Genetic Basis of Congenital Erythrocytosis: Mutation Update and Online Databases
CB, MJP, MFMM, and HC contributed equally to this work.
Communicated by John McVey
ABSTRACT
Congenital erythrocytosis (CE), or congenital polycythemia, represents a rare and heterogeneous clinical entity. It is caused by deregulated red blood cell production where erythrocyte overproduction results in elevated hemoglobin and hematocrit levels. Primary congenital familial erythrocytosis is associated with low erythropoietin (Epo) levels and results from mutations in the Epo receptor gene (EPOR). Secondary CE arises from conditions causing tissue hypoxia and results in increased Epo production. These include hemoglobin variants with increased affinity for oxygen (HBB, HBA mutations), decreased production of 2,3-bisphosphoglycerate due to BPGM mutations, or mutations in the genes involved in the hypoxia sensing pathway (VHL, EPAS1, and EGLN1). Depending on the affected gene, CE can be inherited either in an autosomal dominant or recessive mode, with sporadic cases arising de novo. Despite recent important discoveries in the molecular pathogenesis of CE, the molecular causes remain to be identified in about 70% of the patients. With the objective of collecting all the published and unpublished cases of CE the COST action MPN&MPNr-Euronet developed a comprehensive Internet-based database focusing on the registration of clinical history, hematological, biochemical, and molecular data (http://www.erythrocytosis.org/). In addition, unreported mutations are also curated in the corresponding Leiden Open Variation Database.
Background
Absolute erythrocytosis is defined by an increased red cell mass as reflected by hemoglobin and hematocrit values above the normal range. It can be either primary (intrinsic to the red cell) or secondary (extrinsic to the red cell) and can be acquired or arise from genetic alterations. Polycythemia Vera (PV) is the most common type of acquired primary erythrocytosis with somatic mutations in the JAK2 gene (MIM #147796) being responsible for almost 98% of the described cases (95% of the mutations involve exon 14 with the p.Val617Phe, whereas only 3% involve exon 12) [Cross, 2011]. Acquired secondary erythrocytosis can develop from various diseases, such as cardiac, pulmonary or renal, or conditions of external hypoxia due to smoking and CO poisoning [reviewed by McMullin, 2008; Patnaik and Tefferi, 2009].
Primary erythrocytosis, also known as Primary Familial Congenital Polycythemia (PFCP) is associated with a subnormal serum erythropoietin (Epo) level. It is caused by a molecular defect in the hematopoietic progenitor cells. Previously diagnosed cases have been found to possess germline gain-of-function mutations in the Epo receptor gene (EPOR; MIM #133171) [Huang et al., 2010 and Table 1]. In contrast, secondary congenital erythrocytosis (CE) is often characterized by inappropriately normal or raised serum Epo [van Maerken et al., 2004]. It can be a consequence of tissue hypoxia caused by hemoglobin variants with increased oxygen affinity due to mutations in the α- or β-globin genes (HBB, MIM #141900; HBA1, MIM #141800; HBA2, MIM #141850) [Percy et al., 2009] or defective bisphosphoglycerate mutase (BPGM; MIM #613896) leading to 2,3-bisphosphoglycerate (2,3-BPG) deficiency [Hoyer et al., 2004]. Secondary CE can also result from defects in components of the oxygen sensing pathway, mutations in the genes that encode the hypoxia-inducible factor 2α (HIF-2α; gene EPAS1; MIM #603349), HIF-prolyl hydroxylase 2 (PHD2, gene EGLN1; MIM #606425) and the von Hippel–Lindau tumor suppressor (pVHL; gene VHL; MIM #608537) have been reported [Lee and Percy, 2011] (Table 1).
| Disease group (MIM number) | Gene (MIM number) | Location | Inheritance | Protein | Protein function |
|---|---|---|---|---|---|
| ECYT1 (133100) | EPOR (133171) | 19p13.2 | Dominant (de novo cases described) | Epo receptor (EPOR) |
|
| ECYT2 (263400) | VHL (608537) | 3p25.3 | Recessive (dominant cases described) | Van Hippel Lindau (VHL) |
|
| ECYT3 (609820) | EGLN1 (606425) | 1q42.1 | Dominant | Prolyl hydroxylase domain-containing protein 2 (PHD2) |
|
| ECYT 4 (611783) | EPAS1 (603349) | 2p21 | Dominant | Hypoxia Inducible Factor 2α (HIF2α) |
|
| High oxygen affinity: Variant Hbs, BPGM | HBB, HBA | 11p15.4; 16p13.3 | Dominant | Hemoglobin (Hb) |
|
| BPGM | 7q33 | Recessive | Bisphosphoglycerate mutase |
|
Presently, over 160 mutations (including over 100 causing high affinity Hb variants with the remaining in either the EPOR gene or in genes involved in the oxygen sensing pathway) have been described associated with CE. However, in about 70% of CE patients a molecular cause was not identified. Thus, this condition is referred to as idiopathic erythrocytosis (IE) [Finazzi et al., 2006].
Mutation Nomenclature and Accession Numbers
The mutation nomenclature used in this update follows the guidelines indicated by Human Genome Variation Society (HGVS) [den Dunnen and Antonarakis, 2003]. Mutation descriptions have been checked using the Mutalyzer program (https://mutalyzer.nl/). Nucleotide numbering is based on GenBank reference sequences NM_000518.4 for HBB, NM_000558.3 for HBA1, NM_000517.4 for HBA2, NM_199186.2 for BPGM, NM_000121.3 for EPOR, NM_000551.3 for VHL, NM_022051.2 for EGLN1, NM_001430.4 for EPAS1.
The Oxygen-Sensing Pathway
Red blood cell production is regulated by the glycoprotein hormone Epo, which is mainly synthesized in interstitial tubular kidney cells. Epo production is increased under conditions of hypoxia due to anemia or decreased cellular oxygen tension.
Under normal oxygen tension, the alpha subunits of the hypoxia inducible factor (HIF-1, 2 and 3) are hydroxylated by the dioxygenase PHD (PHD1, 2, and 3) (Fig. 1). Hydroxylated HIF-α is then targeted by the VHL protein (pVHL) for ubiquitin-mediated degradation (the substrate recognition subunit of an E3 ubiquitine ligase complex that, in addition to pVHL, includes Elongin B, C, Rbx1, and Cul2). Under low-oxygen conditions, PHD proteins are unable to modify HIF-α allowing it to escape pVHL recognition and subsequent degradation. HIF-α then forms an active transcriptional complex with nuclear HIF-β (ARNT) and up-regulates expression of more than 200 genes, with one of the target genes being EPO. The major HIF-α isoform involved in the regulation of EPO is HIF-2α, which also regulates genes required for cell survival under low oxygen tension, such as heme synthesis (ALAS2), globin chains production (GATA1) and iron regulation (TRF2, TF) [Lok and Ponka, 1999; Wenger et al., 2005; Haase, 2010; Zhang et al., 2011; 2012]. Once Epo binds its cognate receptor there is initiation of an intra-cellular signaling cascade which inhibits apoptosis and simultaneously promotes the growth and differentiation of erythroid progenitors, thereby adjusting red blood cell mass to oxygen delivery requirements.
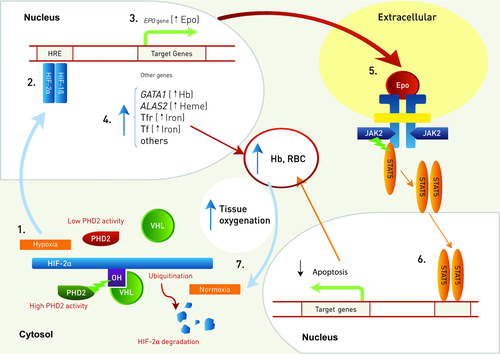
Recently, it has been shown that pVHL also form a heterodimeric E3 ligase complex with SOCS1 (suppressor of cytokine signaling 1) to target phosphorylated (p)JAK2 for proteasomal degradation [Russell et al., 2011]. The VHL p.Arg200Trp mutant has altered affinity for SOCS1 and fails to degrade pJAK2 that overactivates the Epo pathway and explains the Epo hypersensitivity of progenitors from Chuvash patients carring this mutation [Ang et al., 2002a, b].
Erythrocytosis Database
CE is a rare disease where clinical data on patients are sparse and information on clinical progression is not available. Although causative variants have already been identified in eight different genes, causal mutations remain to be identified in about 70% of the patients. A systematic repository will contribute to elucidate the pathogenic manifestations of this disease, and, therefore, the CE working group (WG3), established within the framework of the COST (European Cooperation in Science and Technology) action BM0902 MPN/MPNr-Euronet, developed an internet based erythrocytosis database [www. erythrocytosis.org; Bento et al., 2006]. An EU CE (ECE-C) consortium, which will later be extended to other non-EU countries, was created to include all the clinicians and scientists enrolled in the diagnosis of CE.
The erythrocytosis database aims to collect and share clinical, genetic, and outcome data on patients with absolute erythrocytosis, either idiopathic or with an already established molecular diagnosis. The registry of patients is anonymized and accessible only after validated registration. For report of clinical data, informed consent is obtained from patients. There is open access without registration to the tables summarizing mutations for each gene and information on the laboratories performing molecular studies on CE genes. Thus, www.erythrocytosis.org is a comprehensive and reliable genotype–phenotype database that will fulfill the needs of clinical practitioners, who require reliable markers for disease diagnosis and prognosis. This database will also assist researchers who aim to establish genotype–phenotype correlations for new mutations or genes discovered. Clinical and laboratory data collected at different times can be registered and compared. Statistical evaluations for all given parameters are possible. This database will provide a knowledge base as a prerequisite to develop guidelines and update diagnostic algorithms for new genetic testing and will enhance the understanding of the clinical and molecular mechanisms underlying erythrocytosis. It is not a repository for all CE mutations already previously registered in the literature. Complete information on such previously reported mutations can be obtained from the comprehensive Leiden open variation database [LOVD, http://www.lovd.nl/2.0/; Fokkema et al., 2011].
Current Status of the Database
At the time of submission only patients with mutations already identified have been registered in the database. In a second phase, data from patients with IE will also be included.
Of the 163 patients included in the database, 40 are carriers of a high affinity Hb variant, 27 are heterozygous for an EPOR mutation, 40 are homozygous or compound heterozygous for a VHL mutation, 15 are heterozygous for a mutation in VHL, 15 are heterozygous for an EPAS1, and 26 for an EGLN1 mutation (Table 2). Of these, three EPOR, two VHL and three EGLN1 mutations are reported here for the first time (Tables 3 and 4).
| Gene | Genotype | Patients (Total = 163) | Origin |
|---|---|---|---|
| HBB | Hb Barcelona/WT | 1 | Spain |
| Hb Coimbra/WT | 3 | Portugal, Sweden | |
| Hb Heathrow/WT | 1 | UK | |
| Hb Johnstown/WT | 1 | Spain | |
| Hb Linkoping/WT | 4 | Sweden | |
| Hb Malmo/WT | 2 | France, Sweden | |
| Hb Olympia/WT | 5 | Portugal, Sweden, UK | |
| Hb Pierre Bénite/WT | 1 | UK | |
| Hb San Diego/WT | 7 | Portugal, Sweden, Spain, UK | |
| Hb Santa Clara/WT | 1 | Ireland | |
| Hb Syracuse/WT | 1 | Spain | |
| Hb Trollhattan /WT | 1 | Sweden | |
| Hb Vanderbilte II/WT | 1 | Poland | |
| Hb Vila Real /WT | 3 | Portugal, Sweden | |
| Hb Yakima/WT | 7 | Italy, Portugal | |
| HBA1 | Hb Saratoga-Springs/WT | 1 | Portugal |
| EPOR | Pro380Ala/WT | 1 | Spain |
| Ser412*/WT | 1 | Spain | |
| Ser415Hisfs*18/WT | 1 | Russia | |
| Glu417*/WT | 1 | Italy | |
| Phe424*/WT | 3 | Italy | |
| Tyr426*/WT | 3 | Germany | |
| Arg437His/WT | 5 | Portugal | |
| Pro438Metfs*6 /WT | 4 | Spain | |
| Trp439* /WT | 4 | Germany, Spain, UK | |
| Asn487Ser/WT | 4 | The Netherlands, Portugal | |
| VHL | Glu10*/WT | 1 | France |
| Gly104Val / WT | 1 | Germany | |
| Lys196Glu/Lys196Glu | 1 | Portugal | |
| Gly144Arg/Pro81Ala | 1 | France | |
| Tyr175Cys/WT | 1 | Portugal | |
| Arg200Trp/Arg200Trp | 35 | Italy, Germany, Sweden, UK | |
| Arg200Trp/Gly144Arg | 1 | UK | |
| Arg200Trp/Leu188Val | 1 | UK | |
| Arg200Trp/Pro192Thr | 1 | UK | |
| Arg200Trp/WT | 12 | Italy, Germany, Sweden, UK, The Netherlands | |
| EPAS1/HIF2A | Phe374Tyr/WT | 1 | France |
| Ile533Val/WT | 2 | Italy | |
| Met535Thr/WT | 1 | UK | |
| Met535Val/WT | 1 | UK | |
| Gly537Trp/WT | 3 | UK | |
| Gly537Arg/WT | 5 | The Netherlands, Italy, Germany, UK | |
| Asp539Glu/WT | 1 | The Netherlands | |
| Phe540Leu/WT | 1 | UK | |
| EGLN1/PHD2 | Cys127Ser/WT | 8 | France, Portugal, Spain |
| Gln157His/WT | 5 | The Netherlands, France, Spain, Portugal | |
| Pro200Gln/WT | 1 | France | |
| Lys204Glu/WT | 1 | UK | |
| Asp254His/WT | 1 | France | |
| Gly285Arg/WT | 1 | UK | |
| Pro317Arg/WT | 1 | UK | |
| Trp334Arg/WT | 3 | France | |
| Val338Glyfs*18/WT | 1 | UK | |
| Arg371His/WT | 2 | UK, France | |
| His374Arg/WT | 1 | France | |
| Arg398*/WT | 1 | France |
| Nucleotide exchange | Exon | Protein effect | References |
|---|---|---|---|
| c.1138C>G | 8 | p.Pro380Ala | Almeida (this report) |
| c.1141_1142del | 8 | p.Pro381Glnfs*2 | Al-Sheikh et al. (2008) |
| c.1195G>T | 8 | p.Glu399* | Arcasoy et al. (2002) |
| c.1234delT | 8 | p.Ser412Argfs*41 | O'Rourke et al. (2011) |
| c.1235C>A | 8 | p.Ser412* | Bento et al. (2013a) |
| c.1242_1276del35 | 8 | p.Ser415Hisfs*18 | Minkov M (this report) |
| c.1249G>T | 8 | p.Glu417* | Perrotta et al. (2010) |
| c.1252_1255del | 8 | p.Gly418Profs*34 | Petersen et al. (2004) |
| c.1271_1272del | 8 | p.Phe424* | Al-Sheikh et al. (2008) |
| c.1273G>T | 8 | p.Glu425* | Kralovics and Prchal (2001) |
| c.1278C>G | 8 | p.Tyr426* | Kralovics et al. (1998); Rives et al. (2007) |
| c.1281dupT | 8 | p.Ile428Tyrfs*17 | Kralovics et al. (1997a) |
| c.1285del | 8 | p.Leu429Trpfs*24 | Al-Sheikh et al. (2008) |
| c.1288dupG | 8 | p.Asp430Glyfs*15 | Sokol et al. (1995) |
| c.1282_1289dup8 | 8 | p.Asp430Glufs*26 | Watowich et al. (1999) |
| c.1300C>T | 8 | p.Gln434* | Furukawa et al. (1997) |
| c.1299_1305del | 8 | p.Gln434Cysfs*17 | Arcasoy et al. (1997); Kravolics et al. (1997a) |
| c.1310G>A | 8 | p.Arg437His | Bento C (this report) |
| c.1311_1312del | 8 | p.Pro438Metfs*6 | Bento et al. (2013a) |
| c.1316G>A | 8 | p.Trp439* | la Chapelle et al. (1993); Percy et al. (1998) |
| c.1317G>A | 8 | Rives et al. (2007) | |
| c.1460A>G | 8 | p.Asn487Ser | Le Couedic et al. (1996); Al-Sheikh et al. (2008) |
| c.1462C>T | 8 | p.Pro488Ser | Sokol et al. (1994); Kralovics et al. (1997b) |
| Gene | Nucleotide exchange | Exon | Protein effect | References |
|---|---|---|---|---|
| VHL | c.28G>T | 1 | p.Glu10* | Vainchenker (this report) |
| c.235C>T | 1 | p.Arg79Cys | Bento et al. (2005) | |
| c.241C>G | 1 | p.Pro81Ala | Casadevall (this report) | |
| c.311G>T | 1 | p.Gly104Val | Cario et al. (2005) | |
| c.370A>G | 2 | p.Thr124Lys | Lorenzo et al. (2013) | |
| c.376G>A | 2 | p.Asp126Asn | Bond et al. (2011) | |
| c.376G>T | 2 | p.Asp126Tyr | Pastore et al. (2003a) | |
| c.388G>C | 2 | p.Val130Leu | Pastore et al. (2003a) | |
| c.413C>T | 2 | p.Pro138Leu | Lanikova et al. (2013) | |
| c.430G>A | 2 | p.Gly144Arg | Randi et al. (2005) | |
| c.524A>G | 3 | p.Tyr175Cys | Bento et al. (2005) | |
| c.548C>T | 3 | p.Ser183Leu | Bond et al. (2011) | |
| c.562C>G | 3 | p.Leu188Val | Pastore et al. (2003b) | |
| c.571C>T | 3 | p.His191Asp | Pastore et al. (2003b) | |
| c.574C>A | 3 | p.Pro192Thr | Percy et al. (2007) | |
| c.574C>T | 3 | p.Pro192Ser | Pastore et al. (2003b) | |
| c.586A>G | 3 | p.Lys196Glu | Bento et al. (2013b) | |
| c.598C>T | 3 | p.Arg200Trp | Ang et al. (2002a) | |
| EGLN1(PHD2) | c.12C>A | 1 | p.Asp4Glu# | Lorenzo et al. (2010) |
| c.380G>C | 1 | p.Cys127Ser# | Lorenzo et al. (2010) | |
| c.471G>C | 1 | p.Gln157His# | Albiero et al. (2011); Ladroue et al. (2012) | |
| c.599C>A | 1 | p.Pro200Gln | Ladroue et al. (2012) | |
| c.606delG | 1 | p.Met202Ilefs*72 | Al-Sheikh et al. (2008) | |
| c.609C>G | 1 | p.Asn203Lys | Albiero et al. (2012) | |
| c.610G>A | 1 | p.Lys204Glu | McMullin (this report) | |
| c.760G>C | 1 | p.Asp254His | Ladroue et al. (2012) | |
| c.840dupA | 1 | p.Arg281Thrfs*4 | Al-Sheikh et al. (2008) | |
| c.853G>C | 1 | p.Gly285Arg | McMullin (this report) | |
| c.872A>T | 1 | p.Lys291Ile | Albiero et al. (2012) | |
| c.950C>G | 2 | p.Pro317Arg | Percy et al. (2006) | |
| c.1000T>C | 2 | p.Trp334Arg | Bento et al. (2013b) | |
| c.1010dup | 3 | p.Val338Glyfs*18 | McMullin (this report) | |
| c.1112G>A | 3 | p.Arg371His | Percy et al. (2007); Ladroue et al. (2012) | |
| c.1121A>G | 3 | p.His374Arg | Ladroue et al. (2008) | |
| c.1129C>T | 3 | p.Gln377* | Al-Sheikh et al. (2008) | |
| c.1192C>T | 4 | p.Arg398* | Ladroue et al. (2012) | |
| c.1267A>G | 5 | p.Lys423Glu | Albiero et al., (2012) | |
| EPAS1(HIF2a) | c.1121T>A | 9 | p.Phe374Tyr | Lorenzo et al. (2012) |
| c.1597A>G | 12 | p.Ile533Val | Perrotta et al. (2013) | |
| c.1601C>T | 12 | p.Pro534Leu | Percy et al. (2008a) | |
| c.1605G>A | 12 | p.Met535Ile | Martini et al. (2008) | |
| c.1603A>G | 12 | p.Met535Val | Percy et al. (2012) | |
| c.1604T>C | 12 | p.Met535Thr | Percy et al. (2008a) | |
| c.1609G>A | 12 | p.Gly537Arg | Percy et al. (2008a); Gale et al. (2008) | |
| c.1609G> T | 12 | p.Gly537Trp | Percy et al. (2008a) | |
| c.1617C>G | 12 | p.Asp539Glu | van Wijk et al. (2010) | |
| c.1620C>G | 12 | p.Phe540Leu | Percy et al. (2012) |
High Oxygen Affinity Hemoglobin Variants
HBB, HBA2, and HBA1
The genes that encode the alpha (HBA) and beta (HBB) globin chains of hemoglobin are located on chromosomes 16 (locus 16p13.3) and 11(locus 11p15.4), respectively (Table 5).
| Gene | OMIM number | Chromosome locus | Number of exons | Transcript size (bp) | Number of amino acids | Molecular weight (kD) |
|---|---|---|---|---|---|---|
| HBA2 | 141850 | 16p13.3 | 3 | 605 | 142 | 15 |
| HBA1 | 141800 | 16p13.3 | 3 | 577 | 142 | 15 |
| HBB | 141900 | 11p15.4 | 3 | 754 | 147 | 16 |
| BPGM | 613896 | 7q33 | 3 | 1753 | 259 | 30 |
| EPOR | 133171 | 19p13.2 | 8 | 2056 | 508 | 66 |
| VHL | 608537 | 3p25.3 | 3 | 4700 | 213 and 160 | 30 and 18 |
| EGLN1 | 606425 | 1q42.1 | 5 | 7097 | 426 | 46 |
| EPAS1 | 603349 | 2p21 | 16 | 5160 | 870 | 96 |
The first described molecular defect associated with CE was in an 81-year-old man with hemoglobin of 19.9 g/dl who was seen at the Hematology Clinic in Johns Hopkins Hospital by Samuel Charache [Charache et al., 1966]. A thorough family study revealed 15 other members with increased hemoglobin levels, all of them showing an abnormal hemoglobin band on electrophoresis. In addition, the oxygen dissociation curve was significantly displaced to the left, indicating increased oxygen affinity of hemoglobin. Structural analysis established that there was an alpha-chain variant with a substitution of leucine for arginine at position 92 and this variant was subsequently called Hb Chesapeake. Meanwhile, more than 100 mutations have been described in the globin genes, with the majority being present at the HBB locus, that give rise to high oxygen affinity hemoglobin variants. Mutations are dominantly inherited and there are only a few cases reported arising de novo. Most of the high-affinity variants described thus far have substitutions at one of three regions that are crucial for hemoglobin function (1) the α1β2 interface; (2) the C-terminal end of the β-chain; (3) the 2,3-BPG binding site [reviewed in Thom et al., 2013].
All the described Hb variants are compiled in a complete and updated database, Hb Var (http://globin.bx.psu.edu/hbvar/menu.html). As only patients with new mutations are registered, it is not possible to estimate the real incidence and prevalence of the high affinity hemoglobin variants.
BPGM
The BPGM enzyme is encoded by the BPGM gene (MIM #613896; locus 7q33; Table 5) and is important in the regulation of hemoglobin's affinity for oxygen because it controls the level of 2,3-BPG, which is generated in the Rapoport-Luebering Shunt, a bypass of glycolysis. When 2,3-BPG is bound to hemoglobin it decreases hemoglobin's affinity for oxygen [Benesch et al., 1969]. Consequently, it allows the efficient oxygen delivery to the tissue. Deficiency of BPGM enzyme results in reduced synthesis of 2,3-BPG and red cell production is increased to compensate for less available oxygen.
Reported cases in the literature of erythrocytosis due to BPGM mutations are very rare with only three variants being described. Compound heterozygosity for a missense mutation c.268C>T (p.Arg90Cys) and a small deletion c.61delC (p.Arg21Valfs*28) was found in four members of the same family [Rosa et al. 1978; Lemarchande et al., 1992]. Hoyer et al. (2004) reported a patient homozygous for a missense mutation c.185G>A (p.Arg62Gln).
EpoR Signaling Pathway Variants
The EPOR gene (MIM #133171; locus 19p13.2) encodes the Epo receptor protein, which is a member of the cytokine receptor family. EPOR is composed of 8 coding exons (Table 5). The primary transcript is 2,056 bp long and encodes a protein of 508 amino acids (MW ∼ 66 kDa). Alternatively spliced forms of the Epo receptor have been identified, one of which has a truncated cytoplasmic domain. The shortened transcript is expressed at high levels in immature erythroid progenitor cells. In contrast, the expression of the full-length receptor increases as progenitor cells mature [Nakamura et al., 1992].
The first mutation reported in the EPOR gene was in a successful Finnish sportsman and 29 family members as described by de la Chapelle et al. (1993). Subsequently, more than 22 heterozygous variants have been found in patients with CE. All of these mutations are located in exon 8, which encodes the C-terminal negative regulatory domain of the protein. In total, 18 are frameshift mutations (due to small deletions or insertions) or nonsense mutations leading to cytoplasmic truncation of the receptor and loss of the C-terminal negative regulatory domain (Table 3). These mutations induce a gain-of-function and are associated with PFCP, which is also known as familial erythrocytosis type 1 (MIM #133100; Table 1). Of the remaining variants, four are missense mutations (c.1138C>G, c.1310G>A, c.1462C>T, c.1460A>G) in which the association with erythrocytosis has not yet been established.
Oxygen Sensing Pathway Variants
The VHL gene (MIM #608537) is located on chromosome 3 (locus 3p25.3) and spans 10 kb (Table 5). The VHL gene encodes a 4.7 kb mRNA translated from two translational initiation sites (+1 and +54). The larger protein consists of 213 amino acids (pVHL30 MW ∼ 30 kDa), whereas the shorter protein consists of 160 amino acids (pVHL18), both are functionally active [Iliopoulos et al., 1998]. pVHL is the substrate recognition subunit of an E3 ubiquitin ligase and interacts with elongin C and B and Cullin 2, in a complex referred as VCB-CUL2.
More than 400 germline mutations in the VHL gene that have been described as associated with the VHL disease (MIM #193300) [Nordstrom-O'Brien et al., 2010]. VHL disease is an autosomal dominantly inherited syndrome predisposing to the development of a panel of benign and malignant, highly vascularized tumors including hemangioblastomas, pheochromocytomas (or paragangliomas) and renal cell cancer, but VHL disease is outside the scope of this article. The association of CE VHL mutations with tumors will be discussed below in the section entitled “Risk of tumor development.”
The first loss-of-function mutation in the VHL gene associated with CE was found in the Chuvash autonomous republic of Russia where polycythemia is an endemic disorder. Chuvash polycythemia arose from a homozygous c.598C>T (p.Arg200Trp) VHL mutation [Ang et al., 2002a]. Later, homozygosity for the VHL c.598C>T mutation was also observed in several non-Chuvash patients, and notably in a large cohort on the island Ischia outside Italy. Both non-Chuvash and Italian patients had the same haplotype as the Chuvash cohort, suggesting a common ancestor, which suggested this mutation may be endemic in other parts of the world [Liu et al 2004; Perrotta et al., 2006]. Sixteen additional VHL variants associated with CE have also been described (Table 4). Four of them presented in the homozygous state, whereas the other cases were either compound heterozygotes or heterozygotes. Although VHL associated erythrocytosis (CE type 2; MIM #263400; Table 1) is considered a recessive disease some cases have been described where only one mutation was detected (see “Carriers of VHL mutations with CE” paragraph).
EGLN1 (PHD2)
There are three PHD isoenzymes (PHD1, PHD2, and PHD3), but PHD2 was found to be the key enzyme in catalyzing the prolyl hydroxylation of HIF-α, using oxygen as a cosubstrate [Kunz and Ibrahim, 2003; Percy et al., 2006]. PHD2 is encoded by the EGLN1 gene (MIM #606425), which is located on chromosome 1q42.1, and it is comprised of five exons (Table 5). Loss-of-function mutations in EGLN1 cause CE type 3 (MIM #609820) (Table 1) with autosomal-dominant inheritance. Mutations were first described by Percy et al. (2006) who identified a heterozygous c.950C>G transversion in two generations from one family (three family members). The mutation resulted in a p.Pro317Arg substitution in a highly conserved region of the protein. In vitro functional expression studies showed that the mutant protein had significantly decreased enzyme activity. Epo levels in the son and daughter were inappropriately normal even though the Hct was elevated, suggesting deregulated Epo production. Since then, more than 22 patients were found to carry 16 mutations in this gene, all of them heterozygous, and the mutations include: 12 missense, two nonsense, one small deletion, and one small duplication (Table 4). One of the missense mutations, c.471G>C (p.Gln157His) was found to coexist with the JAK2 p.Val617Phe somatic mutation, the latter probably being the primary cause of the disorder. Meanwhile, the c.471G>C mutation has been categorized as a SNP (rs61750991) with a frequency of around 2% in the normal population although some studies refer to a higher frequency [Astuti et al., 2011; Ladroue et al., 2012]. Interestingly, one particular mutation (p.His374Arg) has been described in a patient with an erythrocytosis associated with a recurrent paraganglioma [Ladroue et al., 2008].
EPAS1 (HIF-2α)
The HIF transcription factor has three isoforms, HIF-1α, HIF-2α, and HIF-3α. HIF-1α was first identified as a mediator of Epo induction in response to hypoxia in vitro [Wang et al., 1995], however HIF-2α was later confirmed as the primary transcription factor that induces Epo expression [Scortegagna et al., 2003; Warnecke et al., 2004; Hickey et al., 2007, Percy et al., 2008a]. The degradation of HIF-2α occurs via the hydroxylation of the residues Pro 405 and Pro 531.
The EPAS1 gene (MIM #603349), which encodes the transcription factor HIF-2α, is located on chromosome 2p21, contains 16 exons and spans at least 120 kb (Table 5). Gain-of-function mutations in exon 12 of EPAS1 cause familial erythrocytosis type 4 (MIM #611783) with autosomal-dominant inheritance. The first EPAS1 mutations found in erythrocytosis patients were the missense mutations p.Gly537Trp, p.Gly537Arg, p.Met535Val, and p.Pro534Leu [Percy et al. 2008a, 2008b, 2009]. Martini et al. (2008) described another pathogenic mutation, p.Met535Ile, and more recently, three additional missense mutations have been described, p.Asp539Glu, p.Met535Thr, p.Phe540Leu [van Wijk et al., 2010, Percy et al. 2012]. In total, 22 patients (eight sporadic cases and four families) heterozygous EPAS1 mutations have been reported (Table 4). Recently, Lorenzo et al. (2012) identified a germline heterozygous missense mutation c.1121T>A (p.Phe374Tyr) in exon 9 in a polycythemic patient who developed pheochromocytoma/paraganglioma. This variant was already reported in the NCBI dbSNP database (rs150797491; http://www.ncbi.nlm.nih.gov/SNP/) with a minor allele frequency of 0.1%. Somatic mutations associated with paraganglioma and erythrocytosis have been described [Zhuang et al., 2012; Yang et al., 2013] but they are not within the scope of this article.
Biological Significance
The identification of CE causal mutations in the HIF pathway genes has established the PHD2:HIF-2α:VHL pathway as the key regulator of adaptation and survival of both cells and the whole organism to hypoxia through Epo regulation [Lee and Percy, 2011].
The first insight into CE came from the studies on Chuvash polycythemia patients where the p.Arg200Trp mutation in the VHL gene led to an autosomal recessive form of erythrocytosis. The VHL p.Arg200Trp loss-of-function mutation results in diminished ubiquitination of the HIF transcription complexes and less proteasomal regulation in normoxia.
Further studies screening individuals with erythrocytosis for defects in HIF-1α, HIF-2α, and the three isofroms of PHD hydroxylases detected mutations in only PHD2 and HIF-2α genes [Percy et al., 2006; 2008a; 2008b]. These results indicated that PHD1 and PHD3 isoforms were unable to compensate for the loss of PHD2 function and there was no redundancy in the oxygen sensing pathway. Furthermore, the different isoforms of HIF and PHD exhibited different specific functions.
The HIF-1 transcription complex was described as the main regulator of Epo from binding studies and for a decade this was believed to be the case. However, the results from erythrocytosis studies caused a paradigm shift resulting in HIF-2α now being recognized as the main isoform that controls Epo. This was borne out by mice and RNA interference studies [Scortegagna et al., 2003; Warnecke et al., 2004]. It is now acknowledged that HIF-1α and HIF-2α regulate different target genes [reviewed by Mole and Ratcliffe, 2008].
Inherited mutations in HIF-2α are all located close to the site of prolyl hydroxylation at Pro531 and this region is crucial for the binding of PHD2 for hydroxylation and VHL for ubiquitination of HIF-2α [Furlow et al., 2009]. Functional analysis of a series of HIF-2α mutations has shown that in most cases the binding of both PHD2 and VHL is decreased, except for the p.Met535Val mutation, whereas VHL binding is retained. Thus, diminishing PHD2 binding alone is sufficient to cause impairment of the oxygen sensing pathway and dysregulation of Epo synthesis.
At the physiological level, VHL mutations may have more profound consequences, not just affecting hemopoiesis but also metabolism and exercise capacity, as both HIF-1α and HIF-2α proteins are stabilized in normoxia [Formenti et al., 2010]. Consequently, a broader range of target genes is upregulated in subjects carrying VHL mutations compared with those with HIF-2α mutations, further highlighting the differing functions of the HIF-α isoforms.
Clinical Significance of Mutation Identification
In CE patients, the elevated number of red blood cells and high hematocrit with a subsequent hyperviscosity, may lead to symptoms and signs ranging from headaches, dizziness, epistaxis, and exertional dyspnea to pruritus after bathing. Moreover, thrombotic and hemorrhagic events leading to premature morbidity and mortality have been reported. Hyperviscosity symptoms are effectively relieved by phlebotomy, but the increased risk of cardiovascular morbidity is not necessarily ameliorated by maintaining a normal hematocrit [McMullin et al., 2005; Finazzi et al., 2006].
Concerning the clinical presentation, the Chuvash cohort, homozygous for the VHL p.Arg200Trp mutation, has been most extensively studied. Homozygous patients were compared with a spouse control group and with age and sex matched community controls including VHL heterozygotes. Chuvash patients had reduced survival rates as compared with the control groups and presented a higher prevalence of arterial and venous thromboses and of hemorrhagic events [Gordeuk et al., 2006]. They also had a higher risk to develop venous varicosity. Patients with Chuvash polycythemia had lower blood pressures than heterozygote carriers whereas those had lower blood pressures than the controls [Gordeuk et al., 2004]. Cancer incidence in the Chuvash polycythemia cohort was not increased. There have been no reports on tumor development in patients with CE type 2 (VHL related), except for two cases of isolated hemangioblastoma [Woodward et al., 2007].
Cardiopulmonary physiology has been investigated in Chuvash polycythemia patients and compared with two control groups. Participants were studied at baseline and then subjected to hypoxia. Mild hypoxia induced a greater increase in ventilation in the Chuvash patients compared with the controls and they did not tolerate moderate hypoxia. They had abnormally high pulmonary artery pressures and hypoxia provoked a further abnormal rise. Physiological studies showed that Chuvash patients appeared to be in a situation characteristic of acclimatization to the hypoxia resulting from high altitude [Bushuev et al., 2006; Smith et al., 2006]. These patients should be regularly monitored for cardiopulmonary function.
Retrospective studies in the original Chuvash population failed to show significant effects of phlebotomy treatment and aspirin on the occurrence and severity of thromboembolic (and hemorrhagic) events [Gordeuk et al., 2004; Gordeuk et al., 2006]. Further studies are needed, as particularly in patients with pulmonary hypertension (PHT) discernable risks of phlebotomy treatment have to be calculated very carefully with regard to a possible negative influence of iron deficiency on PHT [Sable et al., 2012].
EGLN1 and EPAS1 mutations are mostly described in single case reports and only sparse clinical information is available. This includes a few thromboembolic events occurring even at young ages [Percy et al., 2008a]. PHT has also been described in individuals with the EPAS1 p.Gly537Arg mutation [Gale et al., 2008]. The underlying physiological changes are similar to those observed in patients with Chuvash polycythemia [Formenti et al., 2011]
Several cases with EGLN1 and EPAS1 mutations developed paraganglioma. This will be discussed in the section entitled “Risk of tumor development in patients with CE.”
Whereas the majority of adult patients with EPOR mutations had only mild symptoms some cases were reported to present with severe and even fatal clinical complications such as arterial hypertension, intracerebral hemorrhage, deep vein thrombosis, coronary disease, and myocardial infarction [Prchal et al, 1995; Sokol et al, 1995; Kralovics et al, 1997a; 1998;]. In the majority of patients, the disease could be controlled by antihypertensive treatment and by phlebotomies, either regularly performed aimed to maintain the hematocrit at an almost normal level or initiated only in the presence of hyperviscosity symptoms. Although a 40-year-old male patient had been phlebotomized regularly, it was reported that he died from myocardial infarction [Prchal and Sokol, 1996].
CE patients with high oxygen affinity hemoglobins usually are asymptomatic but hyperviscosity symptoms and thromboembolic episodes have been reported and related to the high hematocrit [Fairbanks et al, 1971; Weatherall et al., 1977]. In contrast to other CE types, phlebotomy cannot be recommended as general treatment of choice since erythrocytosis in these patients is primarily a requirement due to general tissue hypoxia. Therefore, phlebotomy treatment will be limited to single symptomatic events. In severe symptomatic cases, regular exchange transfusion has to be considered. Cases have been described showing unusual incidences of spontaneous abortion in female carriers caused by the lower oxygenation of the fetus resulting from either the alteration in the physiological oxygen gradient affinity between fetal and maternal blood or by placental infarction caused by the high viscosity of the mother's blood [Koller et al., 1980, Bento et al., 2000].
In conclusion, the raised hematocrit and increased viscosity associated with CE may lead to a number of clinical complications including increased thromboembolic events at young ages, but in the absence of good clinical data and follow up, it is difficult to obtain a clear picture of the clinical situation. At present, it is not possible to make unambiguous general treatment recommendations. However, the identification of the underlying genetic defect aids avoidance of possible pitfalls in the treatment (e.g., cytoreductive treatment for CE, or phlebotomy in hemoglobinopathies). A specified diagnosis also aids organization of adequate monitoring (e.g., for PHT in VHL and EPAS1 cases) and to counsel the patient.
It is advisable that patients document their family histories concerning all known cardiovascular events as well as causes of death and life spans of relatives. Bleeding tendencies should also be noted and further investigated when indicated. In the absence of clinical data, phlebotomy should be considered in CE mostly in special situations such as relief of possible hyperviscosity symptoms and as secondary prophylaxis if a thromboembolic event has occurred [Finazzi et al., 2006].
Our database will attempt to gather information on treatments and outcomes in pregnancies, with special risks for mother and fetus in CE, to aid future counseling. Genetic counseling is important and contraceptive methods with low thromboembolic risk should be chosen when indicated.
Carriers of VHL Mutations with CE
Although CE type 2 is considered a recessive disease the occurrence of individuals heterozygous for VHL mutations with erythrocytosis has been described. Eleven independent cases of erythrocytosis patients heterozygous for VHL mutations were reported in the literature. In contrast, other carriers of the same VHL mutation exhibited normal hematological parameters. In the case of heterozygous carriers with erythrocytosis the presence of other VHL mutations or a VHL null allele or deletion that could affect the apparently wild-type VHL allele had been ruled out and no mutations in the other genes associated with CE were found [Bento et al., 2005; Cario et al., 2005; Pastore et al., 2003a, b; Percy et al., 2003; Percy et al., 2007; Perrotta et al., 2006; Randi et al., 2005]. In addition 15 patients with erythrocytosis but only heterozygous VHL mutation are registered in the erythrocytosis database (Table 2). Interestingly, in two of these cases, the wild type VHL allele showed an unexplained low RNA expression (data not shown).
Risk of Tumor Development in Patients with CE
The risk of patients with germline mutations in the HIF pathway (VHL, EGLN1, EPAS1) to develop tumors requires consideration, knowing the crucial role that hypoxia plays during tumorigenesis.
In inherited cancer diseases associated with the loss of tumor suppressor genes, the mechanisms of tumor development imply that the first event leads to a loss of function sufficient to induce a selective pressure, which results in the loss of the second allele. In the von Hippel Lindau disease germline heterozygous mutations in the VHL gene predispose to the development of multiple tumors which have subsequently lost the remaining wild type allele.
Concerning the VHL p.Arg200Trp mutation, the heterozygous carriers have no elevated risk to develop malignant tumors and parents of the patients with Chuvash polycythemia are normally healthy. Two rare cases of erythrocytosis with later hemangioblastomas have been described [Woodward et al., 2007]. It is possible to hypothesize that the p.Arg200Trp mutation is not sufficiently deleterious to allow a selective pressure and initiate tumorigenesis. Indeed, the VHL p.Arg200Trp mutation is considered as less severe than classical VHL mutants [Ang et al., 2002a; Rathmell et al., 2004]. Therefore, the risk of patients carrying the p.Arg200Trp mutation to develop malignant tumors can be estimated as very limited. However, stringent follow up is recommended for patient carrying other VHL mutations in which the severity of the loss of function has not been precisely determined. Indeed, a patient, compound heterozygous for the mutations VHL p.Arg200Trp and p.Val130Leu, developed an erythrocytosis and a pheochromocytoma, (a tumor of the VHL disease spectrum) [Capodimonti et al., 2012].
The VHL mutations associated with CE are all missense mutations, except for one truncating mutation, VHL p.Glu10X. This particular mutation is located between the two translation initiation codons and has the capacity to produce a pVHL19 isoform still able to regulate HIF.
Regarding the other genes of the HIF pathway mutated in erythrocytosis (EGLN1, EPAS1), the evaluation of the risk of developing tumors is more complicated because of the restricted number of described cases and the closely related isoforms (PHD1, 3, and HIF-1α), which are theoretically able to compensate for the dysregulation of HIF. Nonetheless, the follow up of the patients carrying such mutations is highly recommended. Indeed, paragangliomas (extra-adrenal pheochromocytoma) have already been described in patients carrying a particular EGLN1 mutation (p.His374Arg) and an EPAS1 mutation (p.Phe374Tyr). Study of the EGLN1 p.His374Arg mutation indicated there was severe loss of function compared with mutations associated with erythrocytosis [Ladroue et al., 2012]. Furthermore, examination of the paraganglioma from the EGLN1 p.His374Arg patient indicated a loss of the remaining EGLN1 wild type allele in the tumor [Ladroue et al., 2008]. EGLN1 is therefore a potential tumor suppressor gene as already been suggested [Kato et al., 2006; Lee, 2008].
Regarding EPAS1 mutations, it should be noted that none of the germline mutations identified in patients with CE target the main hydroxylated prolines (Pro405 and Pro531). Surprisingly, mutations targeting the Pro531 have been described, but only at the somatic level in four cases of pheochromocytomas/paragangliomas [Favier et al., 2012; Toledo et al., 2013]. These observations suggest that total and excessive activation of HIF-2α may be necessary for tumorigenesis.
Performing accurate comparative functional studies of the HIF pathway mutants is required in order to evaluate the risk of the carriers to develop tumors.
Diagnostic Strategies
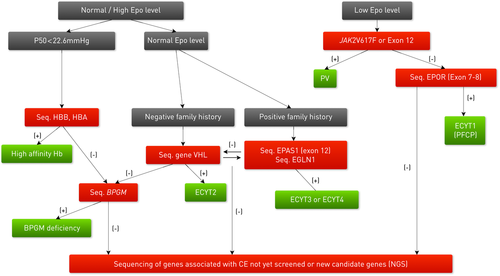
When diagnosing a patient with erythrocytosis it is important to exclude acquired secondary (pulmonary, renal, and cardiac) or acquired primary (PV due to JAK2 mutations) causes. The family history and the determination of serum Epo levels are very useful in the decision regarding which molecular tests should be performed first. If available, determination of p50 (percentage at which Hb is half saturated with oxygen) can be helpful in establishing the presence of a hemoglobin variant with high oxygen affinity. Sequencing of the candidate genes is mandatory for a definitive diagnosis. Information on the laboratories performing molecular studies on CE genes is available at www.erythrocytosis.org. Based upon the serum Epo level and familial data, it is possible to establish an algorithm to decide which genes should be sequenced in each case of erythrocytosis (Fig. 2). A comparable algorithm with a specific focus on diagnostics in affected children and adolescents has been published recently [Cario et al., 2013].
Future Prospects
Significant advances have been made during the past decade in the CE field with the identification of causal mutations in the EPOR gene and the elucidation of the genes directly implicated in the hypoxia sensing mechanism (VHL, EGLN1, and EPAS1). Presently, over 160 mutations have been associated with CE but despite this about 70% of the CE patients, and 12–35% of PFCP cases, still remain unexplained at the molecular level. The absence of erythrocytosis in a child heterozygous for a deleterious nonsense EPOR mutation [Kralovics et al., 1998] and the observation of individuals heterozygous for VHL mutations with erythrocytosis confirm that other genes or epistatic factors must be implicated in the clinical manifestation of CE. The incoming use of next-generation sequencing is expected to further expand the number of genes involved in CE. With the implementation of the internet-based erythrocytosis database, it is hoped that it will allow the establishment of clinical and genotype–phenotype correlations in larger groups of individuals.
Acknowledgments
We thank all the members of the European Congenital Erythrocytosis Consortium (clinicians, research scientists and diagnostic laboratories) who published their patient data on the CE database. The ECE-C members are listed as follows: Aurelie Chauveau, Anne-Paule Gimenez-Roqueplo, Brigitte Bressac-de-Paillerets, Didem Altindirek, Felipe Lorenzo, Frederic Lambert, Fulvio Della Ragione, Harlev Dan, Milen Minkov, Silverio Perrotta, Sophie Gad-Lapiteau, Susana Rives, Sylvie Hermouet, Ana Catarina Oliveira, Betty Gardie, Britta Landin, Cédric Rossi, Celeste Bento, Cristina Fraga, Drorit Neuman, François Girodon, Gennadiy Taradin, Guillermo Martin-Nuñez, Helena Almeida, Helena Vitória, Herrera Diaz Aguado, Holger Cario, Jan Palmblad, Julia Vidán, Luis Relvas, Maria Astrom, Maria Leticia Ribeiro, Maria Luigi Larocca, Maria Luigia Randi, Maria Pedro Silveira, Mary Frances McMullin, Melanie Percy, Mor Gross, Nicole Casadevall, Ricardo Marques da Costa, Stéphane Richard, Richard van Wijk, Soheir Beshara, Susanne Schnittger, Tabita Magalhães Maia, Tal Ben-Ami, Valérie Ugo, and William Vainchenker.




