Shelter-Based Integrated Model Is Effective in Scaling Up Hepatitis C Testing and Treatment in Persons Experiencing Homelessness
This study was supported by an investigator-initiated grant through Gilead Sciences Inc. (IN-US-342-4531 to M.K.), and in part by National Institutes of Health (K24AA022523 to M.K., UCSF Liver Center P30 DK026743, and T32DA007250 to J.K.F.).
Potential conflict of interest: Mandana Khalili is a recipient of a research grant (to her institution) from Gilead Sciences Inc, and Intercept Pharmaceuticals and she has served as a consultant for Gilead Sciences Inc. Jesse Powell is a recipient of a research grant from Gilead Sciences Inc and he has served on an advisory board for Gilead Sciences Inc.
Abstract
Hepatitis C virus (HCV) prevalence is high among people experiencing homelessness, but barriers to scaling up HCV testing and treatment persist. We aimed to implement onsite HCV testing and education and evaluate the effectiveness of low-barrier linkage to HCV therapy among individuals accessing homeless shelters. HCV rapid testing was performed at four large shelters in San Francisco (SF) and Minneapolis (MN). Sociodemographic status, HCV risk, barriers to testing, and interest in therapy were captured. Participants received information about HCV. Those testing positive underwent formal HCV education and onsite therapy. Multivariable modeling assessed predictors of receipt of HCV therapy and sustained virologic response (SVR). A total of 766 clients were tested. Median age was 53.7 years, 68.2% were male participants, 46.3% were Black, 27.5% were White, 13.2% were Hispanic, and 57.7% had high school education or less; 162 (21.1%) were HCV antibody positive, 107 (66.0%) had detectable HCV RNA (82.1% with active drug use, 53.8% history of psychiatric illness), 66 (61.7%) received HCV therapy, and 81.8% achieved SVR. On multivariate analysis, shelter location (MN vs. SF, odds ratio [OR], 0.3; P = 0.01) and having a health care provider (OR, 4.1; P = 0.02) were associated with receipt of therapy. On intention to treat analysis, the only predictor of SVR when adjusted for age, sex, and race was HCV medication adherence (OR, 14.5; P = 0.01). Conclusion: Leveraging existing homeless shelter infrastructure was successful in enhancing HCV testing and treatment uptake. Despite high rates of active substance use, psychiatric illness, and suboptimal adherence, over 80% achieved HCV cure. This highlights the critical importance of integrated models in HCV elimination efforts in people experiencing homelessness that can be applied to other shelter settings.
Abbreviations
-
- CI
-
- confidence interval
-
- DAA
-
- direct-acting antiviral
-
- F
-
- fibrosis stage
-
- HBV
-
- hepatitis B virus
-
- HCC
-
- hepatocellular carcinoma
-
- HCV
-
- hepatitis C virus
-
- HIV
-
- human immunodeficiency virus
-
- IQR
-
- interquartile range
-
- ITT
-
- intention to treat
-
- OR
-
- odds ratio
-
- SVR
-
- sustained virologic response
Despite the introduction of direct-acting antiviral (DAA) therapy, hepatitis C virus (HCV) remains a significant public health concern that affects more than 2 million adults in the United States.(1) Without appropriate intervention, persistent HCV infection can progress to cirrhosis and hepatocellular carcinoma (HCC).(2) As a result of multiple risk factors, including injection drug use, incarceration history, and psychiatric illness, individuals experiencing homelessness are more frequently infected with HCV.(3-10) Studies have demonstrated significant challenges in health care delivery for this vulnerable population and have identified barriers to HCV testing and treatment on all individual, societal, and system levels.(11, 12) Nevertheless, motivation and interest for HCV counseling and therapy remain high among patients experiencing homelessness, demonstrating the potential to eradicate the virus through innovative approaches.(13)
Given the significant HCV disease burden, efforts have focused on identifying gaps in the HCV care continuum and evaluating the effectiveness of treatment within vulnerable populations. Despite these efforts, HCV treatment uptake among individuals experiencing homelessness remains low.(14-17) A study evaluating the HCV care cascade found that veterans experiencing homelessness were less likely to receive therapy despite higher rates of testing compared to veterans with a stable residence.(14) In a retrospective study of 885 patients infected with HCV at five health centers (including one that provided care for those experiencing homelessness) in an urban US setting, history of homelessness was associated with lower odds of medical evaluation by an HCV provider.(15) In addition, the rate of HCV treatment initiation among centers that included patients experiencing homelessness was low at 27%.(15) Similarly, another study evaluating treatment outcomes for individuals experiencing homelessness in a community center in Boston showed low rates of treatment initiation but high rates of treatment completion and sustained virologic response (SVR) at 95% and 85%, respectively.(16) A high SVR rate (97%) following completion of DAA therapy was also noted in another retrospective study from the Boston Health Care for the Homeless Program.(18) These studies suggest that tailored interventions are needed to reduce barriers to treatment access and to enhance treatment engagement in the homeless population. Additionally, integrated care models have been shown to be important not only in delivering health care to persons experiencing homelessness but also in treating HCV among patients with substance abuse or psychiatric illness comorbidities.(19, 20)
To inform the design and implementation of an effective HCV care model within the homeless shelters setting, we previously conducted a needs assessment through engagement of stakeholders, including shelter clients, shelter leadership, medical staff, and providers.(11, 21) We then implemented HCV testing and treatment within four homeless shelters in two diverse urban cities, San Francisco, CA, and Minneapolis, MN. In this prospective study, we aimed to evaluate the prevalence of HCV among patients accessing homeless shelters as well as the efficacy of this integrated and shelter-based HCV testing and treatment intervention.
Participants and Methods
Study Population and Study Design
This prospective study was conducted by a multidisciplinary team at four large homeless shelters, two in San Francisco, CA, and two in Minneapolis, MN, from August 1, 2018, to January 30, 2021. These homeless shelters provided supportive services on a daily basis to more than 300 residents in San Francisco and between 170 to 350 residents in Minneapolis. The services provided included shelter, meals, case management, and some level of medical care ranging from basic triage to specialist consultation.
Following informed consent, adults 18 years of age and older seeking shelter services and who were either treatment naive or had not received HCV treatment within the prior 12 weeks were enrolled. In addition, clients experiencing homelessness who were HCV positive and who accessed low-threshold temporary shelters and safety-net liver specialty care were also recruited. Patients with significant medical or psychiatric conditions that prevented consenting or participation in the study were excluded.
Study Procedures
Clients who agreed to HCV testing and who met study eligibility criteria were enrolled, completed a questionnaire, and underwent point of care HCV testing (OraQuick HCV Rapid Antibody Test; OraSure Technology, Bethlehem, PA). Participants who tested negative for HCV antibody were provided information about HCV and its prevention.(22, 23)
Those who tested positive for HCV antibody received a confirmatory HCV RNA test and a standardized 30-minute HCV education. This in-person comprehensive education was delivered using a PowerPoint slide format and was led by a designated nurse, pharmacist, or advanced practice provider. In addition, pre-education and post-education questionnaires(24, 25) were administered, and information on HCV risk factors and HCV awareness was captured. Standard-of-care HCV therapy was offered to all participants with detectable HCV RNA through insurance or patient-assistance programs for HCV by the prescribing provider. Treatment was primarily delivered on-site at the shelters or through coordination with their primary care provider, liver specialty provider, or addiction services. The treatment plan was made at the discretion of the treating provider and in accordance with clinical practice guidelines and insurance restrictions. The medication dispensation, therefore, varied and ranged from directly observed therapy (within addiction services), to weekly or monthly dispensing, to dispensation of all medication supply at the start of therapy. If needed, the study team assisted participants in obtaining insurance and facilitated linkage to primary care services. In San Francisco, a designated HCV registered nurse coordinator managed patients on treatment within the shelters in collaboration with shelter clinic providers, primary care providers, or the San Francisco safety-net liver specialty clinic. In Minneapolis, a Doctor of Pharmacy embedded within shelter clinics managed patients on treatment in collaboration with shelter clinic staff and the Minneapolis safety-net liver specialty clinic. Patients were followed throughout therapy and completed laboratory tests and questionnaires at the end of treatment and at 12 weeks following completion of therapy (SVR). Clinical data, including bloodwork, imaging, medical history, and adherence to medications, were collected from the participants’ medical records. A US $25 incentive was given for HCV testing, and a total of $75 was given following HCV education and completion of HCV therapy, including SVR blood work.
Institutional review board approvals were obtained from the University of California San Francisco and Hennepin Healthcare Human Subjects Research Committee, and all participants provided written consent.
Assessment of Clinical Variables
Active HCV infection was confirmed with a detectable HCV RNA, and response to therapy was evaluated by HCV RNA testing at the end of therapy and at SVR time points. Diagnosis of cirrhosis was made based on abdominal imaging or presence of fibrosis stage 4 (F4) on transient elastography (FibroScan), FibroSURE (Laboratory Corporation of America, Burlington, NC), or FIB-4 index >3.25.(26) In addition, fibrosis stage F0-F3 was captured when transient elastography or FibroSURE was available. Cirrhotic decompensation was assessed by presence of ascites, hepatic encephalopathy, or history of variceal hemorrhage. Presence of HCC was captured from clinical records and by liver imaging.
Nonprescription drug use, alcohol consumption, and receipt of substance-use therapy before treatment, at the end of therapy, and SVR time point were assessed by self-report. Alcohol consumption was categorized as (1) none or minimal (<1 drink per month), (2) moderate (more than none or minimal but no more than four drinks/day or 14 drinks/week in men, no more than three drinks/day or seven drinks/week in women), (3) heavy (more than four drinks/day for men, more than three drinks/day for women; or binge drinking [five or more drinks for men, four or more drinks for women, on the same occasion]).(27)
Statistical Analysis
Descriptive analyses of cohort characteristics were performed to obtain frequency for categorical variables and median (interquartile range [IQR]) or mean (SD) for continuous variables. Patient characteristics between those with and without receipt of therapy and those who achieved SVR at 12 weeks and those who did not achieve SVR were compared using the chi-squared test or the Fisher’s exact test if appropriate for categorical variables and the Mann-Whitney test for continuous variables. Univariate and multivariable modeling was performed with the outcome measures of receipt of therapy and SVR as well as an a priori list of predictors and those predictors with a P < 0.2 on univariate analysis. The multivariable model for the outcome of receipt of therapy was adjusted for age, sex, race, illicit drug or alcohol use within the past year, receipt of HCV education, and severity of liver disease, while the outcome of SVR was adjusted for age, sex, and race. All analyses were performed in Stata 15 statistical software (Stata Corp LP, College Station, TX).
Results
Participants
Participant recruitment strategies included advertisement for availability of HCV testing in the shelters as well as directly approaching 1,199 shelter clients. A total of 772 clients agreed to participate in the study; of these, 766 were deemed eligible. There were no significant differences (P > 0.05) in age, sex, race, or shelter site among those who met or did not meet enrollment eligibility (data not shown).
HCV Screening
During the study, a total of 120 HCV testing sessions were performed (72 in San Francisco and 49 in Minneapolis). Of the 766 participants, 162 (21.1%) tested positive for HCV antibody; of these, 107 (66.0%) had detectable HCV RNA. Patient characteristics overall and by HCV antibody status are summarized in Table 1. Those who were HCV antibody positive were older (median age 55.8 vs. 52.6 years), a higher proportion were men (75.3% vs. 66.2%), and of non-Hispanic White race (39.1% vs. 24.4%) compared to those who were HCV antibody negative. In addition, patients who were HCV positive (vs. HCV antibody negative) were more likely to report having a health care provider (82.6% vs. 73.8%), receipt of prior HCV testing (79.6% vs. 45.2%), history of injection drug use (66.7% vs. 13.0%), history of substance-use therapy (62.4% vs. 37.6%), and illicit drug use within the prior year (84.4% vs. 66.7%). The proportion of participants who reported receipt of prior HCV testing in San Francisco and Minneapolis was 63.4% and 38.6%, respectively; of these, 17.6% and 12.9%, respectively, reported testing positive. Overall, 60.5% of participants who reported having previously tested positive for HCV had active infection with detectable HCV RNA. There was no significant difference in willingness to engage in HCV therapy or in subsequent receipt of HCV therapy between those patients who were viremic who did or did not report prior knowledge of a positive HCV test (90.8% vs. 93.0% and 68.2% vs. 65.9%, respectively).
| Total (n = 766†) | HCV antibody positive (n = 162†) | HCV antibody negative (n = 604†) | P Value* | |
|---|---|---|---|---|
| Age in years, median (range) [IQR] | 53.7 | 55.8 | 52.6 | 0.0001 |
| (19.5-82.1) | (21.2-82.1) | (19.5-82.1) | ||
| [44.1-59.9] | [49.4-62.7] | [42.9-59.4] | ||
| Male sex (%) | 68.2 | 75.3 | 66.2 | 0.03 |
| Race (%) | (n = 763) | (n = 161) | (n = 602) | 0.007 |
| Black/African American | 46.3 | 41 | 47.7 | |
| White, non-Hispanic | 27.5 | 39.1 | 24.4 | |
| Hispanic | 13.2 | 9.3 | 14.3 | |
| Native American/Alaska Native | 2.4 | 3.1 | 2.2 | |
| Asian/Pacific Islander | 2.4 | 1.9 | 2.5 | |
| Multiple races | 8.3 | 5.6 | 9 | |
| Education (%) | (n = 757) | (n = 161) | (n = 596) | 0.2 |
| Less than high school | 20 | 24.8 | 18.6 | |
| High school | 37.7 | 37.9 | 37.6 | |
| More than high school | 42.4 | 37.3 | 43.8 | |
| Insurance type (%) | (n = 709) | (n = 150) | (n = 559) | 0.09 |
| Public | 83.4 | 89.3 | 81.8 | |
| Private | 6.5 | 4 | 7.2 | |
| Uninsured | 10.2 | 6.7 | 11.1 | |
| Has a health care provider (%) | (n = 765) | (n = 161) | (n = 604) | 0.02 |
| 75.7 | 82.6 | 73.8 | ||
| History of prior HCV testing (%) | (n = 764) | (n = 162) | (n = 602) | <0.0001 |
| 52.5 | 79.6 | 45.2 | ||
| History of injection drug use ever (%) | (n = 759) | (n = 159) | (n = 600) | <0.0001 |
| 24.2 | 66.7 | 13 | ||
| Illicit drug use within the past year (%) | (n = 758) | (n = 160) | (n = 598) | <0.0001 |
| 70.5 | 84.4 | 66.7 | ||
| Alcohol use within the past year (%) | (n = 760) | (n = 160) | (n = 600) | 0.3 |
| None/minimal | 46.2 | 41.9 | 47.3 | |
| Moderate | 24.3 | 23.8 | 24.5 | |
| Heavy/binge | 29.5 | 34.4 | 28.2 | |
| History of substance use therapy (%) | (n = 742) | (n = 157) | (n = 585) | <0.0001 |
- *P < 0.05 is significant.
- † Unless otherwise indicated.
Chronic HCV Therapy
Of the 107 participants with detectable HCV RNA, 66 (61.7%) initiated standard of care HCV treatment (44 in San Francisco and 22 in Minneapolis). The treatment regimens prescribed were as follows: 43 patients (65.2%) received glecaprevir/pibrentasvir, 18 patients (27.3%) sofosbuvir/velpatasvir, 2 patients (3%) elbasvir/grazoprevir, and 1 (1.5%) patient each of sofosbuvir/ledipasvir, sofosbuvir/ledipasvir+ribavirin, and sofosbuvir/velpatasvir/voxilaprevir. Characteristics of participants with detectable HCV RNA by receipt of HCV therapy are summarized in Table 2. There were no significant differences with respect to sociodemographic status, substance use, history of psychiatric illness, or other medical comorbidities, laboratory, or clinical measures in those who initiated therapy compared to those who did not initiate treatment. However, a significantly higher proportion of patients who did not initiate therapy were from Minneapolis compared to San Francisco (56.1% vs. 43.9%, P = 0.03). In comparing the characteristics of patients with detectable HCV RNA by shelter location, a higher proportion of patients in San Francisco were non-Hispanic White whereas a higher proportion of patients in Minneapolis were Black (P = 0.03). There were no other significant differences in patient characteristics between the two sites (Supporting Table S1).
| All Patients With Detectable HCV RNA (N = 107†) | Did Not Receive HCV Therapy (n = 41†) | Received HCV Therapy (n = 66†) | P Value* | |
|---|---|---|---|---|
| Median age in years (range), [IQR] | 55.7 | 56.5 | 55.45 | 0.87 |
| (21.2-82.1) | (21.2-70.2) | (28.2-82.1) | ||
| [48.8-62.3] | [47.8-62.7] | [49.4-60.5] | ||
| Male sex, n (%) | 84 (78.5) | 35 (85.4) | 49 (74.2) | 0.23 |
| Race, n (%) | 0.31 | |||
| White, non-Hispanic | 48 (44.9) | 21 (51.2) | 27 (40.9) | |
| Black/African American | 42 (39.3) | 13 (31.7) | 29 (43.9) | |
| Asian or Pacific Islander | 1 (0.9) | 1 (2.4) | 0 (0) | |
| Hispanic | 9 (8.4) | 5 (12.2) | 4 (6.1) | |
| Native American/Alaska Native | 2 (1.9) | 0 (0.0) | 2 (3.0) | |
| Multiple races | 5 (4.7) | 1 (2.4) | 4 (6.1) | |
| Education, n (%) | (n = 106) | (n = 40) | 0.9 | |
| Less than high school | 24 (22.6) | 9 (22.5) | 15 (22.7) | |
| High school | 45 (42.5) | 16 (40.0) | 29 (43.9) | |
| More than high school | 37 (34.9) | 15 (37.5) | 22 (33.3) | |
| Employment status within the prior year, n (%) | (n = 106) | (n = 40) | 0.34 | |
| Employed | 13 (12.3) | 4 (10.0) | 9 (13.6) | |
| Unemployed | 49 (46.2) | 17 (42.5) | 32 (48.5) | |
| Retired/disabled | 42 (39.6) | 17 (42.5) | 25 (37.9) | |
| Other | 2 (1.9) | 2 (5.0) | 0 (0.0) | |
| Incarcerated within the prior year, n (%) | (n = 106) | (n = 40) | 1 | |
| 9 (8.5) | 3 (7.5) | 6 (9.1) | ||
| Insurance type, n (%) | (n = 99) | (n = 34) | (n = 65) | 1 |
| Public | 88 (88.9) | 31 (91.2) | 57 (87.7) | |
| Private | 4 (4.0) | 1 (2.9) | 3 (4.6) | |
| Uninsured | 7 (7.1) | 2 (5.9) | 5 (7.7) | |
| Has a health care provider, n (%) | (n = 101) | (n = 36) | (n = 65) | 0.11 |
| 82 (81.2) | 26 (72.2) | 56 (86.5) | ||
| History of substance use therapy, n (%) | (n = 106) | (n = 40) | 0.45 | |
| 63 (59.4) | 25 (62.5) | 38 (57.6) | ||
| Drug use within the past year, n (%) | (n = 106) | (n = 40) | 0.69 | |
| 87 (82.1) | 34 (85.0) | 53 (80.3) | ||
| Injection drug use ever, n (%) | (n = 106) | (n = 40) | 0.68 | |
| 69 (65.1) | 25 (62.5) | 44 (66.7) | ||
| Alcohol use within the past year, n (%) | (n = 105) | (n = 39) | 0.43 | |
| None/minimal | 43 (41.0) | 19 (48.7) | 24 (36.4) | |
| Moderate | 26 (24.8) | 9 (23.1) | 17 (25.8) | |
| Heavy | 36 (34.3) | 11 (28.2) | 25 (37.9) | |
| History of psychiatric illness, n (%) | (n = 106) | (n = 40) | 0.42 | |
| 57 (53.8) | 24 (60.0) | 33 (50.0) | ||
| Diabetes type 2, n (%) | (n = 106) | (n = 40) | 0.59 | |
| 16 (15.1) | 7 (17.5) | 9 (13.6) | ||
| Chronic kidney disease, n (%) | (n = 106) | (n = 40) | 0.36 | |
| 5 (4.7) | 3 (7.5) | 2 (3.0) | ||
| HIV coinfection, n (%) | (n = 105) | (n = 40) | (n = 65) | 0.71 |
| 7 (6.67) | 2 (5) | 5 (7.7) | ||
| HBV coinfection, n (%) | (n = 103) | (n = 38) | (n = 65) | 0.25 |
| 8 (7.77) | 1 (2.6) | 7 (10.8) | ||
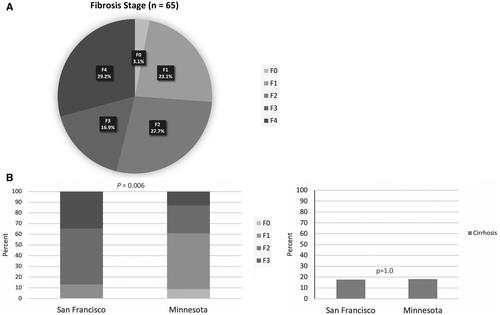
| Fibrosis stage, n (%) | (n = 65) | (n = 18) | (n = 47) | 0.78 |
| F0 | 2 (3.1) | 1 (5.5) | 1 (2.1) | |
| F1 | 15 (23.1) | 4 (22.2) | 11 (23.4) | |
| F2 | 18 (27.7) | 4 (22.2) | 14 (29.8) | |
| F3 | 11 (16.9) | 2 (11.1) | 9 (19.1) | |
| Cirrhosis, n (%) | (n = 106) | (n = 40) | 1 | |
| 19 (17.9) | 7 (17.5) | 12 (18.2) | ||
| History of cirrhosis decompensation, n (%) | (n = 106) | (n = 40) | 1 | |
| 3 (2.8) | 1 (2.5) | 2 (3.0) | ||
| Presence of HCC, n (%) | (n = 106) | (n = 40) | 0.56 | |
| 3 (2.8) | 2 (5.0) | 1 (1.5) | ||
| Platelets, median [IQR] | (n = 105) | (n = 39) | 0.71 | |
| 215 | 209 | 217 | ||
| [169-271] | [172-262] | [161-285] | ||
| AST, median [IQR] | (n = 105) | (n = 39) | 0.67 | |
| 42 | 42 | 42.5 | ||
| [33-72] | [32-72] | [34-79] | ||
| ALT, median [IQR] | (n = 105) | (n = 39) | 0.85 | |
| 41 | 41 | 41 | ||
| [29-68] | [30-72] | [27-68] | ||
| Log10 HCV RNA, median [IQR] | 1.54 × 106 | 1.54 × 106 | 1.54 × 106 | 0.79 |
| [0.29 × 106-4.58 × 106] | [0.27 × 106-4.50 × 106] | [0.34 × 106-5.11 × 106] | ||
| HCV genotype, n (%) | (n = 90) | (n = 33) | (n = 57) | 0.09 |
| 1 | 66 (73.3) | 24 (72.7) | 42 (73.7) | |
| 2 | 6 (6.7) | 4 (12.1) | 2 (3.5) | |
| 3 | 10 (11.1) | 5 (15.2) | 5 (8.8) | |
| 4 | 2 (2.2) | 0 | 2 (3.5) | |
| Indeterminate | 6 (6.7) | 0 | 6 (10.5) | |
| History of prior receipt of any HCV treatment, n (%) | (n = 106) | (n = 40) | 0.78 | |
| 15 (14.2) | 5 (12.5) | 10 (15.2) | ||
| Shelter location | 0.03 | |||
| San Francisco | 62 (57.9) | 18 (43.9) | 44 (66.7) | |
| Minneapolis | 45 (42.1) | 23 (56.1) | 22 (33.3) |
- *P value statistically significant if <0.05.
- † Uness otherwise indicated.
- Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
With respect to severity of liver disease, of the 106 patients with detectable HCV RNA and available data, 19 (17.9%) had cirrhosis, 3 (2.8%) had liver decompensation, and 3 (2.8%) had evidence of HCC; the distribution of these patients was similar in the untreated and treated groups. When evaluating fibrosis stage in participants without cirrhosis by either noninvasive imaging (transient elastography, n = 20) or noninvasive serologic methods (FibroSURE, n = 26), 2 (3.1%) were F0, 15 (23.1%) were F1, 18 (27.7%) were F2, and 11 (16.9%) were F3 (Fig 1A) . Therefore, the overall severity of liver disease by all modalities was 26.2% mild (F0 and F1), 27.7% moderate (F2), and 46.1% advanced fibrosis or cirrhosis (F3 and F4). The distribution of liver disease severity by shelter location is shown in Fig. 1B. Although the proportion of patients with cirrhosis was not statistically different between San Francisco and Minneapolis (P = 1.0), a higher proportion of patients from Minneapolis had F0-F1 and conversely, a higher proportion in San Francisco had F2-F3 severity (P = 0.006). The severity of liver disease did not differ significantly between those who did or did not receive therapy in this study.
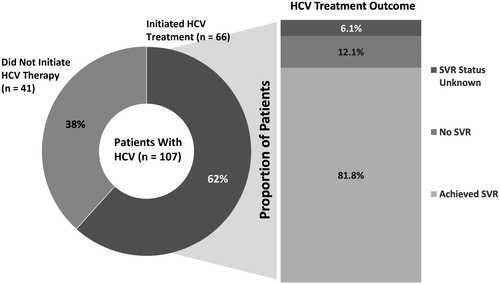
Chronic HCV Treatment Outcomes
Of the 66 patients who were initiated on HCV therapy, 54 patients (81.8%) achieved SVR, 8 (12.1%) did not achieve SVR, and the SVR status was unknown in 4 patients (6.1%) (Fig. 2). The median time to initiation of therapy was 56 (IQR, 32-107) days overall and longer in Minneapolis compared to San Francisco (76 days vs. 43 days, P = 0.04).
At the start of HCV therapy, 10 patients had a history of prior HCV treatment (3 with pegylated interferon [PEG-IFN]/ribavirin, 4 with DAA, and 3 with unknown prior treatment regimen). Of those with prior PEG-IFN/ribavirin therapy, 1 patient was treated with sofosbuvir/velpatasvir and achieved SVR and 2 were treated with glecaprevir/pibrentasvir, of whom 1 achieved SVR and 1 did not. Of the 4 with prior DAA therapy, 3 had received sofosbuvir/ledipasvir; 1 of these patients was treated with sofosbuvir/velpatasvir and did not achieve SVR, and 2 were treated with glecaprevir/pibrentasvir or elbasvir/grazoprevir and both achieved SVR. One patient was previously treated with glecaprevir/pibrentasvir and treated with sofosbuvir/velpatasvir in the study and achieved SVR. Among those with unknown prior treatment regimen, 2 achieved SVR and 1 patient had an unknown SVR status.
There were no significant differences in rate of SVR among those with known SVR status by treatment site (82.5% in San Francisco, 95.5% in Minneapolis; P = 0.2). However, on intention to treat (ITT) analysis that included all patients, a higher proportion of patients in Minneapolis achieved SVR compared to San Francisco (95.5% vs. 75.0%, P = 0.049). Of the 8 patients who did not achieve SVR, 7 were treatment nonresponders and 1 achieved response at the end of therapy but experienced virologic relapse after discontinuation of therapy. Four patients (1 with relapse and 3 with nonresponse) were retreated for HCV with sofosbuvir/velpatasvir/voxilaprevir; 2 of these patients subsequently achieved SVR, SVR status was unknown in 1 patient, and another patient was deemed noncompliant to retreatment.
Factors Associated With Receipt of Therapy
Univariate and multivariate models of factors associated with receipt of therapy are shown in Table 3. On univariate analysis, shelter location was associated with receipt of therapy. On multivariate analysis, having been enrolled in a shelter in Minneapolis compared to San Francisco was negatively associated with receipt of therapy (odds ratio [OR], 0.3; 95% confidence interval [CI], 0.1-0.7; P = 0.01), while having identified a health care provider was positively associated with receipt of therapy (OR, 4.1; 95% CI, 1.2-14.1; P = 0.02). These associations were independent of age, sex, race, illicit drug or alcohol use within the past year, receipt of HCV education, and severity of liver disease.
| Variables | Univariate Analysis | Multivariate Analysis (n = 100) | |||||
|---|---|---|---|---|---|---|---|
| n | OR | 95% CI | P Value* | OR | 95% CI | P Value* | |
| Age per decade | 107 | 1.06 | 0.75-1.51 | 0.72 | 0.8 | 0.50-1.28 | 0.35 |
| Sex | 107 | 0.17 | |||||
| Male | Ref. | Ref. | |||||
| Female | 2.02 | 0.72-5.65 | 2.14 | 0.63-7.29 | 0.23 | ||
| Race | 104 | 0.12 | |||||
| White, non-Hispanic | Ref. | 0.45 | 0.17-1.22 | ||||
| Black/African American | 1.74 | 0.73-4.13 | 0.21 | ||||
| Hispanic | 0.62 | 0.15-2.61 | 0.52 | Ref. | (all other races) | ||
| Multiple races | 3.11 | 0.32-29.94 | 0.33 | ||||
| Shelter location | 107 | 0.02 | 0.01 | ||||
| San Francisco | Ref. | Ref. | |||||
| Minneapolis | 0.39 | 0.18-0.87 | 0.28 | 0.11-0.74 | |||
| Has a health care provider | 101 | 2.39 | 0.87-6.59 | 0.092 | 4.12 | 1.21-14.06 | 0.02 |
| History of psychiatric illness | 106 | 0.67 | 0.30-1.48 | 0.32 | 0.52 | 0.19-1.4.0 | 0.19 |
| Illicit drug use within the past year | 106 | 0.72 | 0.25-2.07 | 0.54 | 0.69 | 0.20-2.37 | 0.55 |
| Alcohol use within the past year | 105 | ||||||
| None/minimal | Ref. | Ref. | |||||
| Moderate | 1.5 | 0.55-4.10 | 0.43 | 2.02 | 0.63-6.48 | 0.24 | |
| Heavy/binge | 1.8 | 0.71-4.56 | 0.22 | 2.09 | 0.68-6.44 | 0.2 | |
| Completed HCV education | 107 | 2.27 | 0.48-10.71 | 0.3 | 1.44 | 0.19-11.06 | 0.73 |
| Cirrhosis | 106 | 1.05 | 0.37-2.93 | 0.93 | 0.95 | 0.26-3.43 | 0.93 |
| Education | 106 | ||||||
| Less than high school | Ref. | ||||||
| High school | 1.09 | 0.39-3.04 | 0.87 | ||||
| More than high school | 0.88 | 0.31-2.53 | 0.81 | ||||
| Employment status within the prior year | 104 | ||||||
| Employed | |||||||
| Unemployed | Ref. | ||||||
| Retired/disabled | 0.84 | 0.22-3.12 | 0.79 | ||||
| 0.65 | 0.17-2.47 | 0.53 | |||||
| Incarcerated within the prior year | 106 | 1.23 | 0.29-5.23 | 0.78 | |||
| Insurance | 99 | ||||||
| Public insurance | Ref. | ||||||
| Private insurance | 1.63 | 0.16-16.36 | 0.68 | ||||
| Uninsured | 1.36 | 0.25-7.42 | 0.72 | ||||
| History of prior receipt of any HCV treatment | 106 | 1.25 | 0.39-3.96 | 0.71 | |||
| History of substance use therapy | 103 | 0.73 | 0.32-1.61 | 0.46 | |||
| Injection drug use ever | 106 | 1.2 | 0.53-2.72 | 0.66 | |||
| HIV coinfection | 105 | 1.58 | 0.29-8.58 | 0.59 | |||
| HBV coinfection | 102 | 4.54 | 0.54-38.46 | 0.17 | |||
| HCV genotype | 82 | ||||||
| 1 | Ref. | ||||||
| 2 | 0.29 | 0.05-1.68 | 0.17 | ||||
| 3 | 0.57 | 0.15-2.18 | 0.41 | ||||
| Log10 HCV RNA | 107 | 1.18 | 0.82-1.71 | 0.37 | |||
| History of cirrhosis decompensation | 106 | 1.22 | 0.11-13.89 | 0.87 | |||
| Fibrosis stage | 65 | ||||||
| F0 | Ref. | Ref. | |||||
| F1 | 8.67 | 0.58-130.11 | 0.12 | ||||
| F2 | 6.5 | 0.46-91.92 | 0.17 | ||||
| F3 | 8 | 0.46-139.29 | 0.15 | ||||
| Length of homelessness in days | 103 | 1 | 0.99-1.00 | 0.12 | |||
| Has heard of HCV (HCV awareness) | 106 | 0.4 | 0.11-1.47 | 0.17 | |||
| Patient confidence in being able to adhere to medication | 99 | ||||||
| Not confident | Ref. | ||||||
| Slightly confident | 2.1 | 0.25-17.59 | 0.49 | ||||
| Moderately confident | 1.5 | 0.14-16.54 | 0.74 | ||||
| Extremely confident | 3.06 | 0.48-19.50 | 0.24 | ||||
- * P value statistically significant if <0.05.
- † Unless otherwise indicated.
Factors Associated with SVR
Characteristics of patients by known SVR status are summarized in Table 4. The median duration of treatment was similar in the SVR and no SVR groups at 58.5 and 58 days (P = 0.1), respectively. In addition, there were no statistically significant differences in patient sociodemographic, clinical, and laboratory characteristics or length of stay at shelter between the SVR and no SVR groups, except those with SVR had significantly lower HCV RNA at treatment initiation (median log10, 6.1 vs. 6.8; P = 0.01) and a higher proportion were adherent to HCV medication by provider report (56.7% vs. 12.5%, P = 0.03). There were no differences in HCV genotype among those who did or did not achieve SVR. Importantly, all 5 patients with genotype 3 achieved SVR. Moreover, 9 of 11 patients with cirrhosis, including both patients with decompensation, had SVR.
| Treated and Did Not Achieve SVR (n = 8†) | Treated and Achieved SVR (n = 54†) | P Value* | |
|---|---|---|---|
| Median age, years (range), [IQR] | 56.55 | 55.45 | 0.63 |
| (40.6-70.1) | (28.2-82.1) | ||
| [54.15-63.6] | [49.4-60.3] | ||
| Male sex, n (%) | 8 (100.0) | 38 (70.4) | 0.1 |
| Race, n (%) | 0.14 | ||
| White, non-Hispanic | 3 (37.5) | 21 (38.9) | |
| Black/African American | 2 (25.0) | 26 (48.2) | |
| Asian or Pacific Islander | 0 (0.0) | 0 (0.0) | |
| Hispanic | 1 (12.5) | 3 (5.6) | |
| Native American/Alaska Native | 0 (0.0) | 2 (3.7) | |
| Other | 2 (25.0) | 2 (2.7) | |
| Education, n (%) | 0.89 | ||
| Less than high school | 1 (12.5) | 13 (24.1) | |
| High school | 4 (50.0) | 22 (40.7) | |
| More than high school | 3 (37.5) | 19 (35.2) | |
| Employment status within the prior year, n (%) | 0.25 | ||
| Employed | 1 (12.5) | 6 (11.1) | |
| Unemployed | 2 (25.0) | 29 (53.7) | |
| Retired/disabled | 5 (62.5) | 19 (35.2) | |
| Incarcerated within the prior year, n (%) | 1 (12.5) | 5 (9.26) | 0.58 |
| Spent every night in shelter | (n = 3) | (n = 36) | 1 |
| 2 (66.7) | 24 (66.7) | ||
| Changed shelter during treatment | (n = 4) | (n = 36) | 0.43 |
| 0 (0.0) | 6 (16.7) | ||
| Extended shelter stay during treatment | (n = 4) | (n = 36) | 0.16 |
| 2 (50.0) | 4 (11.1) | ||
| Insurance type, n (%) | (n = 53) | 0.28 | |
| Public | 6 (75.0) | 47 (87.0) | |
| Private | 1 (12.5) | 2 (3.7) | |
| Uninsured | 1 (12.5) | 4 (7.4) | |
| Unknown | 0 (0.0) | 1 (1.9) | |
| Has a health care provider, n (%) | 7 (87.5) | 45 (83.3) | 1 |
| History of substance use therapy, n (%) | 6 (75.0) | 29 (53.7) | 0.51 |
| Drug use within the past year, n (%) | 7 (87.5) | 42 (77.8) | 1 |
| Injection drug use ever, n (%) | 6 (75.00) | 35 (64.8) | 0.71 |
| Alcohol use within the past year, n (%) | 0.99 | ||
| None/minimal | 3 (37.5) | 19 (35.2) | |
| Moderate | 2 (25.0) | 14 (25.9) | |
| Heavy | 3 (37.5) | 21 (38.7) | |
| History of psychiatric illness, n (%) | 3 (37.5) | 28 (51.9) | 0.71 |
| Diabetes type 2, n (%) | 2 (25.0) | 7 (13.0) | 0.33 |
| Chronic kidney disease, n (%) | 0 (0.0) | 2 (3.7) | 1 |
| HIV coinfection, n (%) | (n = 53) | 1 | |
| 0 (0.0) | 5 (9.4) | ||
| HBV coinfection, n (%) | (n = 53) | 0.17 | |
| 2 (25.0) | 4 (7.55) | ||
| Fibrosis stage, n (%) | 0.87 | ||
| F0 | 0 (0.0) | 1 (2.7) | |
| F1 | 1 (14.3) | 11 (29.7) | |
| F2 | 3 (42.9) | 10 (27.0) | |
| F3 | 1 (14.3) | 6 (16.2) | |
| Cirrhosis, n (%) | 2 (25.0) | 9 (16.7) | 0.62 |
| History of cirrhosis decompensation, n (%) | 0 (0.0) | 2 (3.7) | 1 |
| Presence of HCC, n (%) | 1 (12.5) | 0 (0.0) | 0.13 |
| Platelets, median [IQR] | 230.5 | 210.5 | 0.43 |
| [197.5-307.5] | [161-285] | ||
| AST, median [IQR] | 45.5 | 41.5 | 0.15 |
| [30-90.5] | [34-66] | ||
| ALT, median [IQR] | 36 | 41 | 0.12 |
| [27.5-92.5] | [27-68] | ||
| Log10 HCV RNA, median [IQR] | 6.8 | 6.1 | 0.01 |
| [6.46-6.92] | [5.45-6.63] | ||
| HCV genotype, n (%) | (n = 46) | 0.63 | |
| 1 | 7 (87.5) | 34 (73.9) | |
| 2 | 1 (12.5) | 1 (2.2) | |
| 3 | 0 (0.0) | 5 (10.9) | |
| 4 | 0 (0.0) | 2 (4.4) | |
| Indeterminate | 0 (0.0) | 4 (8.7) | |
| History of prior receipt of any HCV treatment, n (%) | 2 (25.0) | 7 (13.0) | 0.33 |
| Time to initiation of HCV therapy, days, median [IQR] | n = 51 | 0.09 | |
| 38.5 | 66 | ||
| [24.5-55.5] | [35-120] | ||
| Median duration of therapy, days [IQR] | 58 [35.5-77] | 58.5 [56-85] | 0.14 |
| Adherent to HCV medication (%) | 12.5 | 56.7 | 0.03 |
- *P value statistically significant if <0.05.
- † Unless otherwise indicated.
- Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
On ITT analysis, the only independent predictor of achieving SVR when controlling for age, sex, and race was adherence to HCV medication (OR, 14.5; P = 0.01). Although a higher viral load was associated with lower odds of achieving SVR (median log10 OR, 0.4; 95% CI, 0.2-1.2; P = 0.09), this did not reach statistical significance.
Discussion
This is the first prospective study to our knowledge that implemented HCV testing and treatment within homeless shelters. Among the 766 participants screened, 21% tested positive for HCV antibody, the majority (66.0%) of whom were actively infected with detectable HCV RNA, supporting the previously reported high prevalence of HCV in the homeless population.(3, 8, 28, 29) In addition, similar to prior studies,(28, 30) history of injection and illicit drug use represented a main risk factor for HCV in this population. Importantly, nearly 18% had evidence of cirrhosis, and among those with available data, another 17% had advanced stage fibrosis (F3). This highlights the potential impact of delayed HCV therapy despite ongoing engagement in care among the homeless and emphasizes the need for optimized HCV care delivery in this vulnerable population.(31) Using an integrated and homeless shelter-based intervention, we were able to engage 62% of eligible patients in HCV therapy and achieve HCV cure in over 80% of this difficult-to-reach and vulnerable population.
We observed significant gaps in the HCV care cascade among those experiencing homelessness. First, half of all enrolled participants experiencing homelessness had reported prior HCV testing, likely due to screening in high-risk groups, such as those with recent illicit drug use as reported by 70% of our study population. Second, there were geographic differences in receipt of prior HCV testing, with San Francisco clients experiencing homelessness reporting a higher testing rate compared to Minneapolis (63.5% vs. 38.6%, P < 0.001). This is not surprising as important public health community-based initiatives, such as End Hep C SF, in San Francisco have resulted in increased HCV awareness and rates of HCV testing in the city.(32) Third, although nearly 80% of those who tested positive for HCV antibody following enrollment reported receipt of HCV testing in the past and over 80% reported having a health care provider, the majority of the patients who were actively infected had not engaged in HCV therapy. The disparity between the rates of HCV screening and linkage to HCV therapy in this vulnerable population is similar to that reported in other homeless populations.(14)
Our integrated and shelter-based approach that included HCV education was highly effective in engaging patients in HCV therapy; over 60% of patients with active infection received standard-of-care HCV therapy, which is significantly higher than reports in homeless populations infected with HCV.(14-17) Characteristics, including demographics and comorbidities, varied within the treatment cohort. However, participants who reported having a health care provider were 4 times more likely to receive therapy compared to those who did not. Research has shown that a high proportion of patients who remain engaged in health care also engage in HCV therapy.(33) Our findings also highlight that primary care or health care provider engagement in the HCV cascade of care is critical to reducing HCV burden in this highly marginalized population.
The majority of patients in our study achieved HCV cure, with the only independent predictor of response being adherence to HCV therapy. Real-world studies in underserved populations report high rates of SVR in the era of DAA therapy, similar to that reported in the general population.(17, 34-36) In addition, lack of patient compliance with treatment is associated with lower treatment response rates,(37) as observed in our study. Although the overall rate of SVR of 81.8% in our prospective study is similar to that reported in a retrospective study of patients with a history of homelessness,(16) it was lower than another study of patients (SVR of 97%) who were either currently homeless or had marginalized housing.(18) Aside from population differences, the latter study selected patients based on treatment readiness as determined by adherence to appointments and collaborative decision making among care providers.(18)
Although treatment willingness and readiness were also assessed in our study, the threshold for treatment initiation was low, especially in the San Francisco sites. This may have influenced differences in treatment initiation rates observed between the cities, resulting in inadvertent patient drop-off before treatment initiation as well as SVR rates. Indeed, on ITT analysis, the SVR rates were lower at 75.0% in San Francisco compared to 95.5% in Minneapolis where patient selection was more restrictive. Moreover, patients in San Francisco were more likely to initiate treatment within a shorter time compared to Minneapolis. These geographic differences likely reflect potential provider or system factors that may influence treatment initiation despite similarly implemented and integrated infrastructure for HCV treatment within shelters and similar rates of patient acceptance of HCV therapy following education across sites. Furthermore, at the time of the study, access to HCV therapy through insurance varied between sites, with requirements of at least 6 months of substance use sobriety in Minneapolis. Applying for exemption from this requirement on a case-by-case basis may have resulted in further delays in treatment initiation. Alternatively, there was a higher rate of task shifting with respect to HCV therapy from specialty to primary care and community engagement in low-barrier treatment within San Francisco that may have influenced rates of treatment initiation, especially among the most challenging groups, such as those with active substance use, psychiatric illness, and instability of shelter access.
This study has several limitations. First, patients who agreed to participate in the study and received education may have been more motivated to engage in HCV care. Second, the majority of the patients enrolled in this study were English speaking, and therefore we cannot generalize our results to non-English speaking individuals. Third, our treatment response rates may not be generalizable to all homeless populations. There were site differences in HCV treatment initiation and response rates on ITT. While it is unclear what led to the delay in HCV treatment initiation in some patients at the Minneapolis site, this factor coupled with other barriers unique to Minneapolis may have led to an unintentional selection bias favoring patients that were more likely to adhere to treatment appointments and laboratory testing but not necessarily medication dosing, accounting for the differences in ITT SVR rates between sites. Lastly, adherence to medication was not captured using pill count due to lack of feasibility. Nevertheless, we were able to implement an effective model of shelter-based HCV education, testing, and treatment in uniquely diverse patient populations from two geographically distinct locations, enhancing the generalizability of our findings to shelters in other urban settings.
Despite numerous reported barriers to HCV therapy spanning patient, provider, and system factors in individuals experiencing homelessness,(31, 38, 39) our integrated model of on-site HCV testing, education, and treatment following extensive needs assessment involving stakeholders(11, 21) was successful in enhancing HCV screening and linking to HCV therapy. Our study illustrates that tailored models of care that reduce barriers to patient engagement, including colocalization of HCV care within shelters or supportive housing and simplified steps for HCV treatment as recently proposed by guidelines,(40) are critical to HCV elimination efforts in this highly vulnerable population.
Acknowledgment
We thank participating shelter staff, providers, and leadership as well as GLIDE’s HIV/Hep C and Harm Reduction Services program (San Francisco); the San Francisco Department of Public Health; Ms. Katie Burke, M.P.H., from the San Francisco Department of Public Health Population Health Division; and Minneapolis Healthcare for the Homeless.




