Nonalcoholic Fatty Liver Disease Impairs the Liver–Alpha Cell Axis Independent of Hepatic Inflammation and Fibrosis
Supported by the Novo Nordisk Foundation (NNF) Challenge Grant “MicrobLiver” (grant number NNF15OC0016692 to J.S.P., M.O.R., and F.B.), NNF Tandem Program (funding number 31526 to N.J.W.A. and J.J.H.), NNF Project Support in Endocrinology and Metabolism, Nordic Region (funding number 34250 to N.J.W.A. and J.J.H.), and NNF Excellence Emerging Investigator Grant, Endocrinology and Metabolism (grant NNF19OC0055001 to N.J.W.A.).
Potential conflict of interest: Dr. Bendtsen consults for Ferring A/S. The other authors have nothing to report.
Abstract
Nonalcoholic fatty liver disease (NAFLD) is associated with impaired hepatic actions of glucagon and insulin. Glucagon and amino acids are linked in an endocrine feedback circuit, the liver–alpha cell axis, that may be disrupted by NAFLD. We investigated how NAFLD severity affects glucagon and insulin resistance in individuals with obesity and whether bariatric surgery improves these parameters. Plasma and liver biopsies from 33 individuals with obesity (collectively, OBE) were obtained before and 12 months after bariatric surgery (Roux-en-Y gastric bypass [RYGB] or sleeve gastrectomy [SG]). Nine healthy control individuals (collectively, CON) undergoing cholecystectomy were used as a comparison group. The NAFLD activity score (NAS) was used to subdivide study participants into the following groups: OBE-no steatosis, OBE+steatosis, and nonalcoholic steatohepatitis (NASH) and/or grade 2 fibrosis (Fib) (OBE-NASH-Fib). Measurements of amino acids by targeted metabolomics and glucagon were performed. Glucagon, amino acids (P < 0.05), and the glucagon-alanine index, a validated surrogate marker of glucagon resistance, were increased in OBE by 60%, 56%, and 61%, respectively, when compared with CON but irrespective of NAFLD severity. In contrast, markers of hepatic insulin resistance increased concomitantly with NAS. Hyperglucagonemia resolved in OBE-no steatosis and OBE+steatosis but not in OBE-NASH-Fib (median, 7.0; interquartile range, 5.0-9.8 pmol/L), regardless of improvement in insulin resistance and NAS. The type of surgery that participants underwent had no effect on metabolic outcomes. Conclusion: Glucagon resistance to amino acid metabolism exists in individuals with NAFLD independent of NAS severity. Patients with NASH showed persistent hyperglucagonemia 12 months after bariatric surgery, indicating that a disrupted liver–alpha cell may remain in NAFLD despite major improvement in liver histology.
Abbreviations
-
- %EBWL
-
- percentage excess body weight loss
-
- AA
-
- amino acid
-
- ALT
-
- alanine aminotransferase
-
- ANOVA
-
- analysis of variance
-
- AST
-
- aspartate aminotransferase
-
- BMI
-
- body mass index
-
- CON
-
- control group
-
- Fib
-
- fibrosis
-
- GGT
-
- gamma-glutamyltransferase
-
- HbA1c
-
- hemoglobin A1c
-
- HDL
-
- high-density lipoprotein
-
- HOMA-IR
-
- Homeostatic Model Assessment for Insulin Resistance
-
- IQR
-
- interquartile range
-
- LDL
-
- low-density lipoprotein
-
- NAFL
-
- nonalcoholic fatty liver
-
- NAFLD
-
- nonalcoholic fatty liver disease
-
- NAS
-
- nonalcoholic fatty liver disease activity score
-
- NASH
-
- nonalcoholic steatohepatitis
-
- OBE
-
- obesity group
-
- RYGB
-
- Roux-en-Y gastric bypass
-
- SG
-
- sleeve gastrectomy
-
- T2D
-
- type 2 diabetes
-
- VLDL
-
- very low-density lipoprotein
Nonalcoholic fatty liver disease (NAFLD) affects one out of four adults worldwide(1) and covers a broad histopathological spectrum, from simple steatosis (nonalcoholic fatty liver [NAFL]) to steatosis plus necroinflammation (nonalcoholic steatohepatitis [NASH]) with/without liver fibrosis to NAFLD cirrhosis.(2) Obesity and type 2 diabetes (T2D), both characterized by insulin resistance and increased plasma concentrations of glucagon (hyperglucagonemia),(3-5) have been linked with NAFLD and vice versa. Little is known about the relationship between hepatic metabolic alterations and the severity of histopathological NAFLD changes or about how bariatric surgery affects such parameters.
In recent years, disruption of the liver–alpha cell axis, especially as it occurs in NAFLD, has received growing attention from researchers.(6, 7) The liver–alpha cell axis refers to the physiological feedback loop between circulating amino acids (AAs) and pancreatic alpha cell secretion of glucagon.(7-12) Under normal circumstances, glucagon regulates hepatic AA turnover by increasing ureagenesis, resulting in lowered plasma concentrations of AAs.(13-15) In turn, certain glucagonotropic AAs, like alanine, stimulate glucagon secretion from the pancreas, thereby completing the feedback loop.(11)
Glucagon increases hepatic glucose production(16) and hyperglucagonemia contributes to the fasting hyperglycemia observed in patients with T2D.(17-19) The co-existence of hyperglucagonemia and hyperaminoacidemia has been linked to impaired effects of glucagon on hepatic AA metabolism, i.e., a disruption of the liver–alpha cell axis.(8) Interestingly, hyperglucagonemia and hyperaminoacidemia have also been documented in patients with mild NAFLD but without T2D.(20) The latter finding supports the hypothesis that even mildly impaired liver function resulting from hepatic steatosis and inflammation as seen in NAFLD could reduce hepatic glucagon sensitivity, resulting in reduced ureagenesis, increased plasma concentrations of AAs, and hyperglucagonemia. However, the impact of the severity of NAFLD on glucagon sensitivity and hepatic AA metabolism or whether these alterations are reversed after major weight loss induced by bariatric surgery and improvement in NAFLD have yet to be investigated.
We investigated the relationship between plasma glucagon and plasma AAs (by targeted metabolomics) in participants with severe obesity with various degrees of NAFLD and assessed the influence of histologic severity on the liver–alpha cell axis and glucagon resistance. Finally, we followed the metabolic changes, including effects on the liver–alpha cell axis, after a significant weight loss and improvement in NAFLD induced by bariatric surgery.
Participants and Methods
Subjects and Study Design
We recruited 33 adult individuals with obesity who were scheduled for bariatric surgery at Copenhagen University Hospital Hvidovre (Hvidovre, Denmark) and 9 healthy controls.
Study participants were required to fulfill the general criteria for bariatric surgery (laparoscopic gastric bypass [Roux-en-Y gastric bypass; RYGB] or laparoscopic sleeve gastrectomy [SG]) according to the Danish national bariatric guidelines, namely a body mass index (BMI) greater than 35 kg/m2 and the presence of at least one obesity-related comorbidity (T2D, uncontrolled hypertension, polycystic ovarian syndrome, documented sleep apnea, or arthrosis in the lower extremities) or a BMI greater than 40 kg/m2 in the absence of the aforementioned comorbidities. In addition, eligible bariatric candidates must complete an 8% mandatory weight loss before referral for the RYGB or SG procedure in Denmark. Choice of surgical procedure (RYGB or SG) was based on the patients’ own preference, the endocrinologist’s recommendation, and any relevant comorbidities. A further study requirement was that participants be of North European ethnicity. Exclusion criteria were former or ongoing regular alcohol intake (2.5 units/day for men and 1.5 units/day for women), former or ongoing long-term use of medication known to induce secondary NAFLD/NASH, known preexisting liver disease (except preoperative NAFLD), disease in the lipid metabolism, and/or acute or chronic inflammatory disease.
Participants in CON consisted of 9 healthy adults scheduled for planned (not acute) laparoscopic cholecystectomy. CON participants were required to be between 18 and 65 years old, have a BMI between 18.0 and 27.5 km/m2, and have a waist circumference less than 88 cm for women and less than 102 cm for men. Additionally, this group had to be healthy (apart from gall bladder stones) and with zero intake of prescribed medication other than allergy medicine, mild pain killers, and/or oral contraceptives.
Ethics
The study was conducted in accordance with the Helsinki Declaration and approved by the Ethics Committee for the Capital Region of Denmark (H-16030784 and H-16030782). All participants received oral and written information regarding the experimental procedures and their potential risks. Oral and written consent were obtained from all participants.
Study Design and Anthropometrics
Study participants with obesity were assessed twice: at baseline (on the morning of the day of bariatric surgery) and 12 months after surgery (follow-up). At both visits, we registered anthropometrics and phenotypic data and collected fasting blood samples.
At baseline, a wedged liver biopsy from the edge of the right liver lobe was collected intraoperatively immediately after trocar placement but before the actual bariatric procedure. The same surgical team, consisting of three senior bariatric surgeons, performed all biopsies.
At the 12-month follow-up, an ultrasound-guided TruCut percutaneous liver biopsy was sampled from the right liver lobe in all 33 study participants with obesity.
Participants in CON were studied once, on the day of their planned cholecystectomy. Anthropometrics, phenotypic data, blood samples, and an intraoperatively wedged liver biopsy were collected as described above.
Three study subjects were enrolled before their required 8% weight loss. Fasting blood samples were collected and a liver biopsy was obtained by TruCut, before the weight loss, at the time of surgery, and 12 months after surgery.
Biochemical Analyses
Plasma alanine aminotransferase (ALT), gamma-glutamyltransferase (GGT), aspartate aminotransferase (AST), plasma-glucose, low-density lipoproteins (LDLs), very low-density lipoproteins (VLDLs), high-density lipoproteins (HDLs), and triglycerides were measured using the Roche/Hitachi Cobas c 8000 system (Roche Diagnostics GmbH, Mannheim, Germany) and Cobas calibrators and reagents according to the manufacturer’s instructions. Serum insulin and C-peptide concentrations were measured by immunoassay with the Cobas e 602.
Hemoglobin A1c (HbA1c) was measured in plasma with the Tosoh TSKgel G8 Variant His on the Tosoh Automated Glycohemoglobin Analyzer HLC-723G8 (Tosoh Corporation, Tokyo, Japan)
Plasma Glucagon and Targeted Metabolomics
Plasma concentrations of glucagon were quantified using a validated enzyme-linked immunosorbent assay (catalog no. 10-1271-01; Mercodia, Uppsala, Sweden).(21) Plasma concentrations of l-amino acids (termed total AAs) were quantified using the enzymatic l-Amino Acid Assay Kit (catalog no. ab65347; Abcam, Cambridge, United Kingdom). The assay was evaluated by recovery experiments using pooled human plasma (n = 4) with known amounts of AAs (catalog no. A6282; Sigma Aldrich, Copenhagen, Denmark). Recovery of AAs was on average (± SD) 79 ± 9% in human plasma. The individual AAs were measured in a targeted metabolomic approach (using retention time and mass to charge) by mass spectrometry, as described elsewhere.(20)
Liver Biopsies and Histologic Examination
The liver biopsies were fixed in neutrally buffered formalin at room temperature for 24-48 hours, processed, and embedded in paraffin. Slides were cut at 3 µm and evaluated according to standard pathology guidelines. The biopsies were examined independently by three experienced liver pathologists who were blinded to clinical details. Consensus was sought in the event of disagreement among scores. Liver fibrosis (Fib) was staged from F0 to F4 based on sirius red staining, and the NAFLD activity score (NAS) was calculated using standard guidelines for histologic scoring of NAFLD.(22)
To identify how NAFLD severity affects metabolism, we subdivided the group of study subjects with (bariatric) obesity (OBE; n = 33) into three groups (termed NAFLD groups) based on liver histology: (1) OBE-no steatosis comprised 17 patients without liver steatosis but with a median NAS of 2; (2) OBE+steatosis comprised 8 patients with liver steatosis but an overall NAS of less than 5; (3) OBE-NASH-Fib comprised 8 patients with a NAS of 5 or above and/or fibrosis grade 2.
CON consisted of 9 healthy, normal weight/near normal weight, control subjects without hepatic steatosis and with NAS between 0.5 and 1.5
Calculations
To generate a surrogate marker for the hepatic actions of glucagon on ureagenesis, we calculated a previously evaluated glucagon-alanine index using the following formula: glucagon-alanine index = (fasting plasma glucagon [pmol/L] × fasting plasma alanine [µmol)/L]).(8)
Because C-peptide and insulin are secreted from pancreatic beta cells in equimolar amounts while only insulin binds to hepatic insulin receptors and hence is subjected to first-pass metabolism in the liver, calculating the hepatic insulin extraction (insulin clearance) can be used as a proxy for insulin sensitivity of the liver.(23) Hepatic insulin clearance is calculated as the ratio between C-peptide and insulin. A higher ratio indicates a higher clearance and a higher degree of hepatic insulin sensitivity.
The Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) was calculated according to the formula (fasting glucose [mg/dL] × insulin [mU/L]/405), which primarily is an index of hepatic insulin resistance.(24)
At the 12-month follow-up visit, we calculated percentage excess body weight loss (%EBWL) as ([{baseline BMI – follow-up BMI}/{baseline BMI-25}] × 100%).
Statistics
The criteria for normal distribution were not met, therefore we carried out nonparametric testing. Comparisons between more than two groups were made using the Kruskal-Wallis one-way analysis of variance (ANOVA) test, and comparisons within groups (baseline versus 12-month follow-up) were made using the Wilcoxon signed-rank test for paired data. The results of the Kruskal-Wallis tests were adjusted with the Bonferroni correction for multiple comparisons in cases of significant overall outcome. Correlations were assessed using Spearman’s rank correlation.
Study participant characteristics are presented as medians with interquartile range (IQR) or as frequencies. P < 0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics 25 64-bit. Graphs and illustrations were made using GraphPad Prism version 8.1.1 for Windows 10 (GraphPad Software, Inc., www.graphpad.com).
Results
Anthropometric, clinical, and biochemical features of the study population are summarized in Tables 1 and 2. Data on targeted metabolomics are shown in Supporting Table S1.
| Baseline | Follow-Up | ||||||
|---|---|---|---|---|---|---|---|
| OBE-No Steatosis (n = 17) | OBE+Steatosis (n = 8) | OBE-NASH-Fib (n = 8) | CON (n = 9) | OBE-no Steatosis (n = 17) | OBE+Steatosis (n = 8) | OBE-NASH-Fib (n = 8) | |
| Age (years) | 44 (37-53) | 42 (32-45) | 44 (41-54) | 39 (34-43) | |||
| Sex (female/male) | 12/5 | 5/3 | 2/6 | 7/2 | |||
| Type of surgery (SG/RYGB) | 13/4 |
1/7† |
6/2‡ | ||||
| T2D (yes/no) | 2/15 | 3/5 | 5/3† | 0/9§ | 0/17 | 1/7 | 2/6 |
| Weight (kg) | 121 (112-135) | 136 (118-140) | 115 (100-127) | 71 (62-80)†-¶ | 90 (83-100)† | 96 (87-108)‡ | 90 (87-107)§ |
| BMI (kg/m2) | 43.8 (38.5-45.3) | 45.7 (39.5-52.9) | 36.7 (33.1-40.8) | 24.5 (22.3-26.4)†, ‡, ||, ¶ | 32.8 (29.2-34.8)† | 34.0 (27.3-40.9)‡ | 29.9 (26.1-33.6)§ |
| Waist:hip ratio | 0.84 (0.80-0.95) | 0.89 (0.87-0.95) | 1.03 (0.96-1.09)† | 0.84 (0.75-0.90)§ | 0.81 (0.75-0.86)† | 0.84 (0.81-0.90)‡ | 0.97 (0.90-1.00)|| |
| %EBWL | 56.6 (47.6-77.5) | 57.7 (42.8-86.2) | 50.3 (38.7-90.2) | ||||
| Systolic blood pressure (mmHg) | 129 (120-134) | 127 (118-147) | 126 (115-135) | 113 (108-130) | 122 (101-134) | 111 (107-120)‡ | 117 (113-132) |
| Diastolic blood pressure (mmHg) | 83 (76-92) | 83 (78-88) | 79 (74-93) | 75 (70-84) | 71 (67-85)† | 74 (65-78)‡ | 71 (68-83)§ |
| Heart rate (BPM) | 70 (66-81) | 77 (71-93) | 81 (62-96) | 71 (64-76)‡ | 60 (52-69)† | 63 (47-79)‡ | 61 (58-69)§ |
| ALT (U/L) | 29 (22-37) | 35 (19-54) | 34 (26-45) | 19 (18-27) | 17 (16-27) | 29 (24-38) | 25 (19-33) |
| AST (U/L) | 25 (20-31) | 26 (15-35) | 23 (17-31) | 20 (17-26) | 24 (21-28) | 24 (20-33) | 23 (21-26) |
| GGT (U/L) | 28 (17-39) | 24 (19-78) | 32 (25-41) | 28 (26-38)|| | 17 (11-25)† | 15 (10-37)‡ | 24 (20-32)§ |
| HbA1c (mmol/mol) | 34 (32-38) | 36 (33-53) | 39 (36-42) | 33 (29-35)§ | 33 (31-35) | 34 (32-43)‡ | 36 (32-40)§ |
| Fasting glucose (mmol/L) | 5.8 (5.6-6.1) | 6.6 (5.6-8.4) | 6.9 (6.4-9.1)† | 5.3 (5.2-6.0)§ | 5.4 (5.1-5.5)† | 5.5 (5.1-6.3)‡ | 5.7 (5.2-6.7)§ |
| C-peptide (pmol/L) | 1,080 (953-1,345) | 1,220 (980-1,398) | 1,290 (1,213-2,045) | 863 (650-921)†-§ | 735 (648-831)† | 813 (614-980)‡ | 816 (749-997)§ |
| Fasting insulin (pmol/L) | 106 (85-163) | 141 (99-183) | 147 (121-252) | 64 (47-96)‡, § | 61 (41-74)† | 54 (42-94) | 70 (58-137)§ |
| HOMA-IR | 4.0 (3.0-6.6) | 5.3 (4.4-6.5) | 6.9 (6.0-10.9) | 2.1 (1.6-3.6)‡, § | 2.0 (1.3-2.4)† | 1.7 (1.5-3.2) | 2.4 (1.9-5.3)§ |
| Fasting plasma glucagon (pmol/L) | 8.0 (6.0-11.5) | 7.5 (6.0-9.8) | 7.5 (6.0-9.8) | 5.0 (4.0-9.0) | 6.0 (5.0-7.5)† | 4.5 (4.0-8.0) | 7.0 (5.0-9.8) |
| Fasting plasma AAs (μmol/L) | 1,153 (877-1,324) | 1,194 (1,002-1,411) | 1,197 (1,059-1,451) | 749 (548-911)†-# | 1,035 (937-1,305) | 1,242 (1,074-1,368) | 1,054 (997-1,365) |
| Alanine (μmol/L) | 0.361 (0.324-0.448) | 0.385 (0.297-0.455) | 0.421 (0.338-0.537) | 0.299 (0.263-0.369) | 0.311 (0.271-0.377) | 0.317 (0.312-0.344) | 0.319 (0.277-0.369) |
| Total cholesterol (mmol/L) | 4.5 (4.4-5.2) | 3.7 (3.5-4.0) | 4.1 (3.1-4.6) | 4.6 (3.7-5.1) | 4.1 (3.4-5.5) | 3.2 (2.8-3.6) | 4.2 (2.9-4.8) |
| LDL cholesterol (mmol/L) | 2.9 (2.2-3.3) | 2.2 (1.7-2.5) | 2.2 (1.2-2.5) | 2.7 (2.1-3.1)¶ | 2.4 (1.8-3.6) | 1.5 (1.0-2.0)‡, || | 2.1 (1.4-2.8) |
| HDL cholesterol (mmol/L) | 1.2 (1.0-1.4) | 1.1 (0.8-1.2) | 1.2 (1.1-1.3) | 1.3 (1.3-1.5)‡ | 1.5 (1.3-1.6)† | 1.5 (1.3-1.6)‡ | 1.4 (1.2-1.9) |
| VLDL cholesterol (mmol/L) | 0.6 (0.5-0.7) | 0.6 (0.4-0.9) | 0.6 (0.3-1.1) | 0.5 (0.3-0.7) | 0.4 (0.3-0.5)† | 0.4 (0.3-0.4)‡ | 0.3 (0.2-0.7) |
| Triglycerides (mmol/L) | 1.23 (1.00-1.55) | 1.26 (1.01-1.84) | 1.35 (0.60-2.41) | 1.20 (0.65-1.56) | 0.88 (0.66-1.09)† | 0.90 (0.71-0.97)‡ | 0.75 (0.54-1.50) |
| NAS | 2 (2-3) | 3 (3-4) | 5 (3.3-5)† | 1 (0.5-1.5)‡, § | 1 (0-1)† | 1 (1-1.8)‡ | 0 (0-2)§ |
| Steatosis grade | 0 (0-0) | 1 (1-1)† | 1.5 (1-2)† | 0 (0-0)‡, § | 0 (0-0) | 0 (0-0)‡ | 0 (0-1)§, || |
| Inflammation grade | 1 (1-1) | 1 (1-1) | 1 (1-1) | 1 (0.5-1) | 1 (0-1)† | 1 (0.3-1) | 0 (0-0.8)§ |
| Ballooning grade | 1 (1-1) | 1 (1-1.8) | 2 (1.3-2) | 0 (0-0.5)†-§ | 0 (0-0.5)† | 0 (0-0.8)‡ | 0 (0-0)§ |
| Fibrosis grade | 1 (1-1) | 1 (1-1) |
2 (1-2)†, ‡ |
1 (1-1)§ | 1 (1-1) | 1 (0.3-1) | 1 (1-1) |
| Glucagon-alanine index | 2.95 (2.20-4.64) | 2.64 (2.28-3.88) | 3.21 (2.20-4.06) | 1.83 (1.34-2.35) | 1.85 (1.18-2.78)† | 1.69 (1.25-2.57)‡ | 2.21 (1.38-3.74) |
| Hepatic insulin clearance | 9.75 (7.96-12.07) | 9.19 (7.89-11.30) | 9.91 (7.69-10.90) | 13.43 (9.99-14.71) | 12.88 (9.82-15.98)† | 14.25 (11.85-16.72) | 12.34 (9.22-14.07) |
- Data are presented as median (IQR).
- † P < 0.05 compared with the OBE-no steatosis group at baseline.
- ‡ P < 0.05 compared with the OBE+steatosis group at baseline.
- § P < 0.05 compared with the OBE-NASH-Fib group at baseline.
- || P < 0.05 compared with the OBE-no steatosis group at follow-up.
- ¶ P < 0.05 compared with the OBE+steatosis group at follow-up.
- # P < 0.05 compared with the OBE-NASH-Fib group at follow-up.
- Abbreviations: NAFLD, non-alcoholic fatty liver disease. CON, control study subjects; SG, sleeve gastrectomy; RYGB, Roux-en-Y gastric bypass; BMI, body mass index; EBWL, excess body weight loss; mmHg, millimeters of mercury; BPM, beats per minute; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma glutamyl transferase; HbA1c, glycated hemoglobin; HOMA-IR, Homeostatic Model Assessment for Insulin Resistance; LDL, low density lipoprotein; HDL, high density lipoprotein; VLDL, very low density lipoprotein.
| Baseline | Follow-Up | ||||
|---|---|---|---|---|---|
| OBE (n = 33) | CON (n = 9) | OBE (n = 33) | SG (n = 20) | RYGB (n = 13) | |
| Age (years) | 44 (38-51) | 39 (34-43) | — | — | — |
| Sex (female/male) | 19/14 | 7/2 | — | 11/9 | 8/5 |
| T2D (yes/no | 10/23 | 0/9 | 3/30 | 2/18 | 1/12 |
| Weight (kg) | 123 (110-137) | 71 (62-80)†, ‡ | 90 (87-102)† | 90 (87-102) | 91 (81-102) |
| BMI (kg/m2) | 41.7 (37.2-45.3) | 24.5 (22.3-26.4)‡ | 32.5 (28.0-34.8)† | 32.6 (29.2-34.6) | 31.8 (26.7-36.9) |
| Waist:hip ratio | 0.89 (0.83-1.02) | 0.84 (0.75-0.90)† | 0.85 (0.80-0.93)† | 0.85 (0.75-0.96) | 0.85 (0.81-0-90) |
| %EBWL | 52.9 (46.3-80.0) | 50.4 (47.0-74.7) | 60.0 (43.7-90.0) | ||
| Systolic blood pressure (mmHg) | 127 (119-135) | 113 (108-130)† | 119 (107-133)† | 118 (104-134) | 119 (107-121) |
| Diastolic blood pressure (mmHg) | 82 (76-90) | 75 (70-84) | 72 (68-84)† | 74 (68-86) | 70 (65-76) |
| Heart rate (BPM) | 73 (66-86) | 71 (64-76)‡ | 60 (52-70)† | 60 (57-72) | 57 (49-70) |
| ALT (U/L) | 32 (24-39) | 19 (18-27)† | 23 (17-32)† | 18 (16-23) | 31 (26-38)§ |
| AST (U/L) | 25 (18-30) | 20 (17-26) | 24 (21-28) | 23 (21-26) | 25 (21-36) |
| GGT (U/L) | 30 (18-41) | 28 (26-38)‡ | 19 (12-25)† | 22 (11-25) | 16 (14-35) |
| HbA1c (mmol/mol) | 36 (33-39) | 33 (29-35)† | 34 (32-37)† | 34 (32-37) | 34 (32-39) |
| Fasting glucose (mmol/L) | 6.1 (5.7-6.7) | 5.3 (5.2-6.0)† | 5.4 (5.2-5.7)† | 5.4 (5.2-5.7) | 5.4 (5.1-5.7) |
| C-peptide (pmol/L) | 1,210 (1,023-1,395) | 863 (650-921)† | 783 (673-942)† | 820 (651-946) | 749 (674-894) |
| Fasting insulin (pmol/L) | 128 (100-177) | 64 (47-96)† | 62 (45-88)† | 63 (53-93) | 51 (40-66) |
| HOMA-IR | 5.1 (3.7-7.0) | 2.1 (1.6-3.6)† | 2.1 (1.5-2.8)† | 2.1 (1.8-3.2) | 1.7 (1.4-2.2) |
| Fasting plasma glucagon (pmol/L) | 8.0 (6.0-10.0) | 5.0 (4.0-9.0)† | 5.0 (4.0-8.5)† | 5.5 (4.3-8.0) | 5.0 (4.5-10.0) |
| Fasting plasma AAs (μmol/L) | 1,171 (964-1,334) | 749 (548-911)†, ‡ | 1,105 (978-1,331) | 1,157 (959-1,419) | 1,112 (978-1,316) |
| Alanine (μmol/L) | 0.381 (0.322-0.453) | 0.299 (0.263-0.369)† | 0.315 (0.287-0.364)† | 0.318 (0.286-0.409) | 0.315 (0.267-0.330) |
| Total cholesterol (mmol/L) | 4.4 (3.7-4.7) | 4.6 (3.7-5.1) | 3.9 (3.0-4.9) | 4.4 (3.6-5.6) | 3.2 (2.9-3.6)§ |
| LDL cholesterol (mmol/L) | 2.3 (1.8-2.9) | 2.7 (2.1-3.1) | 1.9 (1.5-2.9)† | 2.6 (1.8-3.5) | 1.5 (1.1-1.9)§ |
| HDL cholesterol (mmol/L) | 1.2 (1.0-1.3) | 1.3 (1.3-1.5)†, ‡ | 1.5 (1.3-1.6)† | 1.5 (1.2-1.6) | 1.4 (1.3-1.6) |
| VLDL cholesterol (mmol/L) | 0.6 (0.4-0.7) | 0.5 (0.3-0.7) | 0.4 (0.3-0.5)† | 0.4 (0.3-0.5) | 0.3 (0.3-0.4)§ |
| Triglycerides (mmol/L) | 1.24 (0.99-1.61) | 1.20 (0.65-1.56) | 0.88 (0.66-1.09)† | 0.90 (0.67-1.16) | 0.74 (0.66-0.90) |
| NAS | 3 (2-4) | 1 (0.5-1.5)† | 1.0 (0.5-1.5)† | 1 (0-2) | 1 (0-1) |
| Steatosis grade | 0 (0-1) | 0 (0-0)† | 0 (0-0)† | 0 (0-0) | 0 (0-0) |
| Inflammation grade | 1 (1-1) | 1 (0.5-1) | 1 (0-1)† | 0 (0-1) | 0 (0-1) |
| Ballooning grade | 1 (1-2) | 0 (0-0.5)† | 0 (0-0)† | 0 (0-0) | 0 (0-0.5) |
| Fibrosis grade | 1 (1-1) | 1 (1-1) | 1 (1-1) | 1 (1-1) | 1 (1-1) |
| Glucagon-alanine index | 2.95 (2.25-4.13) | 1.83 (1.34-2.35)† | 1.79 (1.25-2.77)† | 1.82 (1.23-2.82) | 1.75 (1.25-2.77) |
| Hepatic insulin clearance | 9.51 (7.86-11.42) | 13.43 (9.99-14.71)† | 12.78 (10.24-15.71)† | 12.52 (10.01-13.27) | 14.25 (10.25-17.38) |
- Data are presented as median (IQR).
- † P<0.05 compared with the OBE group at baseline.
- ‡ P<0.05 compared with the OBE group at follow-up.
- § P<0.05 compared with the SG group.
- Abbreviations: NAFLD, non-alcoholic fatty liver disease. CON, control study subjects; SG, sleeve gastrectomy; RYGB, Roux-en-Y gastric bypass; BMI, body mass index; EBWL, excess body weight loss; mmHg, millimeters of mercury; BPM, beats per minute; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma glutamyl transferase; HbA1c, glycated hemoglobin; HOMA-IR, Homeostatic Model Assessment for Insulin Resistance; LDL, low density lipoprotein; HDL, high density lipoprotein; VLDL, very low density lipoprotein.
Participants in OBE are Characterized by Hyperglucagonemia and Hyperaminoacidemia
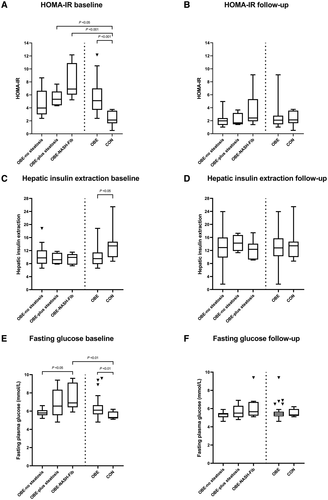
OBE and CON were matched for age and sex. Not surprisingly, participants in OBE were metabolically deranged, reflected by significantly increased fasting glucose, insulin, C-peptide, and HBA1c levels compared with CON (Table 2; Fig. 1E). In total, 10 subjects in OBE had T2D; 6 were treated only with metformin, 2 were treated only with liraglutide, and 2 were treated with a combination of metformin, liraglutide, and long-lasting insulin. Liver histology in OBE was characterized by a significantly higher NAS than in CON, with median NAS scores of 3 (IQR, 2-4) versus 1 (IQR, 0.5-1.5; P < 0.001) and driven by the presence of steatosis and ballooning. Correspondingly, ALT levels were higher in OBE (median, 32 U/L; IQR, 24-39) than in CON (median, 19 U/L; IQR, 18-27; P < 0.05).
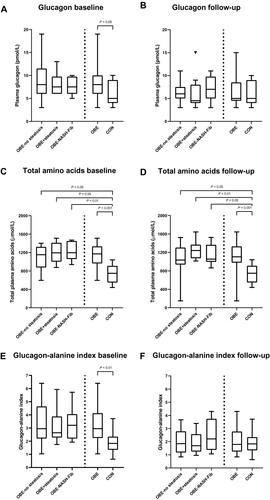
OBE was characterized by increased plasma concentrations of glucagon (60%), i.e., hyperglucagonemia, compared to CON (Fig. 2A; P < 0.05). Similarly, plasma concentrations of the AA pool (termed total AAs) were 56% higher in OBE (Fig. 2C; P < 0.001).
Severity of NAFLD Does Not Worsen Hyperglucagonemia in OBE
We found no significant difference in glucagon concentrations between OBE-no steatosis (median, 8.0 pmol/L; IQR, 6.0-11.5), OBE+steatosis (median, 7.5 pmol/L; IQR, 6.0-9.8), and OBE-NASH-Fib (median, 7.5 pmol/L ; IQR, 6.0-9.8; P = 0.195).
Insulin resistance was numerically higher in OBE-NASH-Fib (median HOMA-IR, 6.9; IQR, 6.0-10.9) versus OBE-no steatosis (median HOMA-IR, 4.0; IQR, 3.0-6.6; P = 0.118). Fasting insulin secretion, evaluated from plasma C-peptide levels, was not found to be different across NAFLD groups (P = 0.486).
The OBE-NASH-Fib group was, as expected, the NAFLD group with the most pronounced metabolic derangement, displaying simultaneous and significantly elevated waist:hip ratio, HbA1c, fasting plasma glucose, insulin, C-peptide, and HOMA-IR levels (Table 1).
Hyperaminoacidemia Is Increased Comparably Among All NAFLD Groups
All NAFLD groups had marked hyperaminoacidemia, with 54% (P < 0.05), 59% (P = 0.01), and 60% (P < 0.01) higher plasma concentrations of AAs in OBE-no steatosis, OBE+steatosis, and OBE-NASH-Fib, respectively. The presence of hepatic steatosis and/or fibroinflammation did not appear to aggravate the hyperaminoacidemia (Fig. 2C; P = 1.0 ).
Liver–Alpha Cell Axis Is Disrupted in Patients With Obesity and NAFLD
The glucagon-alanine index was significantly increased in all NAFLD groups (Fig. 2E) compared with CON, but the severity of NAFLD did not significantly impact the glucagon-alanine indexes (P = 0.109). Hepatic insulin resistance, reflected by fasting HOMA-IR, was increased and the hepatic insulin extraction ratio was markedly lower in OBE (Fig. 1C; P < 0.05), worsening in step with increasing NAFLD severity.
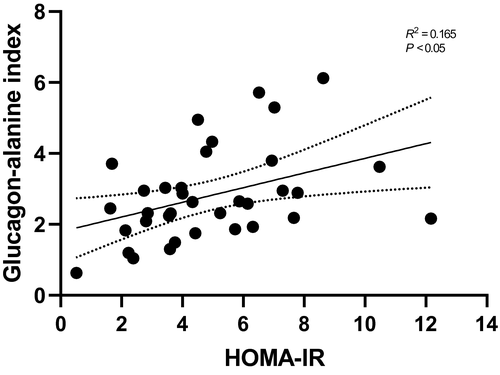
To investigate whether glucagon resistance toward AA metabolism is associated with hepatic insulin resistance, we correlated the glucagon-alanine index to fasting HOMA-IR and found a positive correlation (Spearman’s rank coefficient R = 0.165; P < 0.05) (Fig. 3).
Hyperaminoacidemia Persists in All NAFLD Groups Despite Improvement in NAFLD and Metabolic Derangement 12 Months After Bariatric Surgery
Twelve months after bariatric surgery, all 33 OBE subjects had experienced pronounced weight loss, with a median reduction in %EBWL of 50.3 (IQR, 38.7-90.2) in OBE-NASH-Fib to 57.7 (IQR, 42.8-86.2) in OBE+steatosis (P = 0.850 among NAFLD groups) (Table 1) and an overall decrease in median BMI from 42 to 33 kg/m2 (Table 2).
NAS declined significantly in all three NAFLD groups (delta NAS baseline to 12 months: OBE-no steatosis, 1, IQR, 1-2, P < 0.001; OBE+steatosis, 2.5, IQR, 1.3-3, P < 0.05; OBE-NASH-Fib, 3, IQR, 3-4.8, P < 0.05). OBE-NASH-Fib had a larger reduction in NAS compared to the OBE-no steatosis group (P < 0.001). Of the 17 study participants with steatosis at baseline, 13 had reached grade zero steatosis at 12 months and all three NAFLD groups saw a significant reduction in ballooning grade. Furthermore, the OBE-NASH-Fib group achieved reduced inflammation (P < 0.05), whereas fibrosis did not decrease significantly (P = 0.096). Metabolically, all groups displayed significant and comparable improvement in HOMA-IR, C-peptide, and insulin (Table 1; Fig. 1B). Three participants still had manifest T2D and needed treatment with metformin (2 participants) and a combination of liraglutide, metformin, and long-lasting insulin (1 participant).
Interestingly, bariatric surgery and weight loss had no impact on total AA concentrations, which were elevated and unchanging in all NAFLD groups (Table 1; Fig. 2D).
Hyperglucagonemia Resolves After Bariatric Surgery in Patients With NAFL But Not in Those With NASH
Plasma concentrations of glucagon were significantly reduced from a median of 8.0 pmol/L (IQR, 6.0-10.0) to 5.0 pmol/L (IQR, 4.0-8.5; P < 0.01) 12 months after bariatric surgery. The OBE-no steatosis and OBE+steatosis group normalized their plasma concentrations of glucagon relative to CON. In contrast, glucagon concentrations did not change in OBE-NASH-Fib after 12 months compared with their baseline (OBE-NASH-Fib 12-month median, 7.0 pmol/L, IQR, 5.0-9.8 versus baseline median, 7.5 pmol/L, IQR, 6.0-9.8; P = 0.799) (Table 1; Fig. 2B).
Improvements in Glucagon and Insulin Resistance After Bariatric Surgery
Twelve months after bariatric surgery, the glucagon-alanine index was significantly reduced in OBE-no steatosis and OBE+steatosis but not in OBE-NASH-Fib (Fig. 2F). The decreased glucagon-alanine index was driven by concomitant decreases in both glucagon and alanine plasma concentrations.
Pronounced reductions in HOMA-IR were seen in OBE-no steatosis (P < 0.01) and OBE-NASH-Fib (P < 0.05), but only negligible reductions were seen in OBE+steatosis. Both C-peptide and insulin were significantly lowered from baseline to 12 months; insulin decreased by 52% and C-peptide by 35%, indicating a significant increase in the hepatic insulin extraction in OBE from median 9.51 (IQR, 7.86-11.42) at baseline to 12.78 (IQR, 10.24-15.71) 12 months after surgery (P < 0.01) (Fig. 1C,D).
No Differences Were Observed in the Effect on Biochemical, Anthropometric, or Clinical Parameters Between RYGB and SG Individuals
When comparing the effect of RYGB with SG, we found no difference between the delta changes among any of the parameters assessed (Table 2).
Discussion
We investigated the importance of NAFLD severity, evaluated by liver biopsies, as it relates to glucagon resistance toward AA metabolism and insulin resistance in patients with obesity before and after bariatric surgery. Our primary aim was to characterize the physiological crosstalk between the liver and pancreas, focusing on the liver–alpha cell axis in individuals with different severities of NAFLD. A secondary aim was to determine whether hyperaminoacidemia and hyperglucagonemia can be reversed following major weight loss and whether NAFLD improved after bariatric surgery.
Our primary findings were marked hyperaminoacidemia and hyperglucagonemia as well as significantly increased glucagon-alanine indices indicative of hepatic glucagon resistance to AA metabolism in individuals with obesity with NAFLD. However, the severity of NAFLD did not worsen disruption of the liver–alpha cell axis. Our study therefore expands the understanding of how NAFLD may induce a vicious cycle of diabetogenic hyperglucagonemia.(25, 26)
Given that NAFLD is a disease with a wide histologic spectrum of disease and with worsening metabolic consequences(27-29) as the disease progresses from hepatic steatosis to the fibroinflammatory state, as seen in NASH, it would seem fair to assume that ureagenesis is affected in line with any escalation of the disease. Interestingly, we found no such support for this assumption as all NAFLD groups presented with comparable hyperaminoacidemia and hyperglucagonemia at baseline. Instead, it could be that a certain threshold must be passed before ureagenesis becomes impaired, whether solely due to glucagon resistance or due to a combination of this and other factors, as discussed below. Once this threshold has been met, the liver may not be able to regain control over ureagenesis, as we observed in the individuals in our study with NASH who had persistent hyperglucagonemia 12 months after surgery despite otherwise pronounced histologic as well as metabolic recovery.
Intrahepatocyte build-up of triglycerides is known to interfere with several metabolic functions of the liver.(30) Hepatic steatosis has been hypothesized to impact the development of glucagon resistance to ureagenesis,(25) analogous to hepatic steatosis being a key feature in hepatic insulin resistance.(3, 31, 32)
Glucagon’s impact on ureagenesis includes transcriptional changes in genes (such as carbamoyl-phosphate synthase 1 [CPS1], glutamic-oxaloacetic transaminase 1 [GOT1], and solute carrier family 25 member 18 [SLC25A18]) that regulate hepatic nitrogen conversion in NAFLD, as has been shown in several studies.(26, 33, 34) Eriksen et al.(34) found that the genes most involved with urea cycle-related enzymes were down-regulated in patients with both NAFL and NASH compared to lean and obese controls without NAFLD. The altered expression explains the decrease observed in in vivo functional capacity for ureagenesis. Eriksen et al. concluded that the gene down-regulations seemed attributable to hepatic fat accumulation rather than other metabolic factors ascribed to obesity or evident hepatic necroinflammation/NASH. Possible down-regulation of urea cycle-related enzymes and/or glucagon receptors could play a role in the hyperaminoacidemia observed in our study, both at baseline and 12 months after surgery. It could be speculated that metabolic memory persists for at least 12 months (and is accompanied by transcriptomic changes due to, for example, epigenetic factors) in the livers of subjects with NAFLD, disabling hepatocytes to regain normal metabolic functions (including ureagenesis) even after weight loss and return-to-normal liver histology. The significant metabolic improvement indicated by a decrease in HOMA-IR (i.e., hepatic insulin resistance) and increased insulin extraction rates (i.e., improvement in hepatic insulin sensitivity) point to an overall improvement in insulin-mediated metabolic liver function. It would therefore be rational to assume that hepatic sensitivity to glucagon would also improve and ureagenesis occur unhindered. Nevertheless, in OBE, OBE-no steatosis, and OBE+steatosis, glucagon levels declined, and in all NAFLD groups the glucagon-alanine index also decreased, indicating regained hepatic glucagon sensitivity. Overall, our data point to hepatic glucagon resistance and hepatic insulin resistance being only partly parallel phenomena with different pathophysiological mechanisms.
Another finding in the present study was that total plasma AA concentrations (but not individual plasma AAs, including branched chain AAs [see Supporting Table 1; Supporting Fig. S1]) were overall unaffected by both the severity of NAFLD and prominent weight loss induced by bariatric surgery. From our study it appears that it is the obesity per se rather than the presence and degree of hepatic steatosis and/or accompanying fibroinflammation that primarily drives hyperaminoacidemia in patients with obesity. Obesity (and not solely NAFLD) may directly contribute to hyperaminoacidemia, in line with what was reported by Gaggini et al.(35) In that study, 13 AAs were measured in subjects with and without obesity with biopsy-proven NAFLD as well as in a lean control group. Plasma levels of the individual AAs (including alanine) tended to be increased in subjects with obesity with NAFLD but not in those with NAFLD without obesity. The authors concluded that these observations could be ascribed to worsened peripheral insulin resistance and consequently increased protein catabolism, with an outcome of higher AA levels in those with obesity.
The hyperaminoacidemia observed in our study may partly be explained by increased proteolysis of proteins (primarily muscle protein) due to peripheral insulin resistance(14, 36) leading to the release of AAs, with the circulation of glutamine and alanine being the key transporters of amino groups between muscles and the liver.(36, 37) Although we have no direct measure of peripheral insulin resistance, individuals in OBE were insulin resistant, as indicated by their markedly increased HOMA-IR, and although primarily an index of hepatic insulin resistance, HOMA-IR also correlates with peripheral insulin resistance.(38)
Furthermore, alanine was indeed significantly increased in OBE when compared to CON, and alanine concentrations were elevated according to escalating NAFLD severity. Additionally, the highest alanine values were found in OBE-NASH-Fib, which displayed the highest levels of HOMA-IR. It may therefore be that peripheral insulin resistance contributed to the observed hyperaminoacidemia at baseline.
Bariatric surgery had no effect on total AA concentrations after 12 months. Twelve months after surgery, weight loss, although greatly decelerating, had not yet plateaued. As loss of fat-free mass is a side effect of weight loss induced by RYGB and SG,(39-41) it could be that the persistently elevated AA levels are explained by a negative protein/nitrogen balance resulting from muscle wasting.
Alterations in nutrient absorption after bariatric surgery might also influence AA levels. In a previous study by our group,(42) protein absorption rates and handling as well as whole-body protein balance were measured during and after meal tests in individuals who had had RYGB or SG. We observed increased rates of protein absorption in subjects from RYGB but not from SG when compared to controls. In conclusion, although the data point to increased protein absorption in patients who had RYGB, the results still do not explain the fasting hyperaminoacidemia observed in individuals who had SG. Taken together, further studies are warranted to determine whether the hyperaminoacidemia is explained primarily by hepatic glucagon resistance or is modified by negative protein balance during the major weight loss in the first year after surgery or a change in AA metabolism in the peripheral muscles.
The major strengths of this study are the prospective follow-up with paired and repeat liver biopsies in a well-characterized study cohort and the gold-standard techniques used to analyze glucagon and AAs.
The study also has limitations. Because all participants with obesity had gone through a mandatory 8% weight loss before surgery (baseline visit), and hence before the baseline liver biopsy was taken, it is possible that our study subjects had a worse degree of liver steatosis and higher NAS than before the weight loss occurred. It could be that the absence of marked differences in glucagon, total AAs, and glucagon-alanine index between OBE-no steatosis and CON groups are due to obesity alone or due to previous or “convalescent” NAFLD. The hypothesized improvement in hepatic steatosis at baseline may already have caused our participants with obesity to regain some glucagon sensitivity. Nevertheless, we did measure glucagon and plasma AAs before the 8% weight loss and at baseline in 3 participants, and no differences were found in glucagon or AA concentrations between preweight loss and baseline numbers despite a worse liver histology before the weight loss. Another important limitation of this study is the relatively low number of participants in each NAFLD group, which reduces the statistical power of its results. Future studies with a higher number of participants and a more mechanistic approach are required to address the changes in the liver–alpha cell axis as well as hepatic glucagon and insulin resistance and their mediation at a molecular level.
The liver–alpha cell axis is disrupted in patients with obesity with NAFLD; however, the severity of NAFLD does not seem to increase glucagon resistance to AA metabolism. Insulin and glucagon resistance may develop in parallel, but their etiology may not be identical as our results suggest that major changes in body weight and insulin resistance following bariatric surgery had only a minor effect on hepatic glucagon resistance in patients with NASH, despite significant improvement in their liver histology.
Acknowledgment
We thank Christine Rasmussen (Department of Clinical Biochemistry, Copenhagen University Hospital Rigshospitalet) for laboratory help, project nurse Karen Lisa Hilsted (Gastro Unit, Medical Division) for logistical and administrative assistance, Lili Niu (Novo Nordisk Foundation Center for Protein Research, Copenhagen University) for skillful data assistance, and the MicroBLiver Research Consortium. We also thank Professor Hendrik Vilstrup (Aarhus University, Denmark) for many fruitful discussions related to the topic.




