Adherence to Ideal Cardiovascular Health Metrics Is Associated With Reduced Odds of Hepatic Steatosis
Supported by the National Institutes of Health (NIH) National Heart, Lung, and Blood Institute (NHLBI) and Boston University School of Medicine (NIH award 75N92019D00031 for the Framingham Heart Study), NIH/NHLBI (awards R01HL128914, 2R01 HL092577, 1R01 HL141434 01A1, 2U54HL120163, and 1R01AG066010 to E.J.B.), National Institute of Diabetes and Digestive and Kidney Diseases (award K23 DK113252 to M.T.L.), Gilead Sciences (to M.T.L.), Doris Duke Foundation (to M.T.L.), Boston University School of Medicine Department of Medicine (Career Investment Award to M.T.L.), Boston University Clinical Translational Science Institute (grant 1UL1TR001430 to M.T.L.), and American Heart Association (grant 18SFRN34110082 to E.J.B.).
Potential conflict of interest: Dr. Hoffmann consults for Recor Medical; he received grants from Astra Zeneca, KOWA, Medimmune, and HeartFlow. Dr. Long is on the advisory board for Ionis Pharmaceuticals. The other authors have nothing to report.
Abstract
The American Heart Association (AHA) introduced Life’s Simple 7 as a metric to define ideal cardiovascular health. We examined the association between cardiovascular health score (CHS) and prevalent nonalcoholic fatty liver disease (NAFLD) among Framingham Heart Study participants with varying genetic risk of NAFLD. Framingham Heart Study participants who underwent abdominal computed tomography scans were included (n = 2,773). We defined hepatic steatosis as the mean Hounsfield unit attenuation of the liver compared to a phantom control. We calculated CHS based on adherence to metrics from the AHA’s Life’s Simple 7 guidelines, including blood sugar, total cholesterol, blood pressure, body mass index (BMI), time spent on physical activity per week, and smoking status. We used multivariable-adjusted regression models to evaluate the association between CHS and hepatic steatosis, accounting for covariates and stratifying by NAFLD genetic risk. Overall, 12% of the sample achieved 0-1 goals and 25%, 27%, 21%, 13%, and 2.6% achieved 2, 3, 4, 5, or 6 goals, respectively. For each 1-unit increase in CHS, there was a decrease in the odds ratio (OR) of prevalent hepatic steatosis (OR, 0.54; 95% confidence interval, 0.49-0.59). Individually, BMI had the strongest association with NAFLD. Participants with high or intermediate genetic risk of NAFLD demonstrated higher relative decreases in hepatic steatosis with increased CHS compared to those at low genetic risk. Conclusion: Adhering to the AHA Life’s Simple 7 metrics was associated with reduced odds of prevalent NAFLD, particularly for those at high genetic risk. Additional longitudinal studies are needed.
Abbreviations
-
- AHA
-
- American Heart Association
-
- BMI
-
- body mass index
-
- CHS
-
- cardiovascular health score
-
- CI
-
- confidence interval
-
- CT
-
- computed tomography
-
- GRS
-
- genetic risk score
-
- LPR
-
- liver–phantom ratio
-
- NAFLD
-
- nonalcoholic fatty liver disease
-
- OR
-
- odds ratio
Nonalcoholic fatty liver disease (NAFLD) is characterized by macrovascular steatosis of the liver in the absence of other causes and has rapidly become the most common cause of liver disease in the high-income countries.(1) There is significant morbidity and mortality associated with NAFLD, including cirrhosis and hepatocellular carcinoma.(2) NAFLD is also strongly associated with features of the metabolic syndrome, including obesity, diabetes, and hyperlipidemia.(3-6) The cornerstone of treatment for NAFLD remains diet and lifestyle changes as there are no approved therapies; however, specific recommendations are lacking.(3) Given the increasing prevalence of NAFLD and the limited treatment options, prevention of NAFLD is a major public health concern.
In 2010, the American Heart Association (AHA) developed patient-centered guidelines for cardiovascular health called Life’s Simple 7.(7) The Life’s Simple 7 score defines seven modifiable health factors and behaviors that individuals should target to improve cardiovascular health, including blood pressure, cholesterol, blood sugar, body mass index (BMI), smoking, exercise, and diet. Using Life’s Simple 7 as a surrogate for ideal cardiovascular health, studies observed associations between adherence to Life’s Simple 7 and reduced prevalence of several chronic diseases, including diabetes, heart failure, and chronic kidney disease.(8-11) The association between cardiovascular health, defined by Life’s Simple 7, and NAFLD is not definitively known. Three studies based in Asia observed that improved Life’s Simple 7 was associated with less NAFLD.(12-14) A U.S.-based cohort study used the fatty liver index to define NAFLD, which is less sensitive and specific than imaging and also incorporates BMI, one of the Life’s Simple 7 metrics, into the score.(15) To our knowledge, no studies have examined whether adherence to cardiovascular health metrics modifies genetic risk for NAFLD.
We hypothesized that ideal cardiovascular health, as defined by Life’s Simple 7, is associated with less prevalent NAFLD in a community-based cohort after accounting for covariates. Additionally, we hypothesized that adherence to Life’s Simple 7 metrics modifies genetic risk for NAFLD.
Participants and Methods
Study Sample
The study sample included Framingham Heart Study participants from the Offspring and Third Generation cohorts.(16, 17) A subgroup of 3,206 participants underwent multidetector computed tomography (CT) scanning between June 2002 and March 2005 and had scans interpretable for hepatic steatosis.(18) We recorded covariate information from the nearest corresponding clinical examination for each cohort as follows: examination 1 for Third Generation participants (2002-2005) and examination 7 for Offspring participants (1998-2001). We excluded 262 participants with missing fasting glucose, total cholesterol, systolic and diastolic blood pressure, height, weight, physical activity, or smoking status and 171 participants who reported heavy alcohol consumption, defined as >14 drinks/week for women and >21 drinks/week for men(3); this yielded a total final sample of 2,773 participants. We included a subgroup analysis of participants with all seven health metrics because dietary information was available for Offspring cohort participants only (n = 955). This study was approved by the Boston University Medical Center and Massachusetts General Hospital Institutional Review Board, and individuals gave written informed consent.
Covariates, NAFLD Genetic Risk, and Cardiovascular Health Score
We measured serum glucose and total cholesterol from fasting blood samples. Participants reported alcohol use measured in drinks per week. Trained examiners measured participants’ height, weight, and blood pressure. BMI was calculated by dividing weight in kilograms by height in meters squared. We quantified NAFLD genetic risk based on a genetic risk score (GRS), as discussed in detail elsewhere.(19) In brief, we derived a NAFLD GRS based on five single-nucleotide polymorphisms (rs738409, rs2228603, rs12137855, rs780094, rs4240624) that had a minimum minor allele frequency of 0.05 and were identified and replicated in genome-wide association studies (GWAS). These variants corresponded to the genes patatin-like phospholipase domain containing 3 (PNPLA3), neurocan (NCAN), lysophospholipase-like 1 (LYPLAL1), glucokinase regulator (GCKR), and protein phosphatase 1 regulatory subunit 3B (PPP1R3B). We calculated an individual’s GRS based on the weighted sum of their risk alleles multiplied by the GWAS regression coefficient.(19)
We calculated a cardiovascular health score (CHS) for all individuals based on the AHA’s Life’s Simple 7 guidelines for ideal cardiovascular health. We evaluated participants on six health metrics and awarded a score of 1 if they met the target values as defined by the AHA.(7) We summed the six score components with equal weight, with a score of 0 indicating poor cardiovascular health and 6 indicating ideal cardiovascular health, to define the composite score. Target values were defined as: (1) fasting blood sugar <100 mg/dL without medication; (2) total cholesterol <200 mg/dL without medication; (3) blood pressure <120/80 mm Hg without medication; (4) BMI <25 kg/m2; (5) physical activity index equal to or more than the seventy-fifth percentile, as described in the Framingham Heart Study cohort(20); and (6) never smoker or quit >12 months earlier. We assessed diet using the Harvard semiquantitative food frequency questionnaire.(21)
For the subgroup with available dietary information, we defined adherence to the AHA recommendations as achieving at least four of the following five diet components: (1) ≥4.5 cups of fruits and vegetables per day; (2) ≥2 of 3.5-oz servings of fish per week; (3) ≥3 of 1-oz-equivalent servings of fiber-rich whole grains per day; (4) <1,500 mg of sodium per day; and (5) ≤450 kcal (36 ounces) of sugar-sweetened beverages per week.
For the multivariable analysis, we considered a three-level score, including poor, intermediate, and ideal categories for each health metric except smoking status. We defined ideal cardiovascular health by the same criteria as above. We defined the intermediate category as follows: (1) fasting blood sugar 100-125 mg/dL or on medication; (2) total cholesterol 200-239 mg/dL or on medication; (3) systolic blood pressure 120-139 mm Hg and diastolic blood pressure 80-89 mm Hg or on medication; (4) BMI 25 to <30 kg/m2; (5) physical activity index percentile 25-74; and (6) achieving two to three of the recommended diet components (for Offspring cohort participants).
Liver Fat Measurement
We defined hepatic steatosis based on the average liver fat attenuation measured on an abdominal multidetector CT scan, as described.(18, 22) In brief, liver fat attenuation was measured on an eight-slice CT scanner (General Electric Health Care, Little Chalfont, United Kingdom) along with a calibration phantom (Image Analysis, Lexington, KY), which was present on each image. We quantified liver fat by determining the ratio of the mean fat attenuation (in Hounsfield units) from three areas in the liver and the calibration phantom (liver–phantom ratio [LPR]). Liver fat increases as the LPR decreases. The cutoff for hepatic steatosis was defined at LPR ≤0.33 as in prior studies.(18)
Statistical Analysis
We performed multivariable-adjusted logistic regression models to assess the association between the CHS (as a continuous variable) and prevalent hepatic steatosis (defined as LPR ≤0.33). We also considered the CHS as an ordinal variable in categories of two, three, four, five, or six health metrics achieved (0-1 goals achieved as the reference). For participants with available diet data, we considered up to seven health metrics achieved in a subgroup analysis. All models were adjusted for age, sex, cohort, and alcohol use (in drinks per week).
We performed several prespecified secondary analyses. First, we evaluated the association between each individual component of the CHS and hepatic steatosis to evaluate which health metrics were most strongly associated with hepatic steatosis and adjusted for age, sex, cohort, and alcohol use (in drinks per week). Because BMI correlated with hepatic steatosis in our previous study (r = 0.25),(23) we also repeated our analyses after removing BMI from the CHS. Additionally, we repeated our analyses considering LPR as a continuous variable using multivariable adjusted linear regression models. Finally, we evaluated for an interaction between the CHS and the GRS and performed stratified analysis by tertiles of genetic risk if an interaction was observed. Analyses were performed in SAS software (version 9.4; SAS Institute Inc., Cary, NC). A two-sided α = 0.05 level of significance was used.
Results
Study Sample Characteristics
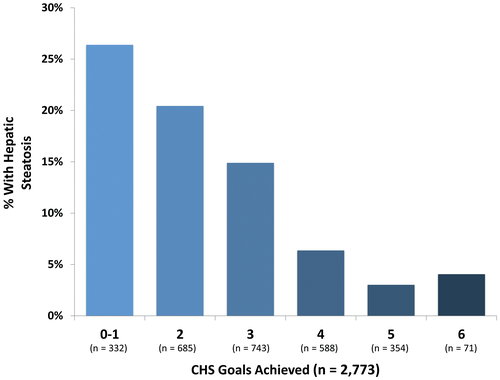
Study sample characteristics based on hepatic steatosis status are shown in Table 1. The prevalence of hepatic steatosis in our cohort was 17%. Among those with hepatic steatosis, the mean age (±SD) was 52 ± 11 years, 52% were women, and 43% were in the Offspring cohort. Overall, 12% of the sample achieved a CHS of 0-1 and 25%, 27%, 21%, and 13% achieved a score of 2, 3, 4, or 5, respectively. Only 2.6% of the total sample achieved all 6 goals. The prevalence of hepatic steatosis based on how many cardiovascular health goals were achieved is demonstrated in Fig. 1. In general, the prevalence of hepatic steatosis was highest for those with a CHS of 0-1 (26%) and decreased as CHS increased.
| Clinical Characteristics* | Hepatic Steatosis (n = 479) | No Hepatic Steatosis (n = 2,294) | Overall (N = 2,773) |
|---|---|---|---|
| Age (years) | 52 ± 11 | 51 ± 10 | 51 ± 11 |
| Women, n (%) | 266 (55.5%) | 1,077 (46.9%) | 1,343 (48.9%) |
| Third Generation cohort, n (%) | 274 (57.2%) | 1,421 (61.9%) | 1,695 (61.1%) |
| Current smoking, n (%) | 51 (10.6%) | 257 (11.2%) | 308 (11.1%) |
| BMI (kg/m2) | 28.7 (0.17) | 26.5 (0.16) | 26.4 (0.19) |
| Ideal (<25 kg/m2) | 49 (10.2%) | 916 (39.9%) | 965 (34.8%) |
| Intermediate (25 to <30 kg/m2) | 169 (35.3%) | 931 (40.6%) | 1,100 (39.7%) |
| Poor (≥30 kg/m2) | 261 (54.5%) | 447 (19.5%) | 708 (25.5%) |
| PAI | |||
| Ideal (PAI ≥75th percentile) | 111 (23.2%) | 599 (26.1%) | 710 (25.6%) |
| Intermediate (PAI 25th-74th percentile) | 236 (49.3%) | 1,145 (49.9%) | 1,381 (49.8%) |
| Poor (PAI <25th percentile) | 132 (27.6%) | 550 (24.0%) | 682 (24.6%) |
| Total cholesterol | |||
| Ideal (<200 mg/dL without treatment) | 257 (53.7%) | 1,344 (58.6%) | 1,601 (57.7%) |
| Intermediate (200-239 mg/dL or treated) | 161 (33.6%) | 726 (31.6%) | 887 (35.0%) |
| Poor (≥240 mg/dL) | 61 (12.7%) | 224 (9.8%) | 285 (10.3%) |
| Blood pressure | |||
| Ideal (SBP <120 mm Hg and DBP <80 mm Hg without treatment) | 118 (24.6%) | 1,128 (49.2%) | 1,246 (44.9%) |
| Intermediate (SBP 120-139 mm Hg, DBP 80-89 mm Hg, or treated) | 328 (68.5%) | 1,077 (46.9%) | 1,405 (50.7%) |
| Poor (SBP ≥140 mm Hg or DBP ≥90 mm Hg) | 33 (6.9%) | 89 (3.9%) | 122 (4.4%) |
| Blood sugar | |||
| Ideal (<100 mg/dL without treatment) | 216 (45.1%) | 1,678 (73.1%) | 1,894 (68.3%) |
| Intermediate (100-125 mg/dL or treated) | 75 (3.3%) | 541 (23.6%) | 755 (27.2%) |
| Poor (≥126 mg/dL) | 49 (10.2%) | 75 (3.3%) | 124 (4.5%) |
| AHA Life’s Simple 7 | 2 ± 1 | 3 ± 1 | 3 ± 1 |
- * Clinical characteristics are represented as mean ± SD or n (%).
- Abbreviations: AHA, American Heart Association; BMI, body mass index; DBP, diastolic blood pressure; PAI, physical activity index; SBP, systolic blood pressure.
Multivariable Logistic Regression Models for CHS and Hepatic Steatosis
Overall, for each 1-unit increase in the CHS, there was a decrease in the odds ratio (OR) of prevalent hepatic steatosis (OR, 0.54; 95% confidence interval [CI], 0.49-0.59) (Table 2). An increase in the CHS from 0-1 to 2 was associated with 38% lower odds of steatosis (OR, 0.62; 95% CI, 0.46-0.82), an increase to 3 associated with 62% lower odds (OR, 0.38; 95% CI, 0.28-0.51), an increase to 4 associated with 87% lower odds (OR, 0.13; 95% CI, 0.09-0.19), an increase to 5 associated with 94% lower odds (OR 0.06; 95% CI, 0.03-0.11), and an increase to 6 associated with 92% lower odds (OR, 0.08; 95% CI, 0.02-0.25). Results from linear regression using the LPR as a continuous variable were generally similar, as shown in Supporting Table S1.
| CHS* | CHS | CHS (Without BMI) | ||||||
|---|---|---|---|---|---|---|---|---|
| Number of Participants | Number With Hepatic steatosis | Adjusted OR (95% CI) | P value | Number of Participants | Number With Hepatic steatosis | Adjusted OR (95% CI) | P value | |
| per 1-unit increase | N = 2,773 | n = 479 | 0.54 (0.49-0.59) | <0.001 | N = 2,773 | n = 479 | 0.68 (0.61-0.76) | <0.001 |
| goals achieved | ||||||||
| 0-1 | 332 | 119 | Reference | - | 391 | 125 | Reference | |
| 2 | 685 | 176 | 0.62 (0.46-0.82) | <0.001 | 812 | 184 | 0.63 (0.48-0.82) | <0.001 |
| 3 | 743 | 130 | 0.38 (0.28-0.51) | <0.001 | 884 | 132 | 0.38 (0.29-0.51) | 0.12 |
| 4 | 588 | 40 | 0.13 (0.09-0.19) | 0.002 | 578 | 34 | 0.14 (0.09-0.21) | <0.001 |
| 5 | 354 | 11 | 0.06 (0.03-0.11) | <0.001 | 108 | 4 | 0.08 (0.03-0.23) | 0.002 |
| 6 | 71 | 3 | 0.08 (0.02-0.25) | 0.03 | ||||
- Data are adjusted for age, sex, cohort, and alcohol drinks/week.
- * CHS without diet variable.
Results from the sensitivity analysis with BMI excluded from the CHS are also shown in Table 2. For each 1-unit increase in the CHS excluding BMI, the odds of prevalent hepatic steatosis decreased by 32% (OR, 0.68; 95% CI, 0.61-0.76). Results from the subgroup analysis including diet in the CHS are available in the Supporting Information. Every 1-unit increase in the CHS including diet was associated with a 33% lower odds of prevalent hepatic steatosis (OR, 0.67; 95% CI, 0.58-0.77) (Supporting Table S2). In the linear regression models, we observed a significantly higher liver fat for each unit increase in the CHS including diet (β = 0.0082; 95% CI, 0.0058-0.0105) (Supporting Table S3).
Association Between Individual CHS Metrics and Hepatic Steatosis
Individually, BMI had the strongest association with hepatic steatosis, with an OR of 10.63 (95% CI, 7.65-14.78) for poor (obese category) and an OR of 3.18 (95% CI, 2.27-4.45) for intermediate (overweight category) compared to the ideal criteria, as shown in Table 3. Fasting glucose was also individually associated with an increased odds of hepatic steatosis, with an OR of 5.10 (95% CI, 3.41-7.63) for poor (diabetes category) and an OR of 2.98 (95% CI, 2.39-3.73) for intermediate (impaired fasting glucose category), as well as blood pressure, with an OR of 3.35 (95% CI, 2.13-5.25) for poor and an OR of 2.78 (95% CI, 2.20-3.51) for intermediate compared to ideal criteria.
| Number of Cases/Number at Risk | OR for Prevalent Hepatic Steatosis (LPR ≤0.33) (95% CI) | Age, Sex, Cohort, Drinks/Week Adjusted OR (95% CI) | |
|---|---|---|---|
| Smoking | |||
| Poor, current | 51/257 | 0.94 (0.69-1.30) | 0.98 (0.71-1.35) |
| Ideal, former or never or quit smoker | 428/2,037 | Ref | |
| BMI | |||
| Poor (≥30 kg/m2) | 261/447 | 10.92 (7.88-15.11) | 10.63 (7.65-14.78) |
| Intermediate(25 to <30 kg/m2) | 169/931 | 3.39 (2.44-4.72) | 3.18 (2.27-4.45) |
| Ideal (<25 kg/m2) | 49/916 | Ref | Ref |
| Physical activity | |||
| Poor (PAI <25th percentile) | 132/550 | 1.30 (0.87-1.42) | 1.40 (1.06-1.86) |
| Intermediate (PAI 25th-74th percentile) | 236/1,145 | 1.11 (0.87-1.42) | 1.18 (0.92-1.52) |
| Ideal (PAI ≥75th percentile) | 111/599 | Ref | Ref |
| Diet* | |||
| Poor (<2 components) | 77/439 | 2.06 (0.61-6.92) | 1.92 (0.57-6.49) |
| Intermediate (2-3 components) | 100/484 | 2.52 (0.75-8.43) | 2.41 (0.72-8.09) |
| Ideal (4-5 components) | 3/32 | Ref | Ref |
| Total cholesterol | |||
| Poor (≥240 mg/dL) | 61/224 | 1.42 (1.04-1.95) | 1.39 (1.01-1.91) |
| Intermediate (200-239 mg/dL or treated) | 161/726 | 1.16 (0.93-1.44) | 1.13 (0.91-1.41) |
| Ideal (<200 mg/dL without treatment) | 257/1,344 | Ref | Ref |
| Blood pressure | |||
| Poor (SBP ≥140 mm Hg or DBP ≥90 mm Hg) | 33/89 | 3.54 (2.28-5.52) | 3.35 (2.13-5.25) |
| Intermediate (SBP 120-139 mm Hg, DBP 80-89 mm Hg, or treated) | 328/1,077 | 2.91 (2.32-3.65) | 2.78 (2.20-3.51) |
| Ideal (SBP <120 mm Hg and DBP <80 mm Hg without treatment) | 118/1,128 | Ref | Ref |
| Fasting glucose | |||
| Poor (≥126 mg/dL) | 216/1,678 | 5.08 (3.45-7.47) | 5.10 (3.41-7.63) |
| Intermediate (100-125 mg/dL or treated) | 214/541 | 3.07 (2.49-3.80) | 2.98 (2.39-3.73) |
| Ideal (<100 mg/dL without treatment) | 49/75 | Ref | Ref |
- * n = 955 for Offspring cohort with diet variable.
- Abbreviations: DBP, diastolic blood pressure; PAI, physical activity index; Ref, reference; SBP, systolic blood pressure.
Stratification by NAFLD GRS
We observed a significant interaction with the GRS for the association between the CHS and continuous liver fat after adjusting for covariates (P = 0.04), so we stratified the results by tertiles of the GRS. For individuals in the lowest tertile of the GRS, the decrease in liver fat per 1-unit increase of CHS was lowest compared to those in the intermediate GRS tertile or the highest GRS tertile (lowest GRS, β = −0.0058; 95% CI, −0.0038 to −0.0079; intermediate GRS, β = −0.0109; 95% CI, −0.0084 to −0.0134; highest GRS, β = −0.012; 95% CI, −0.0091 to −0.0151). Participants with higher genetic risk for NAFLD demonstrated the greatest relative decrease in liver fat as the CHS was higher.
Discussion
In the Framingham Heart Study, we observed that a more favorable CHS was associated with less prevalent hepatic steatosis, and this association remained even after excluding BMI from the score. In a subgroup evaluated for all seven CHS metrics, including diet, a favorable CHS remained associated with less prevalent hepatic steatosis. Our findings highlight that achieving any of the AHA Life’s Simple 7 metrics was associated with lower prevalent NAFLD and that BMI, blood sugar, and blood pressure may be particularly important for NAFLD. Importantly, individuals with high or intermediate genetic risk demonstrated a higher relative decrease in liver fat for each CHS metric achieved compared to those at lower genetic risk for NAFLD. Our findings suggest that adhering to the AHA Life’s Simple 7 metrics may provide the greatest relative benefit for those at increased genetic risk for NAFLD, but additional studies are needed.
Our findings are in line with the many studies demonstrating associations between NAFLD and the metabolic syndrome.(1-3, 24) Without pharmacologic therapy, the focus of treatment for NAFLD is diet and lifestyle modification, although specific lifestyle recommendations for patients with NAFLD are lacking.(3, 25) The AHA Life’s Simple 7 metric defines cardiovascular health metrics that are modifiable by diet and lifestyle choices by using an accessible and easy to understand clinical tool. The benefits of adhering to the Life’s Simple 7 metrics may extend to other cardiovascular-related conditions, including diabetes and chronic kidney disease.(8-11) To date, the AHA’s Life’s Simple 7 metric has not been incorporated into any clinical guidelines for NAFLD. Studies have demonstrated that modifying individual lifestyle factors, such as diet or physical activity, are beneficial and are associated with less prevalent and incident NAFLD.(26, 27) However, less is known regarding the impact of modifying multiple specific cardiovascular risk factors on NAFLD. In a Chinese study of mostly middle-aged and elderly women, those adhering to a higher number of CHS metrics had a lower incidence of ultrasound-defined NAFLD after 5 years of follow-up, although that study lacked dietary information.(14) In a large cohort of younger aged Korean adults, adherence to Life’s Simple 7 was associated with a decreased risk of incident NAFLD; however, the generalizability to older adults or other ethnicities is not known.(12) A U.S.-based cross-sectional study observed that Life’s Simple 7 metrics were associated with lower liver biochemistries and NAFLD, as defined by the fatty liver index; however, the fatty liver index includes BMI and has the potential to be influenced by other medical conditions.(15, 28) Our study adds to the growing literature by demonstrating that favorable cardiovascular health, as defined by seven metrics influenced by diet and lifestyle, is associated with lower prevalent NAFLD in a U.S.-based cohort. By recognizing the strong correlation between NAFLD and BMI in our study, we observed that the CHS remained associated with NAFLD even when BMI was removed from the CHS. Additional studies are needed to determine if adhering to Life’s Simple 7 reduces the risk of NAFLD, slows the progression of NAFLD, or improves rates of NAFLD-related liver complications.
Genetics and lifestyle factors both contribute to an individual’s risk of NAFLD. To our knowledge, our study is the first to explore the interaction between lifestyle, as measured by the CHS, and NAFLD genetic risk. In cardiovascular disease, increasing evidence suggests diet and lifestyle choices can modify the effects of genetic variants on an individual’s risk.(29) In a large study from the United Kingdom Biobank, genetics and health behaviors had a log-additive effect on the risk of developing cardiovascular disease.(30) Although additional studies are needed, it is possible that those with a high genetic risk for NAFLD not only start out with a higher risk but their disease risk may disproportionally increase with poor adherence to a healthy lifestyle. In another United Kingdom Biobank study, measures of cardiorespiratory fitness were inversely associated with cardiovascular disease across categories of genetic risk, suggesting that cardiovascular fitness can help compensate for genetic risk.(31) In a post-hoc analysis of a randomized controlled study of lifestyle modification, those with increased genetic risk based on PNPLA3 were more responsive to the beneficial effects of the lifestyle intervention.(32) In our study, those with the highest genetic risk of NAFLD had the lowest odds of prevalent NAFLD as more cardiovascular health metrics were achieved. Our findings suggest that lifestyle choices may be critically important for those with increased genetic risk for NAFLD, although additional longitudinal studies are needed before personalized lifestyle recommendations can be made based on an individual’s genetic risk.(33)
The strengths of our study are that it was a relatively large community-based cohort of participants well-characterized for NAFLD and the metrics of the CHS. We also had available genetic information to measure NAFLD genetic risk. Our study has many limitations, most notably that it is cross-sectional; we cannot exclude residual confounding and cannot determine causality or temporality. Additionally, defining hepatic steatosis by CT scan may underestimate the true prevalence of NAFLD in our cohort because CT is not sensitive to mild liver fat. The Framingham Heart Study by nature lacks ethnic diversity with a primarily Caucasian population so the generalizability to other ethnic groups is not known. The epidemiology of NAFLD has changed rapidly over time, and our study may not accurately reflect the current situation in the United States. A very small percentage of participants achieved all six health metrics, which limited our ability to determine any relative benefit between achieving a CHS of 5 or 6. Finally, there was no available information about other chronic liver diseases, which may have resulted in misclassification of NAFLD and biased our results toward the null.
Our study showed that individuals who adhere to the AHA’s Life’s Simple 7 metrics had lower odds of NAFLD for each additional metric achieved, regardless of BMI. Those at increased genetic risk for NAFLD had the highest relative decrease in liver fat for each additional cardiovascular health metric achieved. More studies are needed to explore whether achieving AHA Life’s Simple 7 metrics reduces the incidence or progression of NAFLD or improves liver-related complications.