Mutations in the C-Terminal Domain of ColQ in Endplate Acetylcholinesterase Deficiency Compromise ColQ–MuSK Interaction
Contract grant sponsors: Ministry of Education, Culture, Sports, Science, and Technology of Japan, and the Ministry of Health, Labor, and Welfare of Japan.
Communicated by Lars Bertram
ABSTRACT
Acetylcholinesterase (AChE) at the neuromuscular junction (NMJ) is mostly composed of an asymmetric form in which three tetramers of catalytic AChE subunits are linked to a triple helical collagen Q (ColQ). Mutations in COLQ cause endplate AChE deficiency. We report three patients with endplate AChE deficiency with five recessive COLQ mutations. Sedimentation profiles showed that p.Val322Asp and p.Arg227X, but not p.Cys444Tyr, p.Asp447His, or p.Arg452Cys, inhibit formation of triple helical ColQ. In vitro overlay of mutant ColQ-tailed AChE on muscle sections of Colq−/− mice revealed that p.Cys444Tyr, p.Asp447His, and p.Arg452Cys in the C-terminal domain (CTD) abrogate anchoring ColQ-tailed AChE to the NMJ. In vitro plate-binding assay similarly demonstrated that the three mutants inhibit binding of ColQ-tailed AChE to MuSK. We also confirmed the pathogenicity of p.Asp447His by treating Colq−/− mice with adeno-associated virus serotype 8 carrying mutant COLQ-p.Asp447His. The treated mice showed no improvement in motor functions and no anchoring of ColQ-tailed AChE at the NMJ. Electroporation of mutant COLQ harboring p.Cys444Tyr, p.Asp447His, and p.Arg452Cys into anterior tibial muscles of Colq−/− mice similarly failed to anchor ColQ-tailed AChE at the NMJ. We proved that the missense mutations in ColQ–CTD cause endplate AChE deficiency by compromising ColQ–MuSK interaction at the NMJ.
Introduction
Congenital myasthenic syndromes (CMS) are clinically and genetically heterogeneous inherited disorders characterized by neuromuscular transmission defect caused by mutations affecting proteins expressed at the neuromuscular junction (NMJ) [Engel et al., 2003]. The synaptic type of CMS is caused by the absence of the asymmetric form of acetylcholinesterase (AChE) from the endplate [Engel et al., 1977]. Endplate AChE deficiency is characterized by generalized muscle weakness, fatigue, scoliosis, minor facial abnormalities, and episodes of respiratory distress [Mihaylova et al., 2008]. In the absence of AChE, the duration of the endplate currents are prolonged, so that it outlasts the refractory period of the skeletal muscle sodium channel, which in turn evokes repetitive compound muscle action potentials (CMAPs). Four mechanisms lead to defective neuromuscular signal transmission in endplate AChE deficiency [Engel et al., 1977; Ohno et al., 1998]. First, the prolonged endplate currents lead to overload of Ca2+ ions at the postsynaptic sarcoplasm, which causes endplate myopathy with loss of acetylcholine receptor (AChR). Second, excessive ACh at the synaptic space causes desensitization of AChR. Third, repeated opening of AChR causes staircase summation of endplate potentials, which depolarizes the resting membrane potential and makes the muscle sodium channel irresponsive to an endplate potential. Fourth, lack of ColQ at the NMJ diminishes the amount of membrane-bound MuSK and reduces phosphorylation of the AChR β subunit, which compromises AChR clustering [Sigoillot et al., 2010]. Lack of effects of cholinesterase inhibitors, or even worsening of the symptoms with them, in patients with endplate AChE deficiency suggests that lack of AChE rather than lack of AChR is a key underlying mechanism leading to myasthenic symptoms.
Endplate AChE deficiency is not caused by mutations in the ACHE gene (NM_000665.3; MIM #100740) encoding the catalytic subunit but is caused by recessive mutations in the COLQ gene (NM_005677.3; MIM #603033) encoding the collagenic tail subunit [Ohno et al., 1998]. There are two major types of AChE in the skeletal muscle: (1) globular forms consisting of monomers (G1), dimmers (G2), or tetramers (G4) of the T isoform of the catalytic subunit (AChET, NP_000656.1), and (2) asymmetric forms consisting of one, two, or three homotetramers (A4, A8 and A12, respectively) of AChET attached to a triple-stranded collagenic tail (ColQ) [Massoulie, 2002], which are hereafter called ColQ-tailed AChE species. ColQ carries three domains: (1) an N-terminal proline-rich attachment domain that organizes the catalytic AChE subunits into a tetramer, (2) a collagen domain that forms a triple helix and contains heparin-binding domains, and (3) a C-terminal domain (CTD) enriched in charged residues and cysteines.
In previous studies, COLQ mutations were divided into four classes according to their positions in ColQ and their effects on the expression of AChE species in COS cells: (1) N-terminal mutations that prevent association of AChET with ColQ, (2) truncation mutations in the collagen domain that prevent the formation of ColQ-tailed AChE, (3) CTD missense mutations that prevent triple helical formation of ColQ, and (4) CTD mutations that do not abolish formation of ColQ-tailed AChE but affect anchoring of ColQ at the NMJ [Ohno et al., 2000]. We previously reported that p.Asp342Glu, p.Arg410Pro, and p.Arg410Glu, but not p.Cys444Tyr, at the CTD of ColQ compromise anchoring ColQ-tailed AChE to heterologous frog muscle sections [Kimbell et al., 2004]. We, however, did not show how the mutations affect anchoring of ColQ to the synaptic basal lamina. We also failed to prove pathogenicity of p.Cys444Tyr in the heterologous anchoring experiment. Two binding partners for anchoring ColQ-tailed AChE at the synaptic basal lamina have been reported to date: (1) the heparan sulfate proteoglycans such as perlecan, which bind to two heparan sulfate proteoglycan binding domains in the ColQ collagen domain [Arikawa-Hirasawa et al., 2002], and (2) the extracellular domain of MuSK, a muscle-specific receptor tyrosine kinase, on the postsynaptic membrane, which binds to the CTD of ColQ [Cartaud et al., 2004]. We recently demonstrated that anti-MuSK autoantibodies in patients with myasthenia gravis block binding of ColQ to MuSK [Kawakami et al., 2011]. We also reported that intravenous or intramuscular administration of adeno-associated virus serotype 8 (AAV8) carrying human COLQ efficiently anchors ColQ-tailed AChE at the NMJ [Ito et al., 2012]. We proved that ColQ-tailed AChE moves from one muscle to another and anchors to the synaptic basal lamina by exploiting the proprietary binding affinity for synaptic basal lamina, which we named the protein-anchoring therapy.
We here report three patients with AChE deficiency harboring COLQ mutations in the collagen domain and CTD. We examined the effects of the CTD mutations on interaction between ColQ and MuSK by in vitro and in vivo assays and found that the CTD mutations impair this interaction.
Materials and Methods
Patients
All human studies were performed under approvals of the institutional review boards of Nagoya University Graduate School of Medicine, National Center of Neurology and Psychiatry, Juntendo University Faculty of Medicine, and Utano National Hospital. Three patients participated in the study after appropriate informed consents were given. A mutation analysis had been done when they were 7, 12, and 19 years of age, respectively. All had respiratory distress or poor sucking at birth, slight delay in walking, fatigability since early childhood, normal intelligence, no anti-AChR and anti-MuSK antibodies, a decremental electromyographic response, repetitive CMAP to a single nerve stimulus, and no response to anticholinesterase medications (Table 1).
| Pt. | Sex | Age | Onset | Walking | Noc. NIV | Edrophonium i.v. | RNS | rCMAP | Exon | Nucleotide change | Amino-acid change |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 19 y | Birth | 19 m | 12 y | No change | −52% | + | 11 | c.679C>T | p.Arg227X |
| 14 | c.965T>A | p.Val322Asp | |||||||||
| 2 | M | 12 y | Birth | 24 m | 11 y | Respiratory distress | −32% | + | 17 | c.1339G>Ca | p.Asp447Hisa |
| 3 | M | 7 y | 3 y | 18 m | Not used | Improved | −79% | + | 17 | c.1331G>A | p.Cys444Tyr |
| 17 | c.1354C>T | p.Arg452Cys |
- a Homozygous mutation.
- Exon numbers are according to GenBank Accession NM_005677.3. Mutations are numbered according to NM_005677.3 (cDNA) and NP_005668.2 (protein). cDNA number +1 corresponds to the A of the ATG translation initiation codon.
- Pt., patient; Noc. NIV, nocturnal noninvasive ventilation; RNS, repetitive nerve stimulation at 3 Hz of ulnar or accessory nerves; rCMAP, repetitive compound muscle action potential; m, months; y, years.
Mutation Analysis
Genomic DNA was isolated from blood with QIAamp Blood Mini Kit (Qiagen, Hilden, Germany). Poor response to anti-cholinesterases suggested endplate AChE deficiency and slow channel syndrome. We thus directly sequenced 17 constitutive COLQ exons and their flanking regions with CEQ8000 sequencer (Beckman Coulter, Brea, CA). Names of all mutations were checked using Mutalyzer (http://www.lovd.nl/mutalyzer/). Identified mutations were submitted to an LSDB for the COLQ gene (http://www.lovd.nl/COLQ). As mutations were identified in COLQ in all the patients, we did not go into sequencing of CHRNA1 (NM_00079.3; MIM #100690), CHRNB1 (NM_009601.4; MIM #100710), CHRND (NM_021600.2; MIM #100720), and CHRNE (NM_000080.3; MIM #100725) encoding the AChR α, β, δ, and ε subunits, respectively.
Construction of Expression Vectors
Human ACHET, COLQ cDNAs, and LacZ were cloned and introduced into a cytomegalovirus-based mammalian expression vector pTargeT (Promega, Madison, WI) [Ohno et al., 1998]. Each mutation was introduced into COLQ cDNA using the QuikChange site-directed mutagenesis kit (Stratagene). The extracellular domain (aa 1–393) of human MUSK (NM_001166280.1; MIM #601296) cDNA (Open Biosystems/Thermo Scientific, Waltham, MA) was cloned into a mammalian expression vector pAPtag-5 (GenHunter, Nashville, TN) at the NbeI and XboI sites upstream of a myc epitope [Kawakami et al., 2011].
Transfection and AChE Extraction
Wild-type or mutant pTargeT-COLQ was transfected into COS7 cells along with pTargeT-ACHET in a 10-cm dish using X-tremeGENE 9 DNA Transfection Reagent (Roche Diagnostics, Indianapolis, IN). Cells were incubated at 37°C for 48 hr and scraped from dish in Tris–HCl buffer (50 mM Tris–HCl [pH 7.0], 0.5% Triton X-100, 0.2 mM EDTA, 2 µg/ml leupeptin, 1 µg/ml pepstatin, and 0.1 µmol/ml benzamidine) containing 1 M NaCl. The extract was vortexed in a 1.5-ml tube and centrifuged at 14,000g for 5 min, and the supernatant was obtained.
Sedimentation Analysis
A sedimentation analysis was performed as previously described [Ohno et al., 1998]. The AChE-containing supernatant was applied on a 5%–20% sucrose density gradient, which was made in Tris–HCl buffer along with β-galactosidase (16.1 S) and alkaline phosphatase (6.1 S) as internal sedimentation standards. Centrifugation was performed in a Beckman SW41Ti rotor at 4°C for 21 hr at 178300g. The collected fractions were assayed for AChE activities using the Ellman method [Ellman et al., 1961] and determined the absorbance at 420 nm using a Sunrise Absorbance Reader (Tecan, Männedorf, Switzerland).
Isolation of ColQ-Tailed AChE on a Heparin–Agarose Column
For isolation of ColQ-tailed AChE, the extract was diluted in Tris–HCl buffer containing 0.2 M NaCl and loaded onto a HiTrap Heparin HP column (GE Healthcare, Buckinghamshire, UK). We washed the column with five volumes of Tris–HCl buffer containing 0.2 M NaCl, and eluted ColQ-tailed AChE with Tris–HCl buffer containing 1 M NaCl. We concentrated the eluate with an Amicon Ultra-4 Centrifugal Filter (50K) (Millipore, Billerica, MA) [Kawakami et al., 2011; Kimbell et al., 2004].
Transplantation of ColQ-tailed AChE to NMJs of Colq−/− Mice
We obtained approvals of the Colq−/− mice[Feng et al., 1999] studies by the Animal Care and Use Committee of the Nagoya University. We prepared 10-µm-thick sections of quadriceps muscles of Colq−/− mice with a Leica CW3050–4 cryostat at −20°C, and stored at −80°C until used. The muscle sections were fixed in acetone for 15 min. The stock solutions of ColQ-tailed AChE were diluted in Tris–HCl buffer containing 1 M NaCl and 5 mg/ml each of bovine serum albumin, chicken ovalbumin, and gelatin to give an Ellman unit equivalent to 1–2 ng of Torpedo AChE (Sigma, St. Louis, MO), and the NaCl concentration was adjusted to 0.5 M. To adjust the ionic strength of the solution, we added 50 mM Tris–HCl buffer stepwise over a period of 3 hr to 0.3 M NaCl. The slides were placed in a humidified chamber and incubated overnight at room temperature [Kimbell et al., 2004; Rotundo et al., 1997].
Immunofluorescence
For preparation of immunofluorescence, muscle sections were blocked with 5% horse serum in phosphate-buffered saline for 20 min. We detected ColQ-tailed AChE by anti-ColQ antibody [Ito et al., 2012] and anti rabbit-FITC secondary antibody at 1:100 (Vector Lab., Burlingame, CA), along with 2.5 µg/ml Alexa-594-conjugated α-bungarotoxin (Sigma) for visualizing AChR. Signals of ColQ and AChR were examined with fluorescent microscope, BX60 (Olympus, Tokyo, Japan). We analyzed more than 10 muscle sections for each experiment and representative images are indicated in the figures.
Preparation of the Extracellular Domain of MuSK
pAPtag-5 carrying hMuSKect-myc was transfected into HEK293 cells in a 10-cm dish using the calcium phosphate method. The hMuSKect-myc was purified with the c-myc-Tagged Protein Mild Purification Kit version 2 (MBL, Nagoya, Japan) [Kawakami et al., 2011].
In Vitro Plate-Binding assay for ColQ–MuSK Interaction
The Maxi-Sorp Immuno Plate (Nunc/Thermo Scientific) was coated with purified hMuSKect-myc at 4°C overnight and then blocked with phosphate-buffered saline containing 1% bovine serum albumin at room temperature for 1 hr. We incubated an equal Ellman unit of wild-type or mutant ColQ-tailed AChE at 4°C for 4 hr and then quantified the bound ColQ-tailed AChE by the Ellman method. Each time before we moved to the next step, we washed the plate three times with phosphate-buffered saline [Kawakami et al., 2011].
In Vivo Electroporation of pTarget-COLQ
The tibialis anterior muscles of Colq−/− mice were injected with 50 µg each of pTargeT-COLQ and pTargeT-LacZ plasmids. In vivo transfection was performed using an in vivo electroporator (CUY21EDIT; BEX Co., Ltd., Tokyo, Japan). A pair of electrode needles was inserted into the muscle in the longitudinal direction to a depth of 4 mm to encompass the plasmid-injected site. Pulses of 50 V and 25-msec were administered every 1 sec three times in forward polarity and three more times in the opposite polarity [Aihara and Miyazaki, 1998]. Seven days after the electroporation, mice were sacrificed and tibialis anterior muscles were analyzed.
AAV8-Mediated Expression of Mutant ColQ in Colq−/− Mice
We prepared wild-type and mutant pAAV8-COLQ and intravenously administered them to Colq−/− mice as described previously [Ito et al., 2012]. We inserted the wild-type human COLQ or mutant human COLQ harboring p.Asp447His into downstream of a CMV promoter in the pAAV-MCS vector using the AAV Helper-Free system (Stratagene). We employed AAV serotype 8 that can efficiently infect skeletal muscles. We injected 2 × 1012 vector genomes of wild-type or mutant pAAV-COLQ to the tail vein of 4-week-old male Colq−/− mice. Similar copies of wild-type pAAV8-COLQ and mutant pAAV8-COLQ-p.Asp447His genomes were transduced into muscle cells with a mean ratio of 1.07 (mutant transgene/wild-type transgene, n = 3). We quantified motor functions up to 4 weeks after injection. Muscle weakness and fatigability were measured with a rotarod apparatus (Ugo Basile). Mice were allowed to take a rest for 1 hr between each rotarod task and an average of three measurements was taken. Spontaneous running-wheel activities were used to quantify voluntary exercises. Each mouse was placed in a standard cage equipped with a counter-equipped running wheel (diameter, 14.7 cm; width, 5.2 cm; Ohara Medical Corp., Tokyo, Japan). The running distances were recorded every 24 hr. At 6 weeks after injection, mice were sacrificed and sections of skeletal muscles were stained for AChR and ColQ to visualize the transduced ColQ-tailed AChE as described above.
Results
Mutation Analysis
On the basis of combined clinical and electrophysiological features such as early age of onset, negative response to cholinesterase inhibitors, respiratory insufficiency, and repetitive muscle response [Abicht et al., 2012; Engel, 2012], we first sequenced COLQ and identified that each patient carried two mutant COLQ alleles. Patient 1 had heterozygous [c.679C>T, p.Arg227X] + [c.965T>A, p.Val322Asp] mutations. Patient 2 was heterozygous for [c.1331G>A, p.Cys444Tyr] + [c.1354C>T, p.Arg452 Cys]. Patient 3 had a homozygous [c.1339G>C, p.Asp447His] mutation (Fig. 1A, Table 1, Supp. Fig. S1). We traced the mutations in the parents of patients 1 and 3, and found that the mutation on each allele was inherited from unaffected parents. In patient 2, we confirmed heterozygosity by cloning each allele of exon 17 and sequenced them. Among the five mutations, only c.1331G>A, p.Cys444Tyr was previously identified in another Japanese patient [Ohno et al., 2000], but its pathogenicity remained elusive [Kimbell et al., 2004]. The other four mutations were novel. All the amino acids at mutated codons were conserved across species [Ohno et al., 1998].

Sedimentation Profiles of Mutations
Four missense mutations were in CTD and the nonsense p.Arg227X was in the collagen domain. We first examined the effects of the mutations on formation of asymmetric ColQ-tailed AChE species in COS cells. The p.Arg227X mutation that truncates ColQ in its collagen domain did not produce normal A12 species of ColQ-tailed AChE, as has been observed in other truncation mutations in the collagen domain [Ohno et al., 1998; 2000]. The p.Val322Asp mutation close to the N-terminal end of CTD similarly failed to produce A12 species. By contrast, the other three CTD mutations (p.Cys444Tyr, p.Asp447His, and p.Arg452Cys) produced normal or slightly reduced peaks of asymmetric A4, A8, and A12 species compared to the wild-type (Fig. 1B).
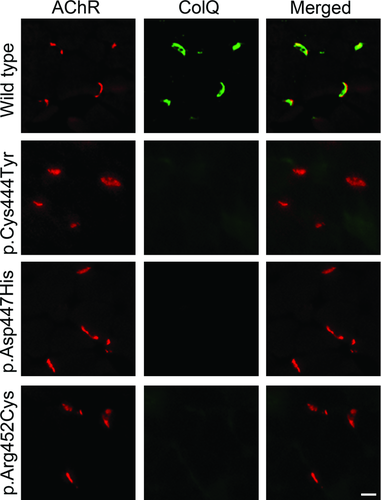
Overlay of CTD Mutants to the NMJ of Colq−/− Mice
We overlaid the purified recombinant human ColQ-tailed AChE protein complex on a section of skeletal muscle of Colq−/− mice. Wild-type ColQ-tailed AChE colocalized with AChR at the NMJs, whereas ColQ-tailed AChE with p.Cys444Tyr, p.Asp447His, and p.Arg452Cys mutations failed to colocalize with AChR (Fig. 2). This assay showed that these CTD mutations impair anchoring of ColQ at the vertebrate NMJs.
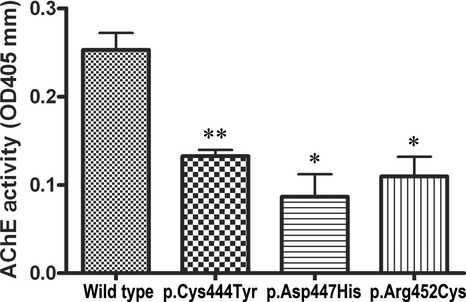
In Vitro Plate-Binding Assay for ColQ–MuSK Interaction
As the synaptic anchorage of AChE by ColQ is partly dependent on the association of ColQ with MuSK [Cartaud et al., 2004], we next examined whether the CTD mutations had an effect on the interaction of human ColQ and human MuSK using an in vitro plate-binding assay. We coated the plate with purified hMuSKect-myc, and added a fixed amount of the purified recombinant human ColQ-tailed AChE protein complex. The bound AChE was quantified by the Ellman method. AChE activities of the CTD mutants carrying p.Cys444Tyr, p.Asp447His, and p.Arg452Cys were significantly lower than that of wild-type CTD (Fig. 3). These results showed that impaired anchoring of the mutant ColQ-tailed AChE was due to the lack of interaction between ColQ and MuSK.
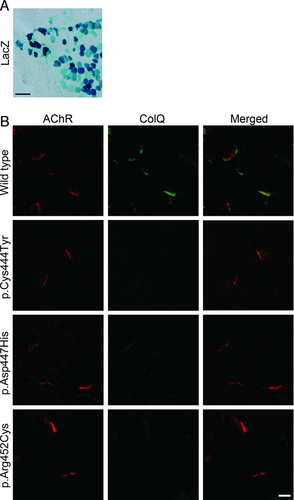
In Vivo Electroporation of COLQ with CTD Mutations into the Muscles of Colq−/− Mice
To confirm whether the three CTD mutants indeed impair anchoring of ColQ-tailed AChE to the NMJ in vivo, we electroporated wild-type and mutant pTargeT-COLQ constructs to tibialis anterior muscles of Colq−/− mice. We first confirmed that the pTargeT-LacZ plasmid is efficiently transduced into muscle fibers (Fig. 4A). ColQ-tailed AChE harboring p.Cys444Tyr, p.Asp447His, and p.Arg452Cys in CTD did not anchor to the NMJs of Colq−/− mice (Fig. 4), although real-time RT-PCR revealed similar expression levels of the wild-type and mutant COLQ mRNAs (ranges of COLQ/Gapdh mRNAs = 0.03 to 0.11 for wild-type and three mutants). These studies revealed that the three mutant ColQ molecules were incompetent for anchoring to the NMJ in vivo.
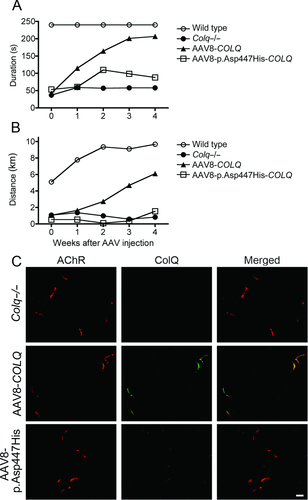
AAV8-Mediated Expression of Mutant ColQ in Colq−/− Mice
We previously reported the protein-anchoring therapy for Colq−/− mice, in which ColQ-tailed AChE is moved to and anchored to remote NMJs using its proprietary binding affinities for perlecan and MuSK [Ito et al., 2012]. To directly prove that the CTD mutations compromise anchoring of ColQ to the NMJ in a model animal, we intravenously administered wild-type and p.Asp447His-mutant AAV8-COLQ to Colq−/− mice, and analyzed motor functions and histological localization of ColQ. Motor functions evaluated by the dwell time on a rotarod (Fig. 5A) and by voluntary movements (Fig. 5B) were prominently improved in Colq−/− mice treated with wild-type COLQ but not with p.Asp447His-COLQ. Histological studies similarly showed that ColQ was colocalized to AChR in Colq−/− mice treated with AAV-wild-type COLQ but not with AAV-p.Asp447His-COLQ (Fig. 5C).
Discussion
CMSs have a variety of causes that lead to defects in the NMJ signal transmission. Elucidation of the molecular pathomechanisms is essential to develop and provide a specific treatment for CMS patients. Ephedrine and albuterol are effective for patients with endplate AChE deficiency harboring COLQ mutations [Chan et al., 2012; Engel et al., 2010]. Cholinesterase inhibitors cannot improve neuromuscular transmission and often worsen myasthenic symptoms and respiratory conditions. Therefore, an early genetic diagnosis is important for the patients. Clinical features of the three patients prompted us to search for mutations in COLQ, and indeed we detected five COLQ mutations. Four of them are novel and p.Cys444Tyr has been previously reported in another Japanese patient [Ohno et al., 2000]. It is interesting to note that the five mutations are unique to Japanese patients, which suggests that all the mutations have recently arisen in the patients’ families and are unlikely to be founder mutations.
In 1998, we cloned human COLQ cDNA, identified its genomic structure, and detected COLQ mutations in patients with endplate AChE deficiency [Ohno et al., 1998]. We also proved that mutations in the collagen domain impair formation of ColQ-tailed AChE [Ohno et al., 1998]. We later reported four classes of COLQ mutations as stated in the introduction, and proved pathogenicity in three classes but not in a class comprising CTD mutations [Ohno et al., 1999; 2000]. We next proved pathogenicity of five mutations (p.Arg315X, p.Asp342Glu, p.Gln371X, p.Arg410Gln, and p.Arg410Pro) in CTD using the heterologous overlay of human ColQ-tailed AChE to the frog muscles, but another mutation, p.Cys444Tyr, had no effect on anchoring of ColQ to the frog NMJ [Kimbell et al., 2004]. To summarize, we have reported 23 COLQ mutations and have succeeded in proving the pathogenicity in 22 mutations [Kimbell et al., 2004; Ohno et al., 1998; 1999; 2000; Shapira et al., 2002]. p.Cys444Tyr in CTD has thus been the only mutation that we failed to prove why the patient was deficient for AChE. Donger et al. (1998) reported p.Tyr430Ser in CTD in a patient with endplate AChE deficiency, but the sedimentation analysis yielded normal asymmetric species of AChE. The p.Tyr430Ser mutation was later employed to prove that CTD binds to MuSK by immunoprecipitation experiments [Cartaud et al., 2004]. Four additional mutations (p.Arg341Gly, p.Cys386Ser, p.Cys417Tyr, and p.Thr441Ala) in CTD have been reported by others but none have been functionally analyzed [Mihaylova et al., 2008; Muller et al., 2004].
In the present study, we found four missense mutations (p.Val322Asp, p.Asp447His, p.Cys444Tyr, and p.Arg452Cys) in CTD and one truncation mutation (p.Arg227X) in the collagen domain in three patients with endplate AChE deficiency. Three mutations (p.Val322Asp, p.Asp447His, and p.Arg452Cys) in CTD have not been functionally characterized and one was p.Cys444Tyr, for which the pathogenicity remained elusive. We characterized the functional consequences of these mutations.
The analysis of sedimentation profiles revealed that p.Arg227X and p.Val322Asp abolish formation of normal asymmetric A12 species of AChE. p.Val322Asp is the only mutation that affects formation of A12 and is the only mutation that introduces a negatively charged residue in CTD. We previously reported that 1082delC in CTD introduces 64 hydrophobic missense residues after the frameshift, which prevents triple helix formation [Ohno et al., 2000]. CTD is enriched with prolines, cysteines, and charged residues. The hydrophobicity profile is likely to be essential in CTD. p.Val322Asp introduces a hydrophilic aspartate residue, and disrupts a hydrophobic cluster close to the N-terminal end of CTD (Supp. Fig. S2A). Prediction of a secondary structure similarly demonstrates shortening of a β-sheet and de novo insertion of a turn before an α-helix (Supp. Fig. S2B). We do not observe these gross alterations in predicted structures with p.Cys444Tyr, p.Asp447His, and p.Arg452Cys (Supp. Fig. S2). p.Val322Asp is thus likely to compromise the tertiary structure of CTD and leads to defective triple helix formation.
The analysis of sedimentation profiles showed that the other CTD mutations, p.Cys444Tyr, p.Asp447His, and p.Arg452Cys, generated normal asymmetric A12 species of AChE. We next examined the anchoring competence of these mutations. We had previously employed frog muscles to analyze anchoring incompetence of CTD mutations, but failed to prove it for p.Cys444Tyr [Kimbell et al., 2004]. We thus used the vertebrate NMJs of Colq−/− mice, and proved that all three mutations are not able to anchor to the mouse NMJ. Anchoring competence of p.Cys444Tyr to frog NMJs but not to mouse NMJs suggests that p.Cys444Tyr does not grossly change the conformation of CTD and is likely to be a mild mutation. Indeed, the onset of patient 3 carrying p.Cys444Tyr was at the age of 3 years, and the patient started walking at 18 months, which are the mildest among the three currently analyzed patients.
To further dissect the underlying molecular bases of anchoring incompetence of p.Cys444Tyr, p.Asp447His, and p.Arg452Cys, we quantified the ColQ–MuSK interaction by the in vitro plate-binding assay, which enabled us to estimate the binding affinities of these two molecules. The three missense mutations decreased the activities of ColQ-tailed AChE bound to MuSK to ∼50% or less, which suggests that ∼50% reduction of the binding affinity is likely to be required to compromise anchoring of ColQ to the NMJ.
Moreover, we demonstrated the pathogenicity of the mutations in a model animal for the first time. We electroporated the three CTD mutations into tibialis anterior muscles of Colq−/− mice, and found that each mutation was indeed anchoring-incompetent. To further prove the pathogenicity of one of the mutations in Colq−/− mice, we intravenously injected AAV8-COLQ-p.Asp447His to Colq−/− mice and found that motor deficits remained essentially the same and anchoring of ColQ was not observed at the NMJs.
In vitro plate-binding assays revealed ∼50% reduction of binding affinity of ColQ for MuSK for p.Cys444Tyr, p.Asp447His, and p.Arg452Cys (Fig. 3), but these mutants were not at all anchored to the NMJ by in vitro overlay assay (Fig. 2), in vivo electroporation (Fig. 4), and AAV8 treatment (Fig. 5). Among the three mutants, p.Cys444Tyr showed the most preserved binding affinity for MuSK, which, however, was not sufficient to anchor the mutant ColQ to the NMJ in in vitro overlay assay (Fig. 2) and in vivo electroporation (Fig. 4). The presence of heparan sulfate proteoglycans like perlecan at the NMJ in these assays was unlikely to sufficiently compensate for the defective CTD-MuSK interaction, which also underscores a pivotal role of CTD-MuSK interaction on binding of ColQ to the NMJ. Although the same amount of ColQ-tailed AChE was used for in vitro plate-binding and in vitro overlay assays, the amount of MuSK (>150 ng/well) used in in vitro plate-binding assay was likely to be much more than that at the NMJ in in vitro overlay assay, which may account for the difference in up to 50% preservation of ColQ binding in in vitro plate-binding assay and complete lack of ColQ binding in in vitro overlay assay.
Acknowledgments
We thank Yasutaka Ohya, Kumiko Yano, and Koji Nomaru at Division for Research of Laboratory Animals of Nagoya University for technical assistance.
Disclosure statement: The authors declare no conflict of interest.




