constituted of 2 or 3 exons, no splicing mutations have been detected so far. None of these variants are cited in the dbSNP or in the 1000 Genome Project.
| Order | GP1BA cDNA mutation (NM_000173.5)a | Number of alleles | Predicted amino acid change in GPIbα (NP_000164.5) | Variant names in mature proteinb | Mutation type | References |
|---|---|---|---|---|---|---|
| 1 | c.1A>C | 2 | p.? | Start codon | BSS Consortium | |
| 2 | c.23_31del | 2 | p.Leu8_Leu10del | Small in frame deletion | BSS Consortium | |
| 3 | c.103A>T | 3 | p.Lys35* | Lys19* | Nonsense | Gohda et al. (2007); BSS Consortium |
| 4 | c.104del | 6 | p.Lys35Argfs*4 | Lys19Argfs*4 | Frameshift | Li et al. (1996); BSS Consortium |
| 5 | c.106A>G | 2 | p.Arg36Gly | Arg20Gly | Missense | BSS Consortium |
| 6 | c.154dup | 2 | p.Leu52Profs*5 | Leu36Profs*5 | Frameshift | Afrasiabi et al. (2007) |
| 7 | c.165_168del | 3 | p.Ser55Argfs*12 | Ser39Argfs*12 | Frameshift | Afshar-Kharghan et al. (2000); Vettore et al. (2008b) |
| 8 | c.236dup | 2 | p.Asp79Glufs*2 | Asp63Glufs*2 | Frameshift | Ali et al. (2014) |
| 9 | c.241T>C | 1 | p.Cys81Arg | Cys65Arg | Missense | Kenny et al. (1998) |
| 10 | c.266dup | 2 | p.Asp89Glufs*63 | Asp73Glufs*63 | Frameshift | Ali et al. (2014) |
| 11 | c.275del | 2 | p.Leu92Argfs*20 | Leu76Argfs*20 | Frameshift | Simsek et al. (1994a) |
| 12 | c.278dup | 2 | p.Val94Serfs*58 | Val78Serfs*58 | Frameshift | Ali et al. (2014) |
| 13 | c.339_340insGA | 2 | p.Thr114Glufs*16 | Thr98Glufs*16 | Frameshift | Sumitha et al. (2011) |
| 14 | c.376A>G | 4 | p.Asn126Asp | Asn110Asp | Missense | Savoia et al. (2011); Vettore et al. (2011) |
| 15 | c.416T>C | 2 | p.Leu139Pro | Leu123Pro | Missense | BSS Consortium |
| 16 | c.434T>C | 5 | p.Leu145Pro | Leu129Pro | Missense | Li et al. (1995); Koskela et al. (1999a); Antonucci et al. (2000) |
| 17 | c.438C>G | 2 | p.Tyr146* | Tyr130* | Nonsense | BSS Consortium |
| 18 | c.515C>T | 3 | p.Ala172Val | Ala156Val | Missense | De Marco et al. (1990); Ware et al. (1993); Margaglione et al. (1999) |
| 19 | c.555_590del | 2 | p.Asn185_Glu197delinsLys | Asn169_Glu181delinsLys | Small inframe deletion | Margaglione et al. (1999); BSS Consortium |
| 20 | c.583_585del | 6 | p.Leu195del | Leu179del | Small inframe deletion | de la Salle et al. (1995); Ulsemer et al. (2000); BSS Consortium |
| 21 | c.586C>T | 1 | p.Gln196* | Gln180* | Nonsense | BSS Consortium |
| 22 | c.667T>G | 2 | p.Trp223Gly | Trp207Gly | Missense | Rosenberg et al. (2007) |
| 23 | c.673T>A | 8 | p.Cys225Ser | Cys209Ser | Missense | Gonzalez-Manchon et al. (2001); Simsek et al. (1994b); Savoia et al. (2011); BSS Consortium |
| 24 | c.785T>G | 2 | p.Val262Gly | Val246Gly | Missense | Ali et al. (2014) |
| 25 | c.882C>G | 2 | p.Tyr294* | Tyr278* | Nonsense | Glembotsky et al. (2012) |
| 26 | c.932_933del | 2 | p.Val311Glyfs*39 | Val295Glyfs*39 | Frameshift | Kanaji et al. (1997) |
| 27 | c.941dup | 2 | p.Thr316Hisfs*35 | Thr300Hisfs*35 | Frameshift | Ali et al. (2014) |
| 28 | c.1012dup | 2 | p.Met338Asnfs*13 | Met322Asnfs*13 | Frameshift | Ali et al. (2013) |
| 29 | c.1039G>T | 1 | p.Glu347* | Glu331* | Nonsense | Yamamoto et al. (2013) |
| 30 | c.1064dup | 2 | p.Pro357Thrfs*2 | Pro341Thrfs*2 | Frameshift | Ali et al. (2014) |
| 31 | c.1077del | 2 | p.Trp359* | Trp343* | Nonsense | Bowers et al. (2006) |
| 32 | c.1077G>A | 1 | p.Trp359* | Trp343* | Nonsense | Ware et al. (1990) |
| 33 | c.1094_1101del | 2 | p.Leu365Argfs*14 | Leu349Argfs*14 | Frameshift | Imai et al. (2009) |
| 34 | c.1257dup | 2 | p.Ser420Glnfs*78 | Ser404Glnfs*78 | Frameshift | Sumitha et al. (2011) |
| 35 | c.1436dup | 2 | p.Leu479Phefs*19 | Leu463Phefs*19 | Frameshift | Ali et al. (2014) |
| 36 | c.1454dup | 6 | p.Ser486Ilefs*12 | Ser470Ilefs*12 | Frameshift | Kanaji et al. (1997); Gonzalez-Manchon et al. (2001); Gohda et al. (2007); Ali et al. (2013) |
| 37 | c.1457C>A | 2 | p.Ser486* | Ser470* | Nonsense | Kunishima et al. (1994) |
| 38 | c.1465del | 2 | p.Glu489Asnfs*64 | Glu473Asnfs*64 | Frameshift | Ali et al. (2014) |
| 39 | c.1480del | 6 | p.Thr494Profs*59 | Thr478Profs*59 | Frameshift | Noda et al. (1995); Kanaji et al. (1997); Mitsui et al. (1998); Yamamoto et al. (2013) |
| 40 | c.1592del | 2 | p.Leu531Argfs*22 | Leu515Argfs*22 | Frameshift | Ali et al. (2014) |
| 41 | c.1600T>C | 1 | p.Tyr534His | Tyr518His | Missense | Sumitha et al. (2011) |
| 42 | c.1601_1602del | 8 | p.Tyr534Cysfs*82 | Tyr518Cysfs*82 | Frameshift | Kenny et al. (1997); Afshar-Kharghan and Lopez (1997); Koskela et al. (1999a); Afshar-Kharghan et al. (2000) |
| 43 | c.1601_1602delinsTGG | 1 | p.Tyr534Leufs*83 | Tyr518Leufs*83 | Frameshift | BSS Consortium |
| 44 | c.1620G>A | 3 | p.Trp540* | Trp524* | Nonsense | Holmberg et al. (1997); Kenny et al. (1998) |
| 45 | c.1759C>T | 2 | p.Gln587* | Gln571* | Nonsense | Yamamoto et al. (2013) |
- a Nucleotide numbering reflects the GP1BA cDNA with +1 corresponding to the A of the ATG translation initiation codon in the reference sequence RefSeq NM_000173.5. Therefore, the initiation codon is codon 1. Novel variants are in bold.
- b To adhere to nomenclature from literature, mutations are named in the mature protein that derives from signal peptide removal of the 16 N-terminal amino acids of GPIbα (NP_000164.5).
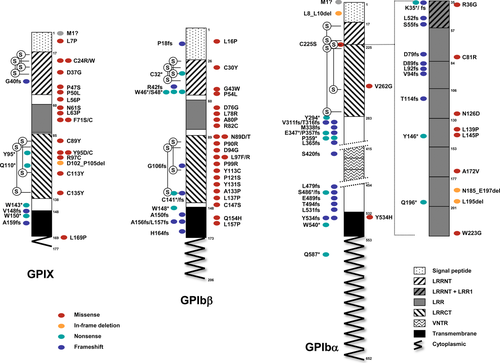
GP1BA
GPIBA spans 2.8 kb on chromosome 17p13.2 and consists of two exons. The entire open reading frame is located on exon 2. In the open reading frame, the presence of a 39 nucleotide variable number tandem repeats (VNTRs), which can be repeated up to four times [López et al., 1992], determines the synthesis of polypeptides ranging from 626 (D allele, 1 VNTR; NM_000173.3), 639 (C allele; 2 VNTR; NM_000173.4), 652 (B allele, 3 VNTR; NM_000173.5) to 665 amino acids (A allele, 4 VNTR; accession number not available). Upon removal of the signal peptide of 16 residues, the mature protein is composed of 636 residues (allele B is the transcript of reference in the Leiden Open Variation Database). According to Blenner et al. (2014), the N-terminus contains eight LRRs (LRR1 to LRR8) flanked by an N-terminus (N-cap or LRRNT), which also overlaps with LRR1, and a C-terminal (C-cap or LRRCT) disulfide looped capping segment (to match structure numbering to the mutational nomenclature, the initiation codon of the native GPIbα, GPIbβ, and GPIX protein is codon 1). The N-cap-LRR-C-cap domain is followed by a short anionic region containing three sulfated tyrosines (292/294/295) and an extended O-glycosylated region. At the junction of the extracellular and transmembrane domains, two cysteines (Cys500 and Cys501) form disulfide interchain bonds with Cys147 of GPIbβ. The cytoplasmic domain interacts with filamins A, B, C, and all the 14–3–3 isotypes [Du et al., 1996; Andrews et al., 1998; Xu et al., 1998; Mangin et al., 2009].
Forty-five different mutations have been identified in this gene (Table 1; Fig. 1). In addition to three in frame alterations, p.Leu8_Leu10del (in BSS3), p.Asn185_Glu197delinsLys (in BSS57), and p.Leu195del (in BSS8, BSS9, and BSS17), 10 are missense mutations mainly spread through almost all the LRRs (Table 1; Fig. 1). Of the 10 nonsense and 21 frameshift mutations, all are located in the extracellular domain except for p.Tyr534Cysfs*82, p.Tyr534Leufs*83, and p.Trp540*, which hit the transmembrane domain, and for p.Gln587*, which truncates the cytoplasmic domain. One novel mutation (c.1A>C) destroys the start codon, whose effect on protein synthesis has not been determined.
GP1BB
The GP1BB gene is localized on 22q11.21 and has two exons, both contributing to the 206 residues of the open reading frame. The 25 N-terminal amino acids represent the signal peptide. In the mature protein, the extracellular region is characterized by a single LRR domain flanked by the LRRNT and LRRCT regions, both containing disulfide bonds [Li and Emsley, 2013].
The 38 distinct point mutations identified in this gene are mainly missense alterations (N = 23; Table 2; Fig. 1). They mainly affect the extracellular regions, including the signal peptide (N = 1), LRRNT (N = 3), LRR (N = 4), or LRRCT (N = 12). There are also nonsense (N = 6) and frameshift (N = 8) mutations within the extracellular or transmembrane domains. No mutation has been identified so far in the short cytoplasmic tail. The last point mutation is g.-160C>G, which affects the promoter region [Ludlow et al., 1996]. It is one rare example of nucleotide substitutions that alter consensus binding sites for transcription factors (see below).
| Order | GP1BB cDNA Mutation (NM_000407.4)a | Number of alleles | Predicted amino acid change in GPIbβ (NP_000398.1) | Variant names in mature proteinb | Mutation type | References |
|---|---|---|---|---|---|---|
| 1 | g.-160C>G | 1 | Reduced protein level | Regulatory (GATA1 binding site disruption) | Ludlow et al. (1996) | |
| 2 | c.47T>C | 2 | p.Leu16Pro | Missense | BSS Consortium | |
| 3 | c.53_65del | 5 | p.Pro18Argfs*23 | Frameshift | Watanabe et al. (2003); Strassel et al. (2004); BSS Consortium | |
| 4 | c.89G>A | 2 | p.Cys30Tyr | Cys5Tyr | Missense | Gonzalez-Manchon et al. (2003) |
| 5 | c.96C>A | 4 | p.Cys32* | Cys7* | Nonsense | Ali et al. (2013) |
| 6 | c.124_145del | 20 | p.Arg42Cysfs*14 | Arg17Cysfs*14 | Frameshift | Sumitha et al. (2011); BSS Consortium |
| 7 | c.127G>T | 2 | p.Gly43Trp | Gly18Trp | Missense | BSS Consortium |
| 8 | c.137G>A | 2 | p.Trp46* | Trp21* | Nonsense | Moran et al. (2000) |
| 9 | c.138G>A | 2 | p.Trp46* | Trp21* | Nonsense | Ali et al. (2014) |
| 10 | c.143C>A | 5 | p.Ser48* | Ser23* | Nonsense | Hadjkacem et al. (2009); Hadjkacem et al. (2010) |
| 11 | c.161C>T | 1 | p.Pro54Leu | Pro29Leu | Missense | Hillmann et al. (2002); Nurden et al. (2003) |
| 12 | c.227A>G | 1 | p.Asp76Gly | Asp51Gly | Missense | Hadjkacem et al. (2010) |
| 13 | c.233T>G | 4 | p.Leu78Arg | Leu53Arg | Missense | BSS Consortium |
| 14 | c.238G>C | 1 | p.Ala80Pro | Ala55Pro | Missense | Hadjkacem et al. (2010) |
| 15 | c.244C>T | 1 | p.Arg82Cys | Arg57Cys | Missense | Sato et al. (2014) |
| 16 | c.265A>G | 16 | p.Asn89Asp | Asn64Asp | Missense | BSS Consortium |
| 17 | c.266A>C | 2 | p.Asn89Thr | Asn64Thr | Missense | Strassel et al. (2003) |
| 18 | c.269C>G | 2 | p.Pro90Arg | Pro65Arg | Missense | Sumitha et al. (2011) |
| 19 | c.281A>G | 2 | p.Asp94Gly | Asp69Gly | Missense | BSS Consortium |
| 20 | c.289C>T | 1 | p.Leu97Phe | Leu72Phe | Missense | Kunishima et al. (2013a) |
| 21 | c.290T>G | 2 | p.Leu97Arg | Leu72Arg | Missense | BSS Consortium |
| 22 | c.296C>G | 2 | p.Pro99Arg | Pro74Arg | Missense | Kunishima et al. (2000) |
| 23 | c.315del | 1 | p.Gly106Alafs*87 | Gly81Alafs*87 | Frameshift | Kenny et al. (1999) |
| 24 | c.338A>G | 4 | p.Tyr113Cys | Tyr88Cys | Missense | Kunishima et al. (1997); Kurokawa et al. (2001); Sato et al. (2014); BSS Consortium |
| 25 | c.361C>T | 1 | p.Pro121Ser | Pro96Ser | Missense | Tang et al. (2004) |
| 26 | c.392A>C | 2 | p.Tyr131Ser | Tyr106Ser | Missense | BSS Consortium |
| 27 | c.397G>C | 1 | p.Ala133Pro | Ala108Pro | Missense | Kunishima et al. (1997) |
| 28 | c.410T>C | 1 | p.Leu137Pro | Leu112Pro | Missense | BSS Consortium |
| 29 | c.418_419dup | 2 | p.Cys141Leufs*53 | Cys116Leufs*53 | Frameshift | BSS Consortium |
| 30 | c.423C>A | 4 | p.Cys141* | Cys116* | Nonsense | Mahfouz et al. (2012); BSS Consortium |
| 31 | c.439T>A | 1 | p.Cys147Ser | Cys122Ser | Missense | Kunishima et al. (2006b) |
| 32 | c.443G>A | 3 | p.Trp148* | Trp123* | Nonsense | Kunishima et al. (2002); Kunishima et al. (2013a) |
| 33 | c.448del | 1 | p.Ala150Argfs*43 | Ala125Argfs*43 | Frameshift | Kunishima et al. (2006b) |
| 34 | c.462G>C | 2 | p.Gln154His | Gln129His | Missense | Sumitha et al. (2011) |
| 35 | c.466dup | 2 | p.Ala156Glyfs*153 | Ala131Glyfs*153 | Frameshift | Strassel et al. (2006) |
| 36 | c.470T>C | 3 | p.Leu157Pro | Leu132Pro | Missense | Sumitha et al. (2011); BSS Consortium |
| 37 | c.[470T>A;472_473del] | 2 | p.Leu157Glnfs*151 | Leu132Glnfs*151 | Frameshift | BSS Consortium |
| 38 | c.491dup | 2 | p.His164Glnfs*145 | His139Glnfs*145 | Frameshift | Savoia et al. (2011) |
| 39 | 22q11.2 deletion | 8 | Large genomic deletion | Budarf et al. (1995); Kenny et al. (1999); Hillmann et al. (2002); Nurden et al. (2003); Tang et al. (2004); Bartsch et al. (2011); Kunishima et al. (2013a) |
- a Nucleotide numbering reflects the GP1BB cDNA with +1 corresponding to the A of the ATG translation initiation codon in the reference sequence RefSeq NM_000407.4. Therefore, the initiation codon is codon 1. Novel variants are in bold.
- b To adhere to nomenclature from literature, mutations are named in the mature protein that derives from signal peptide removal of the 25 N-terminal amino acids of GPIbβ.
In seven BSS patients, alterations are also associated with microdeletions on 22q11.2, which includes GP1BB [Budarf et al., 1995; Kenny et al., 1999; Hillmann et al., 2002; Tang et al., 2004; Bartsch et al., 2011]. In addition to the clinical features of BSS, these patients usually have cardiac defects, dysmorphic facial features, thymic hypoplasia, velopharyngeal insufficiency, which are distinctive features of the DiGeorge syndrome (MIM #188400). However, in two of these patients, macrothrombocytopenia and bleeding tendency were the only clinical features, suggesting that microdeletions on 22q11.2 should be evaluated even in BSS individuals without any features of the DiGeorge syndrome [Kunishima et al., 2013a].
GP9
The GP9 gene is located on chromosome 3q21.3 and organized in three exons with exon 3 coding for the entire 177 amino acid sequence of GPIX. GPIX has a strong sequence homology with GPIbβ and a similar structure with a signal peptide of 16 amino acids, a single LRR flanked by the LRRNT and LRRCT regions in the extracellular domain, a single pass transmembrane domain and a very short eight residue intracellular domain (UniProt P14770).
In patients with defects in the GP9 gene, 28 different mutations have been detected (Table 3; Fig. 1). Eighteen are amino acid substitutions affecting the signal peptide, LRRNT, LRR, LRRCT, or the cytoplasmic region. There are also two short deletions, c.-4_7del that removes the first methionine residue, and c.305_313del, an in frame alteration of LRRCT. A few nonsense (N = 4) and frameshift (N = 3) mutations are due to nucleotide deletions or duplications. The only variant located outside the open reading frame is c.-90G>C, a homozygous substitution of uncertain significance due to the lack of functional studies.
| Order | GP9 cDNA mutation (NM_000174.3)a | Number of alleles | Predicted amino acid change in GPIX (NP_000165.1) | Variant names in mature proteinb | Mutation type | References |
|---|---|---|---|---|---|---|
| 1 | c.-90G>T | 2 | Uncertain significance | BSS Consortium | ||
| 2 | c.-4_7del | 2 | p.? | Start codon | Sandrock et al. (2010) | |
| 3 | c.20T>C | 2 | p.Leu7Pro | Missense | Lanza et al. (2002) | |
| 4 | c.70T>C | 32 | p.Cys24Arg | Cys8Arg | Missense | Rivera et al. (2001); Savoia et al. (2011); Sumitha et al. (2011); Ali et al. (2013); Ali et al. (2014); BSS Consortium |
| 5 | c.72T>G | 2 | p.Cys24Trp | Cys8Trp | Missense | Savoia et al. (2011) |
| 6 | c.110A>G | 1 | p.Asp37Gly | Asp21Gly | Missense | Wright et al. (1993) |
| 7 | c.119del | 2 | p.Gly40Alafs*43 | Gly24Alafs*43 | Frameshift | Sumitha et al. (2011) |
| 8 | c.139C>T | 2 | p.Pro47Ser | Pro31Ser | Missense | BSS Consortium |
| 9 | c.149C>T | 1 | p.Pro50Leu | Pro34Leu | Missense | BSS Consortium |
| 10 | c.167T>C | 2 | p.Leu56Pro | Leu40Pro | Missense | Noris et al. (1998) |
| 11 | c.182A>G | 64 | p.Asn61Ser | Asn45Ser | Missense | Wright et al. (1993); Clemetson et al. (1994); Donner et al. (1996); Koskela et al. (1999b); Vanhoorelbeke et al. (2001); Sach et al. (2003); Liang et al. (2005); Drouin et al. (2005); Dagistan and Kunishima (2007); Zieger et al. (2009); Rand et al. (2010); BSS Consortium |
| 12 | c.188T>C | 2 | p.Leu63Pro | Leu47Pro | Missense | Savoia et al. (2011) |
| 13 | c.212T>C | 18 | p.Phe71Ser | Phe55Ser | Missense | Noris et al. (1997); Suzuki et al. (1997); Suzuki et al. (1999); Afrasiabi et al. (2007); Savoia et al. (2011); Sumitha et al. (2011); BSS Consortium |
| 14 | c.212T>G | 4 | p.Phe71Cys | Phe55Cys | Missense | Sumitha et al. (2011) |
| 15 | c.266G>A | 11 | p.Cys89Tyr | Cys73Tyr | Missense | Noda et al. (1996); Gohda et al. (2007); BSS Consortium |
| 16 | c.283T>G | 2 | p.Tyr95Asp | Tyr79Asp | Missense | Ali et al. (2013) |
| 17 | c.284A>G | 2 | p.Tyr95Cys | Tyr79Cys | Missense | Savoia et al. (2011) |
| 18 | c.285T>G | 2 | p.Tyr95* | Tyr79* | Nonsense | Ali et al. (2014) |
| 19 | c.289C>T | 2 | p.Arg97Cys | Arg81Cys | Missense | BSS Consortium |
| 20 | c.305_313del | 1 | p.Asp102_Pro105delinsAla | Asp86_Pro89delinsAla | Small inframe deletion | Drouin et al. (2005) |
| 21 | c.328C>T | 2 | p.Gln110* | Gln94* | Nonsense | Sumitha et al. (2011) |
| 22 | c.338G>A | 2 | p.Cys113Tyr | Cys97Tyr | Missense | Kunishima et al. (1999) |
| 23 | c.404G>A | 2 | p.Cys135Tyr | Cys119Tyr | Missense | Ali et al. (2013) |
| 24 | c.429G>A | 12 | p.Trp143* | Trp127* | Nonsense | Noda et al. (1995); Iwanaga et al. (1998); Toyohama et al. (2003); Kunishima et al. (2006c) Takata et al. (2012) |
| 25 | c.437_474dup | 2 | p.Ala159Argfs*77 | Ala143Argfs*77 | Frameshift | Sumitha et al. (2011) |
| 26 | c.442dup | 4 | p.Val148Glyfs*67 | Val132Glyfs*67 | Frameshift | Savoia et al. (2011); BSS Consortium |
| 27 | c.450G>A | 2 | p.Trp150* | Trp134* | Nonsense | Xu et al. (2010) |
| 28 | c.506T>C | 1 | p.Leu169Pro | Leu169Pro | Missense | BSS Consortium |
- a Nucleotide numbering reflects the GP9 cDNA with +1 corresponding to the A of the ATG translation initiation codon in the reference sequence RefSeq NM_000174.3, respectively. Therefore, the initiation codon is codon 1. Novel variants are in bold.
- b To adhere to nomenclature from literature, mutations are named in the mature protein that derives from the signal peptide removal of the 16 N-terminal amino acids of GPIX.
Founder Effects
Many families are from the same geographical regions, such as India (n = 50), northern Europe (n = 41), Mediterranean Basin (n = 37), or Japan (n = 29) (Supp. Table S1). Within these groups, there are families carrying the same variants, suggesting that unique mutation events occurred on ancestral chromosomes.
Identified in eight GP1BA alleles, both c.673T>A (p.Cys225Ser) and c.1601_1603del are likely to be associated with distinct founder effects since they have been found in patients from the Iberian peninsula or South America, and from Germany or Finland, respectively. Similarly, the p.Leu196del amino acid deletion and the p.Ser486Ilefrs*12 frameshift mutation characterize Algerian and Japanese alleles, respectively.
The eight apparently unrelated families from Ile de La Réunion, a French island in the Indian Ocean, have the same GP1BB c.265A>G transition, leading to a p.Asn89Asp amino acid change in the GPIbβ subunit. The high frequency of this mutation suggested that these patients shared a common ancestor, as demonstrated by the reconstruction of a genealogy tree. Going back 10–14 generations, the authors were able to connect seven of the families to a common ancestor born in 1671 in India [Lanza et al, 2013]. In the same gene, there is evidence for founder effects of c.124_145del (p.Arg42Cysfs*14) in 10 Indian families, c.143C>A (p.Ser48*) in five alleles from Tunisia, and c.338A>G (p.Tyr113Cys) in four alleles from Japan (Table 2).
In the GP9 gene, five mutations account for 137 of the 184 (75%) GP9 alleles. The p.Asn61Ser missense mutation (64 alleles) is frequent in the European population as the result of at least one founder effect. A common haplotype was found in 14 out of 15 unrelated families from Northern Europe [Liang et al., 2005]. Another mutation, p.Cys24Arg (32 alleles) is relatively frequent in families from India and Pakistan. Two Japanese potential founder effects are likely behind the frequent p.Cys89Tyr and p.Trp143* mutation (11 and 12 alleles in the Kyushu and Okinawa area, respectively) [Kunishima et al., 2006c]. Finally, the p.Phe71Ser has been detected in 18 chromosomes of BSS patients. However, as they are from Iran, India, and Japan, it is likely that distinct mutational events have occurred at this locus.
Variants in Monoallelic BSS
In order to extend the spectrum of deleterious mutations affecting the GPIb-IX complex, we highlight five missense mutations associated with the monoallelic form of BSS [Savoia et al., 2001; Noris et al, 2012]. Of those in GP1BA, p.Asn57His, p.Tyr70Asp, and p.Leu73Phe have been identified in single families [Miller et al., 1992; Kunishima et al., 2006a; Vettore et al., 2008a].
In contrast, the p.Ala172Val allele is relatively frequent at least in Italy [Savoia et al., 2001]. In a recent study aimed at identifying this mutation in a large Italian cohort of 216 probands with macrothrombocytopenia, it was found in 42 apparently unrelated families, all sharing the same haplotype, suggesting that the mutation occurred in an ancestral chromosome [Noris et al., 2012]. Consistent with its relatively high frequency in the Italian population, p.Ala172Val was also identified in two biallelic BSS patients, one homozygous (BSS35) and the other compound heterozygous (BSS57) for the mutation [Ware et al., 1993; Margaglione et al., 1999]. Even in the patient carrying the homozygous p.Ala172Val mutation, both alleles have the same haplotype as that identified in the monoallelic BSS individuals, further supporting a founder effect for this mutation.
The fifth mutation affects the GP1BB gene in one family, substituting arginine at position 42 with a cysteine (p.Arg42Cys) [Kunishima et al., 2001]. It is likely that the number of patients with the monoallelic form of BSS will increase in the future as soon as individuals with mild macrothrombocytopenia are extensively screened for mutations of the GP1BA, GP1BB, and GP9 genes.
Variants in VWDP
Mutations in GPIBA responsible for the autosomal dominant VWDP (MIM #177820) complete the list of alterations affecting the GPIb-IX-V complex [Othman et al., 2011; Hamilton et al., 2011; Dreyfus et al., 2011; Enayat et al., 2012]. Of the six mutations identified so far, one is a rare in frame deletion of residues 462–470 (p.Pro462_Ser470) [Othman et al., 2005]. The other five are amino acid substitutions at residues 249 (known as Gly233Val and Gly233Ser in the mature GPIbα), 255 (known as Met239Val and Met239Ile), and 251 (p.Asp251Tyr) [Enayat et al., 2012]. They are gain of function mutations located in a beta-hairpin loop that increase the affinity of GPIbα for VWF. Therefore, in VWDP patients, platelets bind VWF and agglutinate spontaneously, leading to reduction of plasma VWF and thrombocytopenia for removal of platelets from circulation.
Databases
The mutations enlisted in this report have been deposited in the Leiden Open Variation Database (LOVD) at http://www.lovd.nl/GP1BA, http://www.lovd.nl/GP1BB, and http://www.lovd.nl/GP9 for the GP1BA, GP1BB, and GP9 genes, respectively.
BSS Consortium
Until the institution of the BSS Consortium and the collection of patients’ clinical and molecular data, only scarce information has been available, consisting of original studies reporting one or two patients, reviews, such as that maintained by Orphanet but last updated in 2006 (ORPHA274), or Websites that are no longer accessible. The BSS Consortium, which at present is constituted of research groups from 15 countries worldwide, has helped establish the most comprehensive database with clinical and molecular information of BSS patients (BSS Consortium database). Overall, the BSS Consortium database includes 161 patients from 132 unrelated families, all with ascertained mutations in one of the BSS genes.
Biological Relevance in Biallelic BSS
In platelets of BSS patients, the GPIb-IX-V complex is usually undetectable or expressed at very low levels, as reported in the largest case series of 13 BSS patients [Savoia et al., 2011]. Regardless of which of the GP1BA, GP1BB, or GP9 gene is mutated there is usually no assembly of the complex in the endoplasmic reticulum preventing its localization at the platelet membrane [Li and Emsley, 2013]. Disulfide bond association of GPIbα and GPIbβ in the extracellular domain close to the membrane, as well as interactions among the transmembrane domains of the GP subunits, are required for correct assembly of the complex. As a consequence, nonsense and frameshift mutations above the transmembrane domain of any GP are expected to generate unstable subunits that do not efficiently assemble with the other subunits. Consistent with this hypothesis, some mutant alleles, such as p.Glu347*, p.Trp359*, and p.Ser486* localized in the ectopic domain of GPIbα, are detectable in cell lysates but not on the platelet surface [Ware et al., 1990; Kunishima et al., 1994; Yamamoto et al., 2013]. The only truncated form known, at least to our knowledge, to be expressed at normal levels is p.Gln587*, which affects the cytoplasmic domain of GPIbα [Yamamoto et al., 2013]. Despite this, the mutant weakly binds VWF, suggesting that interaction with the actin cytoskeleton is fundamental for correct binding of VWF to its receptor.
Other mutations lead to abnormal progression of the proteins through the secretory pathway. This is the case of p.Leu8_Leu10del, p.Leu16Pro, and p.Leu7Pro in GP1BA, GP1BB, and GP9, respectively, which being located in the signal peptide are likely to result in an abnormal conformation and, hence, incorrect translocation of subunits into the endoplasmic reticulum or defective signal peptide cleavage [Lanza et al., 2002].
Finally, abnormal expression can also be due to microdeletions, such as those on chromosome 22q11.2, or mutations affecting transcription, RNA processing or translation. An example is g.-160C>G, a nucleotide substitution that disrupts a GATA-1 binding site decreasing the GP1BB promoter activity by 84% [Ludlow et al., 1996]. Another mutation, c.376A>G in GP1BA, may interfere with mRNA stability or processing since the GP1BA mRNA was not detectable in the megakaryocytes of a patient homozygous for this mutation [Vettore et al., 2011]. Finally, c.1A>C (GP1BA) and c.-4_7del (GP9), hitting the start codon and the Kozak sequence would impair initiation of the protein translational process [Sandrock et al., 2010].
More complex is the assessment of the potential pathogenicity of missense mutations, which are relatively common, accounting for 56% of the BSS alleles. Of note, they hit GPIbβ and GPIX more frequently than GPIbα. Although any explanation for this observation is speculative, we hypothesize that folding and stability of GPIbβ and GPIX are more sensitive to amino acid substitution than GPIbα. Compared with the nonsense and frameshift mutations, the effects of missense mutations are more difficult to predict unless functional studies support their deleterious role. As in vitro manipulation of patients’ hematopoietic cells is difficult, heterologous cells have been exploited to develop models for these studies. Chinese hamster ovary (CHO) or other cells have been cotransfected with the GPIbα, GPIbβ, and GPIX subunits to evaluate the effect of mutations on surface expression of mutant subunits. Confirming studies on patients’ platelets, expression of a series of mutations, such as p.Trp233Gly and p.Cys225Ser both in GPIbα, p.Tyr113Cys in GPIbβ or p.Leu7Pro in GPIX, produce unstable subunits [Gonzalez-Manchon et al., 2001; Kurokawa et al., 2001; Lanza et al., 2002; Rosenberg et al, 2007]. The p.Asn89Thr (GPIbβ) mutant is instead stable but retained in the endoplasmic reticulum, preventing surface expression of the complex [Strassel et al., 2003]. In other cases, for instance with p.Cys81Arg (GPIbα), the mutant GPs are stable and expressed on the surface but unable to bind VWF [Kenney et al., 1998]. Combined defects associating abnormal posttranslational processing and less efficient surface expression were observed for the p.Leu195del mutation in GPIbα [Ulsemer et al., 2001].
However, data from these cellular models are not always consistent with those obtained from patients’ cells. This is the case, for instance, of p.Leu145Pro (GPIbα), whose transient expression was associated with inhibition of surface expression of the receptor despite the fact that in platelets of a homozygous individual for the same mutation the expression was 40% of the normal values [Li et al., 1995]. Conversely, p.Cys147Ser (GPIbβ) was expressed in CHO cells but significantly reduced on the platelet surface [Kunishima et al., 2006b]. For these reasons, only a systematic approach combining in vitro and in vivo data will improve our knowledge on the pathogenetic role of mutations by determining their effects on expression and function of the GPIb-IX-V complex.
In the absence of functional studies, deleterious effects of the missense mutations can be predicted by bioinformatic tools using straightforward physical and comparative considerations. Alternatively, it is possible to determine the impact of the amino acid changes on the three-dimensional structure. Therefore, using the crystal structures of GPIbα (Protein Data Base accession name 1gwb), GPIbβ (3rfe), and GPIX (3rez) (to match the PDB numbering with that of mutation nomenclature we refer to the first methionine of the native proteins as residues +1), we mapped the novel missense mutations in the GPs. The majority of the reported mutations potentially cause destabilization of the protein fold and consequent degradation of the unfolded subunits, as detailed below.
GPIbα
In this subunit, the only two novel missense variants, p.Arg36Gly and p.Leu139Pro, are in the N-terminus (Fig. 2). As Leu139 is in a loop of the LRR4 preceded and followed by glycines, which define the backbone structure, its substitution to a proline is predicted to destabilize the fold. Less clear is the effect of the p.Arg36Gly. As it was identified in a patient (BSS3; Supp. Table S1) homozygous for another mutation (p.Leu8_leu10del), it could be a silent variant.
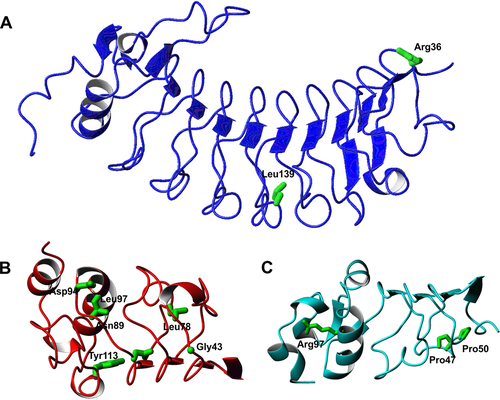
GPIbβ
Excluding a mutation in the signal peptide, seven novel missense mutations (p.Gly43Trp, p.Leu78Arg, p.Asn89Asp, p.Asp94Gly, p.Leu97Arg, p.Tyr131Ser, and p.Leu137Pro) have been identified in this GP. The Gly43 residue is in a tight loop and, although the tryptophan side chain would be exposed, the substitution should have a strong destabilizing effect because the glycine has positive backbone phi and psi values that are strongly disallowed for most of the other residues. Likewise, the hydrophobic Leu78 and Leu97 or the hydrophilic Asn89 amino acids are buried with the side chain pointing toward the hydrophobic core and for this reason their substitutions with charged residues are expected to be highly detrimental. In contrast, Asp94 is exposed and does not have any obvious structural role, although we cannot exclude interference with partner recognition. Finally, Tyr131Ser and Leu137Pro hit the LRRCT domain. The coordinates of this region are currently unavailable but the role of these mutations can be inferred by homology with the LRRCT of GPIbα where Tyr131 and Leu137 correspond to Asn239 and Gln248, respectively. Although Tyr131 would be buried and its substitution would introduce a distortion or cavity, Leu137 is an exposed residue and its mutation to a proline could in principle be better tolerated. The mutation could thus have an effect in distorting the structure and/or have an impact on the function.
GPIX
With the exclusion of p.Leu169Pro, which affects the transmembrane domain, three novel missense variants have been identified in GPIX, including two buried residues, Pro47 and Pro50. The p.Pro47Ser and p.Pro50Leu mutations are expected to introduce distortions in the strand and loop where they are located. It is more difficult to understand the consequence of Arg97Cys though a substitution to a cysteine might interfere with folding for generation of spurious sulfur bridges.
Biological Relevance in Monoallelic BSS
An open question concerns the molecular mechanisms responsible for the monoallelic form of BSS. As mentioned above, five missense mutations are associated with an autosomal dominant pattern of transmission despite the fact that haploinsufficiency is usually not associated with obvious defects in platelet size and number, as well as in GPIb-V-IX levels [Miller et al., 1992; Kunishima et al., 2001; Kunishima et al., 2006a; Vettore et al., 2008a; Noris et al., 2012]. Indeed, microdeletions on chromosome 22q11, which result in the loss of one GP1BB allele, are expected to reduce by 50% surface expression of the receptor without, in most cases, causing an abnormal platelet phenotype [Liang et al., 2007]. Moreover, most of the BSS obligate carriers do not have thrombocytopenia or platelet macrocytosis. Although the dominant nature of the missense mutations involved in this form suggests that they could exert a dominant negative effect, there is no current evidence in support of this hypothesis.
Excluding mutations identified in single families, the p.Ala172Val mutation in GPIbα has been reported in one homozygous (biallelic) individual and in a large group of heterozygous (monoallelic) patients [Ware et al., 1993; Noris et al., 2012]. In the only biallelic case, a functionally inactive GPIb-IX-V complex was expressed, indicating its relatively efficient assembly and transport to the membrane [Ware et al., 1993]. More consistent with the haploinsufficiency hypothesis, the p.Ala172Val mutation was associated with a significant reduction (40%) of its expression level in the monoalleic individuals [Noris et al., 2012], suggesting the need to investigate in more detail this aspect and the role of polymorphisms, such as c.-5T>C (GP1BA), that may interfere with the expression level of GPIb-IX-V [Afshar-Kharghan et al., 1999].
Clinical Relevance
In addition to collect data on mutations, the aim of the BSS Consortium was to increase knowledge on the clinical and laboratory features of the 161 patients enrolled in the database. Clinicians and biologists were asked to provide data on gender, age at diagnosis, previous misdiagnosis of immune thrombocytopenia (ITP) and consequent treatments, Word Health Organization (WHO) bleeding scores, platelet count and size, RIPA assay, and expression of the GPIb-IX-V subunits on the platelet surface. Being collected retrospectively, the information was not complete for all the patients; consequently, the features reported below have been evaluated on numerically different groups of individuals. Notwithstanding, we could derive useful information for our comprehension of the disease.
The mean age of patients at diagnosis was 16 years (range birth to 75 years) with a predominance of females (N = 100) over males (N = 60), indicating a possible diagnostic bias related to occurrence of menorrhagia. Almost 50% (64 out of 131) of the patients had been previously misdiagnosed as having ITP. Of them, almost all were treated with steroids and/or intravenous immunoglobulins and/or splenectomy. Of note, the spleen was removed in 14 cases.
Patients presented with a variable bleeding diathesis as measured by the WHO score. Considering grade 0 with no bleeding, grade 1 with petechiae, grade 2 with mild blood loss (no need for hospital admission or access to emergency); grade 3 with gross blood loss (hospital admission or access to emergency or iron therapy required or red cell transfusions after surgery or delivery) and grade 4 with debilitating blood loss (red blood cell transfusion required for spontaneous hemorrhages), at diagnosis more than 50% of the patients had a severe bleeding diathesis. Of 139 patients with a WHO score, 34 and 43 had grades 3 and 4, respectively. Only a few patients (N = 6) had no bleeding, 17 presented only with petechiae (grade 1) and the remaining 39 had mild bleeding tendency (grade 2). We did not observe any significant difference of the WHO score distribution when we compared patients with mutations in the same BSS gene, though there was a trend of severity within the GP1BB group (P = 0.02) that should be investigated further. Most of the BSS mutations are private and only in a few cases associated with founder effects. However, the number of patients sharing relatively common mutations is limited preventing us from carrying out any additional phenotype and genotype correlation study.
The mean platelet count was 51 × 109/L (range 5–175 × 109/L) as determined by different cell counters in 128 patients. Of note the highest platelet count was reported in BSS120, a patient with only one heterozygous mutation identified (see below). The mean platelet volume available in 84 individuals ranged from 9.3 to 27 fL (mean 14.8 fL). As determined in the peripheral blood smear of 42 patients, the mean platelet diameter was larger (4.8 μm, range 2.9–7.5 μm) than in controls (2.4 μm, range 1.9–3.4 μm). The presence of very large platelets should always lead to a suspicion of BSS, which can be differentiated from other macrothrombocytopenias, such as MYH9-related disease [Kunishima and Saito, 2010; Balduini et al., 2011b], using the RIPA assay and/or the expression of GPIb-IX-V. As for the WHO score, we did not find any correlation of platelet number or size with the disease-causing gene. This is consistent with the fact that expression of the GPIb-IX-V complex is severely affected regardless of which BSS gene is mutated.
Regarding platelet functional aspects, RIPA was absent or markedly reduced (<5% of controls) in 92 cases out of the 107 patients tested. In 14 patients, it ranged from 7% to 22% and in one patient (BSS61) it was 60% of controls. As the concentration of ristocetin used in laboratories was different, we could not compare the RIPA assays among patients, preventing us from studying any correlations between genotypes and platelet agglutination. Moreover, there was no significant difference in the mean platelet count or in the level of GPIb-IX-V expression between the group with partial agglutination and that without agglutination. The GPIbα subunit was not expressed on the platelet surface in most of the cases (103 patients tested). However, in some patients GPIbα was detected at 20%–40% (11 patients) and at >50% (10 patients) of the normal level. Of note, the affected individuals with only one mutation identified (BSS1, BSS61, and BSS120; Supp. Table S1) belong to this group of patients, suggesting that they could have the monoallelic form of BSS. Further investigations will ascertain this condition.
Diagnostic Relevance
Although BSS is a well-defined disease, a significant number of patients are not diagnosed early in life even though their macrothrombocytopenia is congenital. Indeed, the average age at diagnosis of patients from the BSS database is 16 years. Moreover, almost half of the patients have initially been misdiagnosed as having ITP and received undue medical therapies, such as steroids, or even splenectomy. Thus, recognizing BSS can be challenging, and in our opinion, this is due to the current assumption that BSS is always characterized by a severe bleeding diathesis. The increasing attention to patients with mild defects of hemostasis, as well as the systematic investigation of large case series of individuals with inherited thrombocytopenias, has shown that not all BSS patients have frequent and spontaneous hemorrhage [Savoia et al., 2011]. This conclusion is also supported by this study, as nearly half of the patients included in the BSS database have grade 0–2 of the WHO bleeding score, corresponding to no or mild bleeding episodes that do not require medical intervention.
Therefore, BSS should always be suspected in individuals with thrombocytopenia and large platelets regardless of their clinical phenotype. Unfortunately, electronic counters are not reliable in measuring platelet volume, as well as platelet count, in BSS because they fail to recognize very large platelets, underestimating both parameters [Noris et al., 2013]. For this reason, peripheral blood film examination is mandatory to ascertain the presence of platelets larger than half a red cell or even larger than erythrocytes. If this is the case, two simple analyses, RIPA assay and/or flow cytometry of the GPIb-IX-V subunits, are suitable to further support the diagnostic suspicion of BSS before molecular genetic confirmation [Noris et al., 2004; Balduini et al., 2013a]. Identification of a causative mutation is even more important for patients with the monoallelic form of BSS, where the RIPA assay and/or flow cytometry are not always sensitive enough to detect partially decreased amounts of the GPIb-IX-V complex [Noris et al., 2012].
Future Prospects
Improving the management of BSS patients is one of the most important aspects that should be addressed in the future. This could be achieved by defining the disease more accurately in terms not only of bleeding risk, prognosis, and therapies but also of diagnosis. The present BSS database is the start toward this goal but it remains limited and incomplete. The database will be open for a prospective comprehensive collection of data, such as functional in vitro tests, that will be more appropriate to provide further insights into this disease.
In term of diagnosis, BSS patients are appropriately recognized when applying the diagnostic algorithm proposed for inherited thrombocytopenias [Balduini and Savoia, 2012; Balduini et al., 2013a]. However, the diagnostic procedures will greatly benefit from the application of next generation sequencing platforms. Because of the simultaneous analysis of the different genes responsible for thrombocytopenias, mutations will be identified without any prescreening strategies as successfully achieved for other diseases [Albers et al., 2011; Kunishima et al., 2013b; De Rocco et al., 2014].
Regarding biological significance, defects of the GPIb-IX-V complex explain why BSS platelets are dysfunctional. On the contrary, the origin of thrombocytopenia is not well defined in BSS, although in vitro studies have recently suggested that the binding of VWF to GPIbα expressed on the megakaryocyte membrane is essential for proplatelet formation and subsequent platelet release [Balduini et al., 2009]. Consistent with this hypothesis, proplatelet formation was significantly impaired in patients’ megakaryocytes and this defect was more severe in the biallelic than in the monoallelic form [Balduini et al., 2009; Balduini et al., 2011a]. Further studies on megakaryopoiesis and development of novel therapeutic approaches, such as gene therapy and the generation of iPS cell lines, will greatly help our progress in understanding and treating BSS [Kanaji et al., 2012].
Acknowledgments
We are grateful to the following clinicians who gave access to patients: Claire Barro (Service d’Hématologie Hémostase Hémolyse, CHU de Grenoble, Grenoble, France), Sophie Bayart (Centre Régional de Traitement de l’Hémophilie, CHU de Rennes, Rennes, France), Françoise Boehlen (Unité d'hémostase, Hôpitaux Universitaires de Genève, Suisse), Jean-Claude Bordet (Unité d’Hémostase Clinique, Hôpital Edouard Herriot, Lyon, France), Bernadette Boval (AP-HP, Hôpital Lariboisière, Paris, France), Marie-Elisabeth Briquel (Centre de Traitement de l’Hémophilie, Laboratoire d’Hématologie, CHU de Nancy, Nancy, France), Jacques Caen (Fondation franco-chinoise pour la science et ses applications, Paris, France), Jacqueline Conard (Service d’Hématologie Biologique, Hôpital Universitaire Hôtel-Dieu, Paris, France), Yesim Dargaud (Unité d’Hémostase Clinique, Hôpital Edouard Herriot, Lyon, France), Marie Dreyfus (Service d’Hématologie Biologique, CRPP, Hôpital Bicêtre, Assistance Publique-Hôpitaux de Paris, Université Paris-Sud, Le Kremlin Bicêtre, France), Jordi Fontcuberta (Hemostasis and Thrombosis Unit, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain), Jalel Gargouri (Centre Régional de Transfusion Sanguine de Sfax, Sfax, Tunisia), Michel Hanss (Laboratoire d’Hématologie, CBPE, Hospices Civils de Lyon, Lyon, France), Marie-Line Jacquemont (Service de Génétique, Service de Néonatologie-Pôle femme-mère-enfant, GHSR-CHU la Réunion, Saint Pierre, France), Thomas Lecompte (Département d’Hématologie Clinique, Hôpitaux Universitaires de Genève, Suisse), Mercè López Grau (Institut Català de la Salut, Barcelona, Spain), Jose Mateo (Hemostasis and Thrombosis Unit, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain), Paquita Nurden (Plateforme Technologique d’Innovation Technologique, Hôpital Xavier Arnozan, Pessac, France), Corinne Pondarre (Hôpital Intercommunal, Créteil, France), Catherine Ternisien (Laboratoire d’Hématologie Biologique, CHU de Nantes, Nantes, France), Christine Trzeciak (Unité d’Hémostase Clinique, Hôpital Edouard Herriot, Lyon, France), Elisabeth Verdy (Laboratoire d’Hématologie Biologique, Hôpital Tenon, Paris, France), and Christine Vinciguerra (Service d’Hématologie Biologique, HCL, Hôpital Edouard Herriot, Lyon, France). We also thank Marie-Jeanne Baas for her technical support in genotyping.
Disclosure statement: All but one of the authors do not have any conflict of interest to declare. BZ discloses support from CSL Behring.




