Role of Cholesterol-Associated Steatohepatitis in the Development of NASH
Supported by the U.S. Department of Veterans Affairs (BX002910).
Potential conflict of interest: Nothing to report.
Abstract
The rising prevalence of nonalcoholic fatty liver disease (NAFLD) and NAFLD-related cirrhosis in the United States and globally highlights the need to better understand the mechanisms causing progression of hepatic steatosis to fibrosing steatohepatitis and cirrhosis in a small proportion of patients with NAFLD. Accumulating evidence suggests that lipotoxicity mediated by hepatic free cholesterol (FC) overload is a mechanistic driver for necroinflammation and fibrosis, characteristic of nonalcoholic steatohepatitis (NASH), in many animal models and also in some patients with NASH. Diet, lifestyle, obesity, key genetic polymorphisms, and hyperinsulinemia secondary to insulin resistance are pivotal drivers leading to aberrant cholesterol signaling, which leads to accumulation of FC within hepatocytes. FC overload in hepatocytes can lead to ER stress, mitochondrial dysfunction, development of toxic oxysterols, and cholesterol crystallization in lipid droplets, which in turn lead to hepatocyte apoptosis, necrosis, or pyroptosis. Activation of Kupffer cells and hepatic stellate cells by hepatocyte signaling and cholesterol loading contributes to this inflammation and leads to hepatic fibrosis. Cholesterol accumulation in hepatocytes can be readily prevented or reversed by statins. Observational studies suggest that use of statins in NASH not only decreases the substantially increased cardiovascular risk, but may ameliorate liver pathology. Conclusion: Hepatic FC loading may result in cholesterol-associated steatohepatitis and play an important role in the development and progression of NASH. Statins appear to provide significant benefit in preventing progression to NASH and NASH-cirrhosis. Randomized controlled trials are needed to demonstrate whether statins or statin/ezetimibe combination can effectively reverse steatohepatitis and liver fibrosis in patients with NASH.
Abbreviations
-
- 2-Oxo
-
- 2-oxoglutarate
-
- ABC
-
- ATP-binding cassette transporter
-
- ACAT2
-
- acyl-CoA:cholesterol acyltransferase enzyme
-
- ApoB
-
- apolipoprotein B
-
- BSEP
-
- bile salt export pump
-
- CASH
-
- cholesterol-associated steatohepatitis
-
- CE
-
- cholesterol ester
-
- CVD
-
- cardiovascular disease
-
- ER
-
- endoplasmic reticulum
-
- FC
-
- free cholesterol
-
- FDFT1
-
- farnesyl diphosphate farnesyl transferase 1
-
- FXR
-
- farnesoid X receptor
-
- HDL
-
- high-density lipoprotein
-
- HMGCoAR
-
- 3-hydroxy-3-methylglutaryl coenzyme A reductase
-
- HSC
-
- hepatic stellate cell
-
- Ihh
-
- Indian hedgehog
-
- IL
-
- interleukin
-
- KC
-
- Kupffer cell
-
- LD
-
- lipid droplet
-
- LDLR
-
- low-density lipoprotein receptor
-
- LXR
-
- liver X receptor
-
- MBOAT7
-
- membrane-bound-O-acyltransferase domain-containing protein 7
-
- MetS
-
- metabolic syndrome
-
- mRNA
-
- messenger RNA
-
- NAFLD
-
- nonalcoholic fatty liver disease
-
- NASH
-
- nonalcoholic steatohepatitis
-
- nCEH
-
- neutral cholesterol ester hydrolase
-
- NF-κB
-
- nuclear factor kappa B
-
- NLRP3
-
- NOD-, LRR-, and pyrin domain-containing protein 3
-
- NPC1L1
-
- Niemann-Pick type C1 like 1 protein
-
- oxLDL
-
- oxidized low-density lipoprotein
-
- PNPLA3
-
- patatin-like phospholipase domain-containing protein 3
-
- ROS
-
- reactive oxygen species
-
- Scap
-
- SREBP cleavage activating protein
-
- SERCA
-
- sarco/endoplasmic reticulum Ca2+-ATPase
-
- SR-B1
-
- scavenger receptor class B type 1
-
- SREBP
-
- sterol regulatory element binding protein
-
- T2DM
-
- type 2 diabetes mellitus
-
- TGF-β
-
- transforming growth factor β
-
- TNF-α
-
- tumor necrosis factor α
-
- UPR
-
- unfolded protein response
-
- VLDL
-
- very low density lipoprotein
Nonalcoholic fatty liver disease (NAFLD) encompasses a wide histological spectrum of disease ranging from simple steatosis and nonalcoholic steatohepatitis (NASH) to cirrhosis and hepatocellular carcinoma.(1) It is the most common cause of chronic liver disease worldwide, with a prevalence estimated to be 24%-26%.(2, 3) The prevalence of NASH in North America is estimated to be about 24.1%, with the highest prevalence reported in the Middle East (31.7%) and South America (30.4%).(3) About 41% of patients with NASH experience progression of fibrosis with an incidence of stage 3 or 4 fibrosis of 68 per 1,000 person-years.(1, 3, 4) NAFLD/NASH is currently the second leading indication for liver transplantation and is expected to become the number-one indication in the next few years, with excellent long-term posttransplant survival. (1-3, 5, 6)
Many risk factors for developing hepatic steatosis have been identified including the metabolic syndrome (MetS), obesity, insulin resistance with hyperinsulinemia, personal or family history of type 2 diabetes mellitus (T2DM), and dyslipidemia.(1, 3) Of these risk factors, T2DM and insulin resistance are very common in NAFLD and may play a pivotal role in NAFLD/NASH development.(3, 7-10) Despite these known risk factors, we do not yet know the causative factor(s) without which development and progression of NASH cannot possibly occur in certain patients. In this review, we examine the data supporting the hypothesis that hepatic cholesterol is a key pathogenetic factor driving the development of NASH at least in a subset of patients, and propose the term cholesterol-associated steatohepatitis (CASH) to describe this mechanistic pathway by which hepatic cholesterol may result in the development of steatohepatitis. These considerations are critical, because unless a causative agent is uncovered, it is unlikely that a highly effective treatment of NASH will ever be identified. Although there is mounting evidence that cholesterol may also lead to hepatic carcinogenesis, we will not focus on the association between cholesterol and hepatocellular carcinoma in this review.
Pathogenesis of NASH: Conceptual Models and the Role of Cholesterol
Historically, the two-hit hypothesis proposed a stepwise progression from normal liver to hepatic steatosis and then to NASH.(11, 12) This theory postulates that insulin resistance is the “first hit,” which promotes accumulation of fatty acids in the liver, leading to steatosis.(13) Hyperinsulinemia results in increased lipolysis from peripheral adipose tissue and altered hepatic gene transcription, which promotes free fatty acid uptake and de novo lipogenesis.(13-15) Oxidative stress is the “second hit,” resulting from increased oxidation of fatty acids, and causing reactive oxygen species (ROS) formation, lipid peroxidation, DNA damage, mitochondrial dysfunction, and release of proinflammatory cytokines.(16, 17) These cellular mechanisms result in hepatocyte damage, inflammation, and fibrosis, characteristic of steatohepatitis.(16, 17)
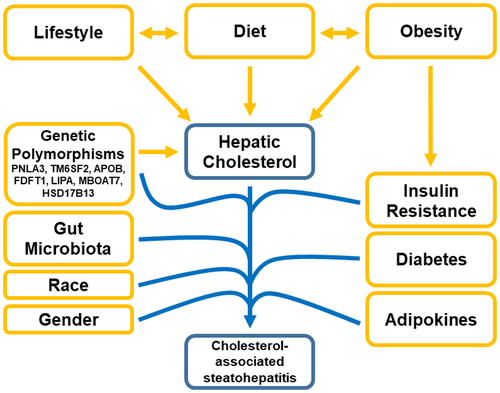
More recently, a “multiple parallel hits” hypothesis has been proposed, in which multiple cellular mechanisms, working simultaneously to cause a “perfect storm,” result in hepatic inflammation and progression to NASH.(18) Cellular mechanisms that could be altered and lead to inflammation include abnormal lipid metabolism, mitochondrial oxidative injury, endoplasmic reticulum (ER) stress, genetic polymorphisms, altered immune responses, and gut microbiome dysbiosis.(18-20) It is postulated that the accumulation of lipotoxic lipids within the liver, which interact with pro-inflammatory signals, causes these cellular abnormalities, which leads to inflammation and fibrosis.(21) Although triglycerides are the most common lipids in the liver by far, it is likely that they represent a “safe” storage molecule for fatty acids.(22) Instead, it is the accumulation of other lipotoxic lipids, such as cholesterol (and potentially free fatty acids, diacylglycerol, ceramides, and others), which are postulated to result in cellular dysfunction.(21, 23, 24) Cholesterol has a relatively “safe” storage option (i.e., its esterification to cholesterol esters [CEs]); however, hepatic free (i.e., unesterified) cholesterol is highly toxic to multiple cellular processes and organelles even if only slightly increased.(25) Thus, we propose that in a subset of patients with NASH, hepatic cholesterol accumulation results in the development of cholesterol-associated steatohepatitis (CASH) and is the main driver of the necroinflammation and fibrosis causing NASH, while dietary, genetic, and lifestyle co-factors either lead to the accumulation of hepatic cholesterol or interact which hepatic cholesterol to promote NASH, as shown in Fig. 1.
Hepatic Cholesterol Metabolism
The liver is the most important organ that controls body cholesterol homeostasis. In the nonpathologic state, the mouse liver has a relatively low cholesterol concentration (132 mg/kg), but it has a high flow of sterols through the liver every day, consistent with its role in lipoprotein and bile acid synthesis and homeostasis (143 mg/kg/day).(26) When the sum total of the pathways involved in synthesis and uptake of cholesterol (FIG. 2A) exceeds the pathways that lead to removal of cholesterol (FIG. 2B), cholesterol accumulates in hepatocytes.(27)
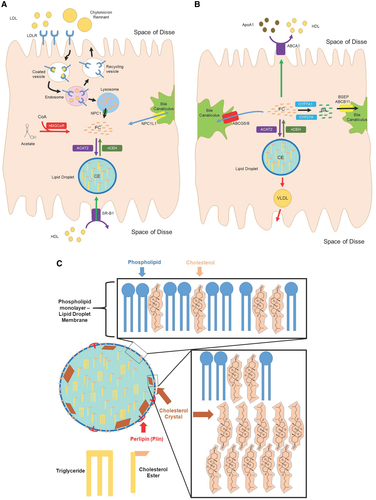
A critical component of the CASH hypothesis is that the liver (not adipose tissue) is the body’s storage site for excess cholesterol. Excess cholesterol is stored in the liver within hepatocyte lipid droplets (LDs) as CEs.(28) Once previously believed to be inert storage vessels, LDs have now been recognized as metabolically active organelles within cells that serve a wide variety of functions. LDs are derived from the ER and consist of a core of neutral lipids (CEs and triglyceride) that are surrounded by a phospholipid monolayer, studded with a diverse array of proteins.(29, 30) The phospholipid monolayer contains FC, which affects LD membrane properties, including surface and line tension, size, and interaction with other LDs(29, 30) (FIG. 2C).
Regulation of Cholesterol Homeostasis
Cholesterol homeostasis is tightly regulated by a number of nuclear transcription factors, three of which have also been linked to NAFLD pathogenesis: sterol regulatory element binding protein-2 (SREBP-2), farnesoid X receptor (FXR), and liver X receptor (LXR) (FIG. 3).
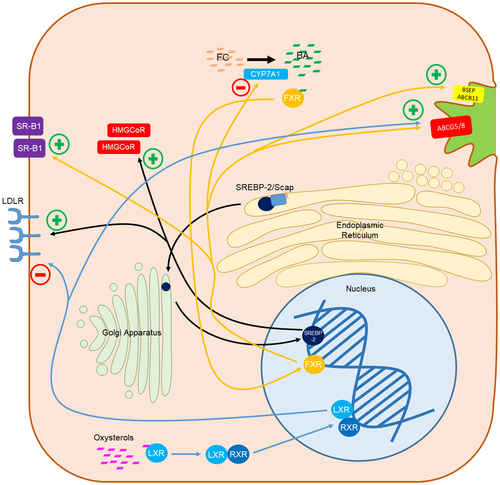
SREBPs are a family of membrane-bound transcription factors that sense membrane cholesterol and fatty acid content and modulate the transcription of genes involved in cholesterol and fatty acid synthesis and uptake.(31, 32) SREBP-1 is primarily involved in fatty acid, triglyceride, and phospholipid pathways, whereas SREBP-2 is involved in cholesterol metabolism.(33, 34) SREBP-2 is a resident of the ER, where it is bound to SREBP cleavage activating protein (Scap).(35) When Scap senses cholesterol depletion, SREBP-2 is transported to the Golgi complex, where it is cleaved to the active form and enters the nucleus to activate the transcription of genes for cholesterol synthesis and uptake, including 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCoAR) and low-density lipoprotein receptor (LDLR).(35, 36)
FXR is a nuclear receptor that senses bile acids and is extensively involved in bile acid, lipid, and glucose homeostasis.(37-39) In the liver, FXR up-regulates scavenger receptor class B type 1 (SR-B1), resulting in increased uptake of high-density lipoprotein (HDL) cholesterol from the circulation, increases ATP-binding cassette transporter (ABC) G5 and G8 (ABCG5/G8) specifically in mice, and bile salt export pump (BSEP) synthesis, resulting in biliary excretion of cholesterol and bile acids, but also inhibits CYP7A1 preventing cholesterol conversion to bile acids.(37, 40, 41) FXR also promotes removal of triglycerides from hepatocytes by increasing β-oxidation and decreasing lipogenesis.(42) In hepatic stellate cells (HSCs), FXR creates a quiescent and pro-apoptotic phenotype, which promotes liver fibrosis resolution.(43)
LXRs are nuclear cholesterol sensors that are activated by high intracellular oxysterols.(44, 45) Following activation by oxysterols, LXRα forms a heterodimer with retinoid X receptor.(44) LXRα results in reverse cholesterol transport and hepatic cholesterol metabolism by increasing the expression of macrophage ABCA1/G1, resulting in increased HDL levels, and increasing hepatic, macrophage, and intestinal ABCG5/G8, causing net cholesterol excretion from the body, but also increases LDLR degradation on hepatocytes.(46-50) In rodent models, LXR also induces expression of CYP7A1, resulting in cholesterol conversion to bile acids; however, this phenomenon is not seen in humans.(51) Additionally, duodenal Niemann-Pick type C1 like 1 protein (NPC1L1) expression has been shown to be negatively correlated with LXR expression, inhibiting intestinal cholesterol absorption, resulting in fecal excretion of cholesterol.(52) LXRα agonists in mice demonstrated reduced hepatic inflammation and fibrosis by decreasing cholesterol-mediated activation of hepatic Kupffer cells (KCs) and HSCs.(53, 54)
Cholesterol Esterification
To prevent the toxic effects of FC within hepatocytes, FC is esterified to CE and stored in hepatocyte LDs.(55) One of the enzymes responsible for cholesterol esterification in hepatocytes is acyl-CoA:cholesterol acyltransferase enzyme 2 (ACAT2).(56) ACAT2 is a transmembrane protein found in the ER in the liver but not in adipose tissue; it integrates newly formed CEs into the ER membrane, which can either be incorporated into apolipoprotein B (ApoB) or bud off to form LDs.(29, 30, 57, 58) When FC is needed by the hepatocyte, ACAT2 is down-regulated and neutral cholesterol ester hydrolase (nCEH) hydrolyzes CE to FC.(55, 59)
Hepatic Cholesterol Accumulation in NASH
In the setting of NAFLD, numerous derangements to hepatic cholesterol homeostasis have been identified, which lead to the accumulation of hepatic cholesterol.(60) In mice, both hyperinsulinemia and inflammation lead to loss of the inhibitory effect of elevated plasma cholesterol on Scap/SREBP-2, resulting in hepatic cholesterol accumulation.(61-63) Increased levels of nuclear SREBP-2, HMGCoAR messenger RNA (mRNA), HMGCoAR protein, and HMGCoAR dephosphorylation, resulting in the active form of the enzyme, have been demonstrated in patients with NAFLD/NASH.(60, 64) Despite elevated nuclear SREBP-2 levels, LDLR levels are actually down-regulated in patients with NAFLD/NASH, but an alternative hepatic scavenger receptor for oxidized low density lipoprotein (oxLDL) particles, CD36, is increased relative to the severity of steatosis.(15, 60, 63, 64) Export of cholesterol out of the cell is also decreased, with decreased mRNA levels of ABCA1, ABCG1, and ABCG5.(60, 63)
Typical esterification/de-esterification activity in healthy individuals is determined by the relative concentration of ACAT2, while the concentration of nCEH remains relatively constant.(55) However, in patients with NAFLD, there is a 6-fold higher concentration of nCEH compared with healthy controls.(60, 63, 65, 66) Increased expression of nCEH in animal models was also associated with reduced expression of CYP7A1 and CYP27A.(59) These cellular abnormalities, coupled with the decreased expression of ABC cholesterol exporters noted previously, result in the accumulation of toxic FC within hepatocytes.
Dietary Cholesterol and NASH
Human studies consistently support the association between cholesterol intake and the development of NASH or cirrhosis (Table 1). A nested case-control analysis of the multiethnic cohort, a large prospective study with over 215,000 older-adult participants in Hawaii and California, showed a positive association between dietary cholesterol intake and development of NAFLD with cirrhosis.(67) Another study, representative of the U.S. population, reported that dietary cholesterol consumption (but not total fat consumption) was significantly associated with the development of cirrhosis from all etiologies of liver disease combined.(68)
| Study | Population | Measurements | Results |
|---|---|---|---|
| Musso et al. 2003(149) | 50 patients | 7-day alimentary record | Dietary intake richer in cholesterol in patients with NASH |
| 25 NASH; 25 controls | NASH: 506 ± 108 mg/dL | ||
| Mean age: 37 | Control: 405 ± 111 mg/dL | ||
| P = 0.002 | |||
| Allard et al. 2008(150) | 73 patients referred for elevated liver enzymes and suspected NAFLD at a single center from Oct 2003 to Oct 2006 | Self-reported dietary intake assessment | Increased dietary intake correlated with histologic disease severity |
| Mean age: | Cholesterol consumption (mg/day): | ||
| Minimal findings: 46.8 ± 2.7 | Minimal findings: 269.5 ± 27.5 | ||
| Simple steatosis: 44.7 ± 2.7 | Simple steatosis: 290.8 ± 28.1 | ||
| NASH: 47.7 ± 2.2 | NASH: 357.9 ± 37.5 | ||
| Ioannou et al. 2009(68) | 9,221 patients without evidence of cirrhosis followed for 13.3 years as part of the National Health and Nutrition Examination Survey | 24-hour dietary recall | Cholesterol consumption positively associated with cirrhosis and liver cancer |
| Age: 25-74 | Cholesterol consumption (mg/day): | ||
| 0-156: 1 | |||
| 157-294: 1.52 | |||
| 295-510: 1.66 | |||
| >511: 2.45 | |||
| P = 0.007 | |||
| Yu et al. 2013(151) | 608 patients with hepatitis C enrolled in the Hepatitis C Antiviral Long-term Treatment Against Cirrhosis trial followed for 1.8 years | Responses to food frequency questionnaires at baseline and 1.8 years later | Each higher quartile of cholesterol intake was associated with a 46% increase in the risk of clinic or histologic liver disease progression |
| Mean age: 51.0 ± 7.0 | Cholesterol consumption (mg/day): | ||
| 32-152: 1 | |||
| 152-222: 1.51 | |||
| 224-310: 2.83 | |||
| >310: 2.74 | |||
| P = 0.004 | |||
| Mokhtari et al. 2017(152) | 169 patients with NAFLD referred to two Hepatology clinics in Tehran, Iran in 2015 and 782 controls | Responses to a validated food frequency questionnaire | Dietary cholesterol intake was higher in cases compared with controls; greater egg consumption was associated with higher dietary cholesterol intake; greater egg consumption was associated with higher OR for NAFLD |
| Mean age: | Cholesterol consumption (mg/day): | ||
| Cases (NAFLD): 42.65 ± 12.21 | Cases: | ||
| Controls: 43.71 ± 14.52 | 263.41 ± 5.35 | ||
| Controls: | |||
| 315.31 ± 11.50 | |||
| P < 0.001 | |||
| Cholesterol consumption (mg/day) per egg consumption: | |||
| <2/week: 226.40 ± 5.75 | |||
| 2-3/week: 291.95 ± 11.60 | |||
| >4/week: 383.90 ± 9.53 | |||
| P < 0.001 | |||
| Noureddin et al. 2019(67) | >215,000 men and women living in Hawaii or California between 1993 and 1996 | Responses to a validated quantitative food frequency questionnaire | Cholesterol intake positively associated with NAFLD with cirrhosis |
| Age: 45-75 years | NAFLD: 1.16 (P = 0.005) | ||
| NAFLD with cirrhosis: 1.52 (P = 0.002) | |||
| Yasutake et al. 2009(153) | 56 patients with NAFLD diagnosed by ultrasound, CT, or liver biopsy at Kyushu Medical Center between Oct 2006 and Oct 2007 | Self-reported dietary intake | Cholesterol intake was significantly higher in nonobese patients with NAFLD compared to obese patients with NAFLD and healthy controls |
| Mean age: | P = 0.0378 | ||
| Obese: 53.5 ± 12.3 | |||
| Nonobese: 47.2 ± 14.8 |
- Abbreviations: CT, computerized tomography; OR, odds ratio.
Experimental animal models (e.g., mice, rats, rabbits, gerbils, pigs) also consistently demonstrate that the addition of dietary cholesterol leads to progression of liver disease to fibrosing steatohepatitis and cirrhosis (Table 2). These studies generally show that while dietary fat intake alone causes the development of only simple steatosis without substantial necroinflammation or fibrosis, the addition of dietary cholesterol causes the progression from steatosis to NASH. Studies in some animal models, such as Ossabaw swine, showed that marked steatosis is not always necessary for the development of dietary cholesterol-induced ballooning degeneration, KC activation, and fibrosis.(69, 70)
| Study | Animal Model | Diet | Age at Onset of Diet | Duration of Diet | Liver Histology Induced by Dietary Cholesterol | Mechanism |
|---|---|---|---|---|---|---|
| Cote et al. 2013(154) | Dawley female rats | 40% fat and 1.25% cholesterol | 8 weeks | 7 weeks | Hepatic steatosis | Hepatic accumulation triglycerides and cholesterol |
| Decreased FXRs | ||||||
| Lower expression of HMGCoAR, FDFT1, and ABCG8 | ||||||
| Ichimura et al. 2015(155) | Sprague-Dawley male rats | High-fat alone or in combination with 1.25% or 2.5% cholesterol | 9 weeks | 9 weeks | Fibrosing NASH and progression to cirrhosis | Diminished CPT activity and ABCG5 |
| Ichimura et al. 2017(156) | Sprague-Dawley male rats | High-fat alone or in combination with 1.25% or 2.5% cholesterol | 9 weeks | 18 weeks | Fibrosing NASH and progression to cirrhosis | Diminished CPT activity, ABCG5, and BSEP |
| Moriya et al. 2012(157) | SHRSP5/Dmcr male rats | High-fat and high-cholesterol diet (25% palm oil, 5% cholesterol, 2% cholic acid) | 10 weeks | 2, 8, and 16 weeks | Fibrosing NASH | Altered TNF-α proinflammatory cytokine and NF-κB pathway |
| Yetti et al. 2013(158) | SHRSP5/Dmcr male rats | High-fat and high-cholesterol diet (25% palm oil, 5% cholesterol, 2% cholic acid) | 10 weeks | 2, 8, and 16 weeks | Fibrosing NASH | Downregulation of caspase activity |
| Hepatic necrosis | ||||||
| Horai et al. 2016(159) | SHRSP5/Dmcr male rats | High-fat and high-cholesterol diet (25% palm oil, 5% cholesterol, 2% cholic acid) | 6 weeks | 2, 4, 6, 8, and 16 weeks | Fibrosing NASH | Eosinophilic inclusion bodies and mega-mitochondria |
| Csonka et al. 2017(160) | Wistar males rats | 2% cholesterol, 0.25% cholate | 6 weeks | 12 weeks | Hepatic steatosis | Increased SCD1 and decreased FADS1 and FADS2 |
| Matsuzawa et al. 2007(161) | C57BL/6J male mice | 1.25% cholesterol and two different amounts fat (7.5% and 60%) | 6 weeks | 6, 12, or 24 weeks | Fibrosing NASH | Down-regulation of antioxidant enzymes |
| Savard et al. 2013(65) | C57BL/6J male mice | 15% fat and/or 1% cholesterol | 6 months | 30 weeks | Fibrosing NASH | N/A |
| Vergnes et al. 2003(162) | C57BL/6J and C57BL/6ByJ male mice | 7.5% fat, 0.5% cholate, and/or 1.25% cholesterol | 3 months | 3 weeks | Fibrosing NASH | Activation of HSCs, SAA family genes, histocompatibility antigens, Il-2rγ, Scyb9, and Samhd1 |
| Desai et al. 2008(163) | C57BL/6J males mice | 1.25% cholesterol, 0.5% cholic acid, and 16% fat | 8-10 weeks | 3 weeks | NASH | Mononuclear leukocyte infiltration in liver |
| Enhanced MCP1, RANTES, and MIP2 | ||||||
| Sumiyoshi et al. 2010(164) | C57BL/6J males mice | 15% milk fat, 1.5% cholesterol, and 0.1% cholic acid | 4 weeks | 25 or 55 weeks | Hepatic steatosis | Elevated levels of MCP1 levels and PDGF-B protein |
| Fibrosis | ||||||
| Focal nodular hyperplasia | ||||||
| Ganz et al. 2015(165) | C57BL/6J male mice | High fat, 10% cholesterol, and high sugar supplement | 8-10 weeks | 8, 27, or 49 weeks | Fibrosing NASH | Enhanced levels of MCP1, TNF-α, and IL-1β |
| Macrophage polarization toward an M1 | ||||||
| Tu et al. 2017(166) | C57BL/6J male and female mice | 15.8% fat, 1.25% cholesterol, and 0.5% cholate diet | 8 weeks | 3 weeks | Fibrosing NASH | Elevated FC, CEs, and cholic acid |
| Changes to metabolism of sphingomyelins and phosphatidylcholines | ||||||
| Henkel et al. 2017(167) | C57BL/6J male mice | Soibean oil, 6-PUFA, and 0.75% cholesterol | 8 weeks | 20 weeks | Fibrosing NASH | Activation of KCs and enhanced expression of Cdl2, Cxcl2, Tnf, and Osm |
| McGettigan et al. 2019(168) | C57BL/6J male mice | One of six diets with variable amounts of fat (10% or 45% of total kilocals) and cholesterol (0.05%, 0.2%, and 2.0% of weight) | 6-8 weeks | 12, 20, or 24 weeks | Fibrosing NASH | Induction of tissue repair and regeneration phenotype in KCs and recruited infiltrating macrophages |
| Andres-Blasco et al. 2015(169) | HL-/- male mice | 10.8% fat and 0.75% cholesterol | 2 months | 16 weeks | NASH | Dyslipidemia |
| Increased NEFA | ||||||
| Enhanced macrophages | ||||||
| Circulating levels of MCP1 and Th17 T-cell subset | ||||||
| Chiu et al. 2010(170) | HL-/- female mice | 21% fat and 0.15% cholesterol | 21-23 weeks | 12 weeks | Decreased hepatic steatosis | No dyslipidemia and IR |
| Wouters et al. 2008(171) | LDLR-deficient and ApoE2 knock-in male and/or female mice | 21% fat and 0.2% cholesterol | 13 weeks | 2, 4, 7, and 21 days or for 7 days according to experiments | NASH | Macrophage accumulation in the liver, increase in lipid and inflammatory genes |
| Subramanian et al. 2011(172) | LDLR-deficient male mice | 36.6% fat, 35.5% carbohydrate, and 0.15% cholesterol | 10 week | 24 weeks with diet | NASH | Macrovesicular steatosis, inflammatory cell foci, and fibrosis |
| Van Rooyen et al. 2011(63) | Alms1 mutant (foz/foz) and wild-type diabetes NOD B10 female mice | 23% fat and 0.2% cholesterol | 8 weeks | 12 or 24 weeks | NASH | Increased macrophage, liver apoptosis, and fibrosis |
| Schierwagen et al. 2015(173) | apoE-/- mice | Western-type diet containing 1.25% of cholesterol | 12 weeks | 7 weeks | NASH | Hepatic fibrosis |
| Up-regulation of TGF-β | ||||||
| Increased hepatic collagen | ||||||
| Activation of HSCs | ||||||
| Rodriguez-Sanabria et al. 2010(174) | apoE-/- vs. LDLR-/- male mice | 20% fat and 0.25% cholesterol | 10 weeks | 6 weeks | NASH | Increased macrophages and inflammatory nodules (apoE, apoE-/-) vs. hepatic steatosis (LDLR-/-) |
| Kampschulte et al. 2014(175) | ApoE-/- LDLR-/- male mice | Western diet containing 5% cholesterol and 21% fat | 4 weeks | 35 weeks | Fibrosing NASH | Macrophage and T-cell infiltration, hepatic ROS accumulation, JNK activation |
| Induction of PPAR-α | ||||||
| Kainuma et al. 2006(176) | Rabbits male | Standard diet containing 1% cholesterol | 10 weeks | 8-12 weeks | Fibrosing NASH | N/A |
| Ogawa et al. 2010(177) | Pathogen-free Japanese White male rabbits | Standard diet supplemented with 0.75% cholesterol and 12% corn oil | 1 year | 2 months | Fibrosing NASH (almost cirrhosis) | Induction of PPAR-γ and aP2, increased mRNA of TNF-β1 and collagen 1A1 |
| Ipsen et al. 2016(178) | Guinea female pigs | 15%-25% sucrose, 20% fat, and 0.35% cholesterol | 10 weeks | 16 or 25 weeks | Fibrosing NASH | Decreased microsomal triglyceride transfer protein mRNA and decreased hepatic VLDL secretion |
| Lee et al. 2009(70) | Ossabaw male and female swine | 20% fructose, 46% fat, 2% cholesterol, and 0.7% cholate | 5-10 months | 24 weeks | Fibrosing NASH | N/A |
| Liang et al. 2015(69) | Ossabaw female swine | 18% fructose, 43% fat, 3500 ppm methionine, and 700 ppm choline | 6 months | 24 weeks | Fibrosing inflammation without steatosis | Caspase 3/7–induced apoptosis |
- Abbreviations: ApoE, Apolipoprotein E; CPT, carnitine palmitoyltransferase; FADS, fatty acid desaturase; FDFT1, farnesyldiphosphate farnesyl-transferase 1; JNK, c-Jun N-terminal kinase; MCP1, monocyte chemotactic protein 1; MIP2, macrophage inflammatory protein 2; PDGF-B, platelet-derived growth factor B; PUFA, polyunsaturated fatty acids; RANTES, regulated on activation normal T cell expressed and secreted; SAA, serum amyloid A; Samhd1, SAM domain and HD domain 1; SCD1, stearoyl coenzyme A desaturase; Scyb9, small inducible cytokine B9.
Genetic Polymorphisms Associated With NASH Are Related to Hepatic Cholesterol Metabolism
Many human genetic polymorphisms that have been strongly linked to NAFLD, NASH, and NASH-related cirrhosis appear to be related to hepatic cholesterol metabolism, although some clearly affect other lipids too. The most common and well-described is the patatin-like phospholipase domain-containing protein 3 (PNPLA3) I148M variant, which causes impairment of very low density lipoprotein (VLDL) secretion, LD remodeling, and hydrolase activity for triglycerides and retinyl esters.(71, 72) Homozygous carries of the PNPLA3 I148M variant have a greater risk of progressive steatohepatitis and fibrosis.(73) Carriers of the TM6SF2 (transmembrane 6 superfamily member 2) E167K variant have impaired hepatic VLDL secretion, and are at higher risk for liver disease; however, they are at lower risk of cardiovascular events.(74) ApoB mutations, characteristic of familial hypobetalipoproteinemia, impair hepatic secretion of VLDL particles, which results in worsening steatosis, steatohepatitis, and cirrhosis.(75) Polymorphisms in farnesyl diphosphate farnesyl transferase 1 (FDFT1), encoding squalene synthase, the first enzyme in the sterol biosynthesis pathway, have been associated with NAFLD activity scores and fibrosis.(76) Patients with mutations in the LIPA (lysosomal acid lipase) gene, encoding lysosomal acid lipase, accumulate CEs and triglycerides in the liver, with progression to hepatic steatosis, fibrosis, and cirrhosis.(77) Nongenetic reductions in lysosomal acid lipase activity have been identified in patients with NAFLD, with higher reductions in lysosomal acid lipase activity, resulting in worsening disease.(78) Finally, a newly investigated protein, HSD17B13 (17β hydroxysteroid dehydrogenase 13), a LD enzyme with retinal dehydrogenase activity that also plays a key role in cholesterol and fatty acid metabolism, was found to have 5.9-fold higher hepatic expression in patients with NASH compared with controls.(79) Although it is intriguing that these polymorphisms appear to affect hepatic cholesterol homeostasis directly or indirectly, it is important to emphasize that some also affect other lipids and that the specific mechanisms by which each polymorphism causes NASH are complex and not fully elucidated.
Mechanisms of CASH Development
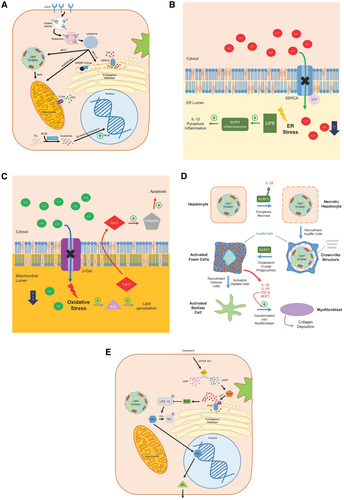
Cholesterol accumulation results in dysfunction of many organelles within hepatocytes and activation of other liver cells critical to fibrosing steatohepatitis, such as KCs and HSCs. The fluidity of a cell’s membranes, both the outer plasma membrane as well as membranes of internal organelles, is dependent on a precise ratio of FC to phospholipids, as well as the saturation status of the phospholipids.(80) FC accumulation within a cell membrane causes liquid-ordered rafts to become too rigid, which affects transmembrane proteins that require a degree of fluidity in order to function properly.(80) Figure 4 summarizes the processes by which hepatic FC accumulation leads to hepatocyte dysfunction (FIG. 4A).
Cholesterol and ER Stress
The ER is responsible for a number of critical cellular functions, including folding and posttranslational modification of proteins, calcium storage, lipid-membrane biosynthesis, drug metabolism, regulating surviving and cell death signals, and signaling the production of cholesterol through Scap/SREBP-2.(33, 34, 81, 82) Multiple cellular aberrations can lead to ER stress and impair the proper folding of proteins, including oxidative stress, calcium dysregulation, hyperglycemia, inflammation, and hypercholesterolemia.(83, 84) Elevated FC/phospholipid ratio in the ER membrane impairs the action of sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) in mice, a pump that maintains high Ca2+ concentration in the ER lumen to facilitate protein folding (FIG. 4B).(83-86) Impaired functionality of SERCA results in decreased luminal calcium concentration, higher levels of unfolded proteins, and ER stress.(83-86) Activation of the unfolded protein response (UPR) leads to up-regulation of key enzymes that alleviate ER stress by decreasing ER secretory load and enhancing protein folding. Conversely, in cases of chronic ER stress in mouse and human models, the UPR can actually promote worsening steatosis, apoptosis, autophagy, or activation of the NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasome causing interleukin (IL) 1β production, pyroptosis, and hepatic inflammation.(83, 87, 88) In this way, ER stress leads to a positive feedback loop of worsening steatosis, ER stress, cell death, and inflammation characteristic of NASH.
Cholesterol in Mitochondrial Stress
The mitochondria membrane contains little cholesterol compared with other cellular membranes and is more susceptible to slight alterations in cholesterol membrane content.(89, 90) Steroidogenic acute regulatory proteins transfer cholesterol from late endosome/lysosome (LE/LY) to the mitochondria for steroid synthesis in steroidogenic cells, and demonstrate a 7-15-fold increase in expression in patients with steatosis and NASH.(66, 91) Increased delivery of cholesterol to the mitochondrial membrane results in dysfunction of membrane proteins such as 2-oxoglutarate (2-Oxo)(90) (FIG. 4C). When mitochondrial cholesterol content increases in mice and human HepG2 cells, the fluidity of the mitochondrial membrane is reduced, impairing the function of 2-Oxo and depleting the mitochondrial glutathione pool.(90, 92) This sensitizes the hepatocyte to tumor necrosis factor α (TNF-α), promoting oxidative stress, lipid peroxidation, increased mitochondrial membrane permeability with cytochrome c release, and signaling for necrosis.(92) Indeed, studies evaluating elevated cholesterol content in mice and human HepG2 cells in mitochondria demonstrate experimental NASH.(90, 92)
Formation of Toxic Oxysterols
Formation of oxysterols within the cell occurs either through auto-oxidation of cholesterol in the setting of oxidative stress, or through hydroxylation by a number of cytochrome P450 monooxygenases, typically as an intermediary in the formation of CEs, bile acids, or steroid hormones.(93, 94) Oxysterols are known to be potent signaling molecules, binding to LXRα in human hepatocytes and promoting reverse cholesterol transport, or binding to SREBP-2 and inhibiting de novo cholesterol synthesis.(36, 44, 95-98) Studies looking at both animal models and humans with biopsy-proven NASH show increased levels of oxysterols within the liver and subsequent liver damage, inflammation, and fibrosis.(99-101) One species of oxysterol, 25-hydroxycholesterol, has been demonstrated to enhance inflammatory signaling in rat hepatocytes through nuclear factor kappa B (NF-κB) activation, a key proinflammatory regulator; however, its sulfate derivative, 25-hydoxycholesterol-3-sulfate, actually has anti-inflammatory properties.(102) In the rat mitochondria, oxysterols can promote signaling for cellular apoptosis.(101) These findings suggest a complex role of oxysterols in the pathogenesis of NASH with room for further experimentation.
Cholesterol Activates KCs and HSCs
In atherosclerotic plaques, cholesterol accumulation within macrophages results in the formation of foamy cells, and has been implicated as a prominent component of the inflammatory response found in these plaques.(103-105) In much the same way, mouse models demonstrate cholesterol accumulation within KCs, the resident macrophages in the liver, and appear to contribute to the inflammation that is characteristic of NASH.(106) As KCs in both mice and humans are not able to synthesize cholesterol de novo, they acquire cholesterol through uptake from the circulation, through LDLR-mediated endocytosis or scavenger receptors that bind oxLDL particles, or from processing remnant LDs of dead steatotic hepatocytes.(107-109) Uptake of oxLDL through the scavenger receptors, CD36 or SR-A, results in trafficking of oxLDL to the lysosome, where it gets trapped and cannot be exported out of the lysosome.(107, 108, 110) Unlike the LDLR pathway for cholesterol accumulation, the scavenger receptor pathway does not possess a negative feedback loop, leading to rapid accumulation of oxLDL in KC lysosomes and triggering hepatic inflammation.(107, 108, 110, 111) Experiments in mouse models with scavenger receptor knockout/inhibition, or alleviation of lysosomal cholesterol accumulation, have shown improvement in the hepatic inflammation characteristic of NASH.(112-114)
HSCs, a type of nonparenchymal hepatic cell located in the space of Disse, are activated by fibrogenic cytokines elaborated by KCs, specifically transforming growth factor β (TGF-β) and TNF-α, resulting in transformation into myofibroblasts, which cause hepatic fibrosis.(115) Similar to KCs, experiments in mice show FC accumulates in HSCs by uptake from scavenger receptors, specifically lectin-like oxidized LDL receptor-1 (LOX-1), which directly activates HSCs via signaling through toll-like receptor 4 (TLR-4).(116, 117) The LOX-1 IVS4-14 AG polymorphism, encoding a nontruncated splice isoform that was previously shown to confer higher cardiovascular disease (CVD) risk in homozygotes, was associated with increased severity of NASH in a study of 40 patients with biopsy-proven NASH and 40 matched controls.(118) Increased accumulation of FC leads to decreased lysosomal degradation of TLR-4, and sensitizes the cell to TGF-β signaling.(116)
Cholesterol Crystallization
The hepatocyte LD represents one of the body’s main storage sites for excess cholesterol, which is transferred to the LD membrane most likely through direct membrane contact sites with other organelles, including the ER, mitochondria, peroxisomes, and LE/LY. FC transferred to the LD membrane can be esterified to CE for “safe” storage. However, a high FC concentration can be reached in the LD membrane during this process. As the cholesterol concentration in the membrane increases, it eventually exceeds the ability of phospholipid head groups to cover all the cholesterol molecules, and excess molecules precipitate adjacent to the membrane, forming cholesterol monohydrate crystals (FIG. 4D). LD cholesterol crystals have been observed in steatotic hepatocytes in both patients with NASH and animal models of NASH.(28, 105, 109) In patients with biopsy-proven NAFLD, hepatocyte LD cholesterol crystals were observed almost exclusively in patients with NASH and not in patients with simple steatosis, suggesting that these cholesterol crystals are important in pathogenesis rather than innocent bystanders.(28)
Cholesterol crystals in subintimal atherosclerotic plaque macrophages are known to activate the NLRP3 inflammasome in humans and mice, mediating IL-1β and IL-18 release through the caspase 1 pathway.(119, 120) It is plausible that cholesterol crystallization within hepatocyte LD also activates the NLRP3 inflammasome.(121) In mouse hepatocytes, NLRP3 activation causes pyroptosis, a form of programmed cell death marked by NLRP3 activation of caspase 1, DNA damage, and cell membrane pore formation, causing cell swelling and death.(122)
KCs that process dead hepatocytes with cholesterol crystals become exposed to these crystals and their proinflammatory effects. Following pyroptosis or necrosis of steatotic hepatocytes, their remnant LDs are encircled by KCs and form characteristic “crown-like structures” (CLSs), which secrete lysosomal enzymes involved in the extracellular processing of LDs.(123-125) Processing of the LDs by lysosomal acid lipase results in the hydrolysis of CE to FC and further production of cholesterol crystals.(123, 124) KCs that are exposed to cholesterol crystals are transformed into activated lipid-laden foam cells(123, 124) through pathways that likely include activation of the NLRP3 inflammasome.(119, 120, 122-124) Given the central role of the NLRP3 inflammasome, it is no surprise that inhibition of this inflammasome in genetic and diet-induced mouse models of NASH resulted in decreased levels of inflammation and fibrosis.(126)
TAZ Pathway
A pathway has been identified recently that directly connects hepatocyte FC loading with hepatic fibrosis through HSC activation.(127) In 2016, Wang et al. showed that the transcription regulator TAZ was higher in both mouse models and patients with NASH; silencing of TAZ prevented or reversed features of steatohepatitis, but not steatosis; and expression of TAZ in models of steatosis induced steatohepatitis.(127) In most hepatocytes, TAZ is phosphorylated and in the inactive cytoplasmic state. However, models of NASH show increased dephosphorylation of TAZ to the active form, translocation to the nucleus, and transcription of target genes.(127) One of the target gene induced by TAZ is Indian hedgehog (Ihh), which can be secreted from hepatocytes and induces profibrotic genes in HSCs.(127) Wang et al. showed that silencing of TAZ in NASH models decreased gene expression of hepatocyte Ihh and subsequent profibrotic HSC mRNA.(127) A follow-up study published in 2020 showed that the process of TAZ activation is initiated by hepatocyte FC, which blocks proteosomal TAZ degradation through induction of soluble adenylyl cyclase and resulting Ca release from the ER.(128) This pathway (FIG. 4E) provides a direct link between increased hepatocyte FC levels and features of NASH.
Ezetimibe and Statins in NASH
Cholesterol-lowering medications (such as statins and ezetimibe) are very common in patients with NAFLD/NASH due to the high prevalence of hypercholesterolemia, diabetes, and CVD. In addition to their proven cardiovascular benefits, statins and ezetimibe also appear to have beneficial effects on NAFLD/NASH.(129-131) Table 3 summarizes studies that evaluated the effects of cholesterol-lowering medications on NAFLD/NASH, identified through a comprehensive review of the literature. Multiple small prospective studies in patients with either NAFLD or NASH assessed the effect of ezetimibe on steatosis, inflammation, and fibrosis. Although these studies have shown benefit in biochemical, metabolic, and histologic outcomes from ezetimibe therapy, the small size and relatively short follow-up of these studies limit their interpretation.(132-136) A meta-analysis performed in 2017 encompassing six studies and 273 patients with NAFLD or NASH suggested that ezetimibe improved serum liver enzymes, hepatocyte steatosis and ballooning, but had no effect on inflammation or fibrosis.(137)
| Study | Study type | Medication | Study Population | Duration of Treatment | Results |
|---|---|---|---|---|---|
| Chan et al. 2010(132) | Randomized, single-blind placebo controlled trial | Ezetimibe vs. placebo | 25 obese patients (ezetimibe, n = 15; hypocaloric diet alone, n = 10) | 16 weeks | Improved hepatic steatosis, inflammation, and LDL-apoB-100 metabolism |
| Park et al. 2011(135) | Prospective long-term study | Ezetimibe | 45 patients with newly diagnosed biopsy-proven NAFLD | 24 months | Improved biochemical parameters (AST, ALT, hsCRP, TC, LDL, ox-LDL, and TG), visceral fat, and histologic features (steatosis, necroinlammation, ballooning, and NAS) |
| Takeshita et al. 2014(133) | Open-label randomized controlled clinical trial | Ezetimibe vs. placebo | 32 patients with NAFLD (ezetimibe, n = 17; placebo, n = 15) | 6 months | Improved hepatic fibrosis, increased long-chain fatty acids, and Hgb A1c |
| Loomba et al. 2015(136) | Randomized, double-blind, placebo-controlled trial | Ezetimibe vs. placebo | 50 patients with biopsy-proven NASH (ezetimibe: n = 25; placebo: n = 25) | 24 weeks | No significant difference in liver fat as measured by MRI-PDFF; no significant difference in biochemical parameters or histologic response |
| Nakade et al. 2017(137) | Meta-analysis | Ezetimibe | Six studies (two randomized-controlled; four single-arm trials) including 273 patients with NAFLD or NASH | 24 weeks, four studies | Improved serum liver enzymes (AST, ALT, and GGT), hepatic steatosis, and ballooning |
| 48 weeks, one study | |||||
| 96 weeks, one study | |||||
| Athyros et al. 2006(130) | Prospective, open-label randomized study | Atorvastatin vs. fenofibrate vs. combination | 186 nondiabetic patients with MetS and biochemical and ultrasonographic evidence of NAFLD | 54 weeks | Significantly higher percentage of patients who no longer had evidence of NAFLD in the atorvastatin and combination groups, including reduction in hs-CRP, TG, LDL-C, TC, and glucose |
| Nelson et al. 2009(179) | Double-blind, randomized, placebo-controlled trial | Simvastatin vs. placebo | 16 patients with biopsy-proven NASH, 14 completed the study, 10 underwent repeat biopsy at 1 year | 12 months | No statistically significant improvement in serum aminotransferases, hepatic steatosis, necroinflammatory activity, or stage of fibrosis |
| Athyros et al. 2010(129) | Post hoc analysis of prospective, randomized intention-to-treat study (GREACE) | Atorvastatin vs. placebo | 1,600 GREACE patients with coronary heart disease, 437 patients with moderately abnormal liver enzymes possibly associated with NAFLD (227 treated with statin) | 3 years | Statin-treated patients had significant improvement in liver enzymes and reduction in cardiovascular events |
| Athyros et al. 2011(141) | Post hoc analysis of prospective randomized controlled trial comparing two LDL-C targets, <100 mg/dL (A2) or <130 mg/dL (B2) | Atorvastatin | 1,123 ATTEMPT patients with MetS without diabetes or CVD, 326 with modestly elevated liver enzymes and ultrasonographic evidence of NAFLD | 42 months | 86% in the A2 group and 74% in the B2 group had resolution of NAFLD (P < 0.001), mean LDL-C and TG targets were higher in the B2 group compared with the A2 group |
| Foster et al. 2011(144) | Prospective, randomized, placebo-controlled trial as part of the St. Francis Heart Study | Atorvastatin vs. placebo | 1,005 patients, 80 with NAFLD at baseline | 3.6 years | Treatment with atorvastatin plus vitamins C and E, significantly reduced the odds of NAFLD at the end of follow-up (70% vs. 34%, OR 0.29, P < 0.001) |
| Tikkan et al. 2013(140) | Post hoc analysis of a prospective randomized controlled trial (IDEAL) | Atorvastatin 80 mg/day vs. simvastatin 20-40 mg/day | 8,863 IDEAL patients, 1,081 with ALT ≥ ULN | 4.8 years | Major CVD event rates were 11.5% for simvastatin and 6.5% for atorvastatin; in patients with baseline elevated ALT, greater improvement in ALT was noted in atorvastatin group ( 13.4 ± 27.5 vs. 13.4 ± 27.5 vs.  8.8 ± 28.8; P = 0.0073) 8.8 ± 28.8; P = 0.0073) |
| Dongiovanni et al. 2015(142) | Multicenter cohort study | Statins (simvastatin 49%; rosuvastatin 27%; atorvastatin 17%; pravastatin 4%; fluvastatin 2%) vs. no statins | 1,201 European patients who underwent liver biopsy for suspected NASH, 107 on statin therapy for at least 6 months | 6 months | Statin use was associated with lower risk of steatosis (OR 0.09, P = 0.004), steatohepatitis (OR 0.25, P < 0.001), and fibrosis stage F2-F4 (OR 0.42, P = 0.017) |
| Kargiotis et al. 2015(145) | Prospective study | Rosuvastatin | 20 patients with biopsy proven NASH, MetS, and dyslipidemia | 12 months | Postintervention liver biopsy showed complete resolution of NASH in 19 of 20 patients, normalization of AST/ALT and GGT by the third treatment month, and normalization of ALP by the sixth treatment month |
| Nascimbeni et al. 2016(143) | Cross-sectional study | Statins (45%) (simvastatin 15%; pravastatin 6%; fluvastatin 2%; atorvastatin 53%; rosuvastatin 15%) vs. no statins (55%) | 346 patients with diabetes with biopsy-proven NAFLD | N/A | Statins use was associated with a lower risk of NASH (OR 0.57, P = 0.055) and F2-F4 fibrosis (OR 0.47, P = 0.011) |
| Kim et al. 2017(131) | Systemic review and meta-analysis | Statins vs. no statins | 13 studies (10 cohort studies, 3 clinical trials) in 121,058 patients with chronic liver disease, 46% exposed to statins | N/A | In patients with cirrhosis, statin use was associated with a 46% lower risk of decompensation (RR 0.54) and 46% lower morality (RR 0.54). In patients with chronic liver disease without cirrhosis, statin use was associated with a 58% lower risk of development of cirrhosis or fibrosis progression (RR 0.42). Statin use was also associated with a 27% lower risk of variceal bleeding or progression to portal hypertension (HR 0.73) |
- Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; apoB-100, apolipoprotein B-100; AST, aspartate aminotransferase; GGT, gamma-glutamyltransferase; GREACE, Greek Atorvastatin and Coronary Heart Disease Evaluation; Hgb A1c, hemoglobin A1c; HR, hazard ratio; hsCRP, high sensitivity C-reactive protein; MRI-PDFF, magnetic resonance imaging proton density fat fraction; NAS, NAFLD activity score; OR, odds ratio; ox-LDL, oxidized LDL; RR, risk ratio; TC, total cholesterol; TG, triglyceride.
Despite concerns about statin-induced hepatotoxicity, studies reported very rare incidence of statin-related adverse events in patients with liver disease.(138, 139) Post hoc analysis of three large randomized, controlled, trials designed to evaluate the effect of statins on CVD, consisting of 11,587 patients, including 1,844 with elevated aminotransferases, demonstrated that statins resulted in improvement in serum aminotransferase levels and ultrasonographic steatosis.(129, 140, 141) In 2015, a multicenter cohort study consisting of 1,201 European patients who underwent liver biopsy for suspected NASH showed that the 107 patients who were taking statins had a protective effect from steatosis, inflammation, and NASH in a dose-dependent manner.(142) A multicenter, Italian cross-sectional study of 346 patients with diabetes with biopsy-proven NAFLD, confirmed that statins were independently associated with reduced odds of NASH and significant fibrosis.(143) Multiple small prospective trials using atorvastatin and rosuvastatin demonstrated improvement in biochemical, radiological, and histological features of NAFLD and NASH.(130, 144, 145) A systemic review of 121,058 patients with chronic liver disease showed that statins reduced the risk of portal hypertension, progression to cirrhosis or decompensated cirrhosis, and mortality.(131)
Meta-analyses of randomized controlled trials, including very large numbers of participants, demonstrated that statins resulted in a slightly increased risk of development of diabetes,(146, 147) but the risk was low both in absolute terms and when compared with the reduction in coronary events. Specifically, treatment of 255 patients with statins for 4 years resulted in one extra case of diabetes (or approximately one case of diabetes per 1,000 patient-years). In observational studies, statin-treated patients had increased hepatic de novo lipogenesis through activation of SREBP-1c and up-regulation of genes involved in fatty acid and triglyceride metabolism, suggesting that activation of these genes contributes to insulin resistance and diabetes.(148) Because insulin resistance and diabetes are important risk factors for NASH, these findings raise some concern about the role of statins as potential NASH pharmacotherapies.
In summary, this evidence suggests beneficial effect of statins on steatosis, inflammation, fibrosis, portal hypertension and cirrhosis, and confirms the safety of statins for the treatment of dyslipidemia in patients with NAFLD and NASH as recommended by the American Association for the Study of Liver Diseases.(1) However, large, randomized, placebo-controlled trials of statins in NASH adequately powered for histological outcomes are lacking. Such studies are desperately needed but very difficult to design, as it may be considered unethical to randomize patients with NASH to placebo, given that most would fulfill criteria for being on a statin for cardiovascular reasons.
Conclusions
NASH is rapidly rising in prevalence worldwide and currently has no approved pharmacological treatments. In the near future, the number of liver transplantations for NASH will surpass all other indications for liver transplantation. The evidence presented in this review strongly supports the role of cholesterol in causing “cholesterol-associated steatohepatitis” (CASH) and should serve to focus efforts on targeting cholesterol lowering as a therapeutic option. This strategy has multiple advantages. First, statins are widely available, inexpensive medications with a proven track record of safety. Second, statins are proven to reduce cardiovascular mortality, which is the number-one cause of death in patients with NASH, and may have even greater cardiovascular benefits in patients with NASH.(129) Therefore, treatment of patients with NASH with statins would potentially simultaneously ameliorate both cardiovascular mortality as well as liver-related complications (e.g., cirrhosis and portal hypertension) and mortality. Randomized controlled trials of statins in patients with NASH or cirrhosis that are under way are eagerly awaited, while clearly more such studies are urgently needed.




