Liver Diseases in the Perinatal Period: Interactions Between Mother and Infant
Abstract
Liver diseases affecting the mother and infant dyad may present in the perinatal period from 20 weeks of gestation to 28 days of life. This review will focus on the current approach to neonatal acute liver failure and the progress made in the diagnosis and management of gestational alloimmune liver disease. It will highlight mother-to-child transmission of viral hepatitis, both management and public health implications. Emerging concepts implicating maternal obesity and nutrition in the development of a rapidly progressive nonalcoholic steatohepatitis phenotype in the offspring will be discussed. Finally, the presentation and management of acute fatty liver of pregnancy and intrahepatic cholestasis of pregnancy, and their impact on the fetus, will be reviewed.
Abbreviations
-
- AFLP
-
- acute fatty liver of pregnancy
-
- ALF
-
- acute liver failure
-
- ALT
-
- alanine aminotransferase
-
- BA
-
- bile acid
-
- CMV
-
- cytomegalovirus
-
- CoA
-
- coenzyme A
-
- FAOD
-
- fatty acid oxidation disorders
-
- FXR
-
- farnesoid-X receptor
-
- GALD
-
- gestational alloimmune liver disease
-
- GALD-NH
-
- GALD with NH
-
- HBeAg
-
- hepatitis B e antigen
-
- HBV
-
- hepatitis B virus
-
- HCV
-
- hepatitis C virus
-
- HE
-
- hepatic encephalopathy
-
- HEV
-
- hepatitis E virus
-
- HLH
-
- hemophagocytic lymphohistiocytosis
-
- HSV
-
- herpes simplex virus
-
- ICP
-
- intrahepatic cholestasis of pregnancy
-
- INR
-
- international normalized ratio
-
- IVIG
-
- intravenous immunoglobulin
-
- LCHAD
-
- long-chain 3-hydroxyacyl-coenzyme A (CoA) dehydrogenase
-
- LT
-
- liver transplantation
-
- MTCT
-
- mother-to-child transmission
-
- mtDNA
-
- mitochondrial DNA
-
- NAFLD
-
- nonalcoholic fatty liver disease
-
- NASH
-
- nonalcoholic steatohepatitis
-
- NH
-
- neonatal hemochromatosis
-
- OID
-
- obesity-inducing diet
-
- PFIC
-
- progressive familial intrahepatic cholestasis
-
- UDCA
-
- ursodeoxycholic acid
-
- VH
-
- viral hepatitis
Several liver diseases affect the mother and infant dyad during the perinatal period, including neonatal acute liver failure (ALF), gestational alloimmune liver disease (GALD), nonalcoholic fatty liver disease (NAFLD), viral hepatitis (VH), acute fatty liver of pregnancy (AFLP), and intrahepatic cholestasis of pregnancy (ICP). Pathogenesis and onset of these diseases are based on the unique interactions between the maternal-fetal environments. Herein, we highlight advances in neonatal ALF and GALD diagnosis and management, including the role of liver transplantation (LT). We discuss the emerging concepts implicating maternal obesity and nutrition in the development of NAFLD in offspring. We also outline screening and preventive measures for VH acquired through mother-to-child transmission (MTCT) and provide a summary about ICP and AFLP.
Neonatal ALF
Neonatal ALF is a rare condition with subtle inconsistencies in published data because of varied criteria used to identify both neonates (ranging from ages of <30 days to 1 year) and ALF. ALF is manifested by biochemical evidence of hepatocellular injury and rapid onset of coagulopathy resulting in the rapid deterioration of critical liver function.1 The National Institutes of Health–funded Pediatric Acute Liver Failure Study specified clinical and biochemical criteria (international normalized ratio [INR] ≥1.5 with hepatic encephalopathy [HE] or an INR ≥2 without HE) intended to identify children with severe acute liver injury placing them at risk for progressive clinical deterioration that could result in LT or death.1, 2 Although these study criteria may be challenging to apply in this specific population (because of difficulty detecting early HE and lack of normal INR data), neonates with ALF who meet these criteria are at particularly high risk for a poor outcome.
GALD, discussed below, is the most frequently identified cause of neonatal ALF with strikingly normal or near normal aminotransferases.3 Viral infections, including herpes simplex virus (HSV; the most frequent etiology of neonatal ALF with elevated aminotransferases), enterovirus, and cytomegalovirus (CMV), are also commonly implicated in neonatal ALF. Neonatal HSV may be transmitted in utero, though the majority of infections are acquired peri- or postpartum, with the greatest risk to neonates born to mothers with a primary genital infection near the time of delivery. Additional risk factors include a prolonged rupture of membranes or disruption of the mucocutaneous barrier, whereas delivery by C-section may be protective.4 Only 10% of neonates with disseminated disease, including hepatic involvement, survive without treatment.5 Given the significant potential mortality, initiation of acyclovir treatment in all potential cases of neonatal HSV may be life-saving while awaiting definitive diagnostic testing.4 HSV diagnosis requires isolation of HSV in tissue culture or polymerase chain reaction testing. Neonates infected with enterovirus may have a mother with a recent diarrheal or respiratory illness. Although no U.S. Food and Drug Administration–approved therapy exists, pleconaril may be available for compassionate use. Maternal CMV infection during pregnancy most often results from close contact with young children. Risk of MTCT is far higher with primary maternal infection than with recurrent infection (32% vs. 1.4%). Congenital CMV is the primary cause of nonhereditary sensorineural hearing loss and may cause other long-term neurodevelopmental disabilities. Clinical findings of congenital CMV infection, including petechiae, jaundice, and hepatosplenomegaly, occur in 10% of affected neonates at birth.6
Hemophagocytic lymphohistiocytosis (HLH), resulting in abnormal immune activation, may be an under-reported cause of neonatal ALF. HLH, initially described in patients with autoimmune and rheumatological conditions, may be primary (attributed to gene mutations that affect cytotoxic natural killer [NK] and T-cell function) or secondary (to another infection). Consensus recommendations require five of eight criteria for diagnosis: fever (>38.5°C); splenomegaly; cytopenia of ≥2 cell lines (without bone marrow hypoplasia; hemoglobin <10 g/dL; platelets <100,000/uL; and neutrophils <1,000/uL), hypertriglyceridemia (>3.0 mmol/L), hypofibrinogenemia (<150 mg/dL), elevated ferritin (>500 ng/mL), loss/absent NK cell activity, increased interleukin-2 receptor levels (>2,400 U/mL), and histological evidence of hemophagocytosis.7 The rarity of this disease in neonates combined with overlap of some HLH features with malignancy, sepsis, and primary immunodeficiencies may pose a diagnostic dilemma. Early consultation with pediatric hematology-oncology may allow for potential treatment options, including chemotherapy. An undetermined etiology for neonatal ALF accounts for up to 38% of cohorts, although this may reflect an incomplete evaluation in some.3
Heritable causes of neonatal ALF include metabolic diseases, such as galactosemia, tyrosinemia, urea cycle defects, and mitochondrial disorders.3 Diagnostic testing for these etiologies includes the newborn screen, galactose-1-phosphate uridyl transferase assay for galactosemia, and urine succinylacetone for tyrosinemia. Significant hyperammonemia (>80 umol/L and often >250 umol/L) may be indicative of urea cycle defects, with prognosis influenced by both duration and peak ammonia levels at presentation.8 Prompt discontinuation of protein, initiation of intravenous glucose to prevent catabolism, and involvement of metabolic/genetic specialists may optimize outcomes.8 Mitochondrial liver disease, including respiratory chain defects and mitochondrial DNA (mtDNA) depletion syndromes, are increasingly recognized causes of neonatal ALF.9 mtDNA depletion syndromes occur as a consequence of mutations in nuclear genes involved in mtDNA replication or maintenance of deoxynucleotide pools required for de novo mtDNA replication.9 Definitive diagnosis requires the identification of two pathogenic mutations in known disease-causing genes, the most common mutations being deoxyguanosine kinase, DNA polymerase subunit gamma, and MPV17.9 An alternative approach requires demonstration of abnormal respiratory chain function or loss of mtDNA copy number in liver tissue, but this may occur in liver failure without a mitochondrial disorder. In the future, next-generation whole-exome and -genome sequencing may allow more definitive diagnosis of these complex diseases using scalable and affordable technology in a clinically relevant time frame.
The overall outcome of neonatal ALF remains poor, given the primary diagnoses, severity of illness at presentation, and the patient’s small size. Spontaneous survival occurs in 50%-60% of neonates with ALF.3, 8 Mortality (mean age of 16 days) is most commonly attributed to bleeding, hemodynamic instability, or multiorgan failure. LT, the standard of care in older children with ALF, poses unique challenges in neonates because of their critical illness, surgical challenges related to their size, and scarcity of size-appropriate organs, often necessitating reliance on technical variant grafts. Broader questions of equipoise also occur given that for many neonates, etiology of their ALF is unknown and could be caused by a metabolic disease not cured by LT. Centers that lack adequate LT experience for small infants and/or technical variant grafts should consider early transfer to a transplant center with such expertise.
The Pediatric ALF Study Group reported that neonates (n = 148) were much less likely (41%) than older children with ALF (58%) to be listed for LT (P = 0.008).3 Whereas neonates with GALD or undetermined ALF were most likely to be listed for transplant, neonates (40%) were also less likely to be transplanted than older children (66%; P < 0.0001).3, 10 Limited data from the Studies in Pediatric Liver Transplant for 38 neonates receiving an LT at five centers indicated good overall 1-year graft (76%) and patient (88%) survival, similar to older children.11 However, United Network for Organ Sharing (UNOS) data examining 570 infants weighing ≤5 kg at transplant demonstrated lower 1-year graft (78%) and patient survival (66%).12 Similarly, UNOS data suggest that neonates had a higher waitlist mortality and poorer graft survival than older infants undergoing LT (60% at 1 year; 42% at 5 years). Taken together, these data suggest that although center expertise may impact transplant outcomes in neonatal ALF, other opportunities must be explored to improve their overall survival, including careful expansion of the donor pool through use of reduced left lateral segments and monosegment grafts and ABO-incompatible grafts. Survival outcomes for children aged <2 years transplanted with ABO-incompatible grafts are equivalent to those receiving ABO-compatible grafts.13 When combined with desensitization protocols and critical care expertise, ABO-incompatible grafts may expand the donor pool for neonates with ALF.14
GALD
GALD, a leading cause of neonatal ALF, is a maternal-fetal alloimmune disorder. Subacute GALD is most common, presenting at birth with cirrhosis and liver failure secondary to chronic hepatic injury in utero. As a consequence of severe fetal liver injury, extrahepatic iron accumulates in the pattern observed for hereditary hemochromatosis and is referred to as neonatal hemochromatosis (NH). Uptake of excess iron occurs in the pancreas, heart, thyroid, thymus, and minor salivary glands, whereas the reticuloendothelial system (including macrophages and spleen) is spared. Less commonly, GALD may present acutely in the fetus as ALF, characterized by hepatic necrosis without NH in contrast to the subacute form. Accurate diagnosis of the first poor outcome relies on a constellation of clinical and pathological findings and a high index of suspicion.
Although the exact target antigen of alloimmune injury in GALD remains unknown, evidence suggests that the antigen is expressed early in embryological development by young or “nascent” hepatocytes that arise shortly after differentiation from bipotent hepatoblasts (Fig. 1).15 Normally, bipotent cells differentiate into mature hepatocytes that provide negative feedback for ongoing proliferation. In subacute GALD with NH (GALD-NH), damage to nascent hepatocytes results in reduced hepatocyte mass and loss of inhibition, causing a compensatory increase in biliary developmental pathways characterized by parenchymal tubulogenesis and liver fibrosis.15 Lineage markers expressed by these tubules support that they are biliary progenitors derived from the bipotent hepatoblasts spared from alloimmune injury. The tubules exhibit active hedgehog signaling, which drives fibrosis. Lack of functional hepatocytes also results in reduced angiotensinogen synthesis necessary for renal proximal tubule development. Similarly, reduced hepatic hepcidin synthesis, a negative regulator of maternal-fetal iron transport, leads to fetal iron overload and the NH phenotype.16
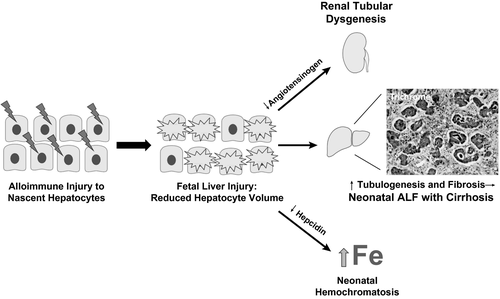
Distinguishing features of GALD-NH reflect the unique onset of liver injury during gestation. Infants commonly present at birth with signs of in utero distress, including intrauterine growth restriction, oligohydramnios, and prematurity. Renal injury and multisystem organ failure may also occur in severely affected infants. Whereas ascites with cirrhosis is common, a patent ductus venosus prevents development of splenomegaly in many infants. Laboratory findings include disproportionately low aminotransferases for degree of liver synthetic dysfunction (commonly <100 IU/L), elevated ferritin, and increased alpha-fetoprotein (usually >300,000 ng/mL with normal newborn values <80,000 ng/mL). Onset of cholestasis is frequent shortly after birth without metabolic acidosis.
Investigation for GALD-NH includes evaluation for iron deposition by loss of signal intensity on T2-weighted magnetic resonance imaging in affected tissues and/or Prussian blue staining of an oral mucosal biopsy (each test ~60% sensitive). Liver biopsy, though diagnostically useful, carries risk in coagulopathic infants. Histological features of GALD-NH include lack of mature hepatocytes, significant parenchymal ductular reaction reflective of increased tubulogenesis, pronounced lobular fibrosis and cirrhosis, and little/no extramedullary hematopoiesis (Fig. 1). Hepatic inflammation and necrosis are not characteristic of GALD and should raise suspicion for an alternative diagnosis. Positive staining for the end complex in the complement cascade (C5b-9 or the membrane attack complex) in residual hepatocytes remains a research tool that supports a mechanistic role for complement in the antibody-mediated liver injury.17 C5b-9 staining is nonspecific for GALD and has limited sensitivity with severe reduction of hepatocyte volume.
In any neonate with suspected GALD, intravenous immunoglobulin (IVIG; 1 g/kg) should be administered with subsequent double-volume exchange transfusion (DVET) and IVIG repeated after NH confirmation.18 First-line medical treatment is supportive care, and spontaneous recovery can occur, although significant improvement in INR may take weeks. Outcomes for LT for GALD are similar to other neonatal ALF etiologies and should be considered in refractory cases.19
Despite treatment advancements, outcomes for GALD remain poor, with 82% mortality of live-born infants.20 Survival after IVIG/DVET (45%) and LT (43%) is significantly improved compared to the historically used cocktail of chelators and antioxidants.20 Even in cases with hepatic recovery, long-term complications from multisystem organ failure may occur. Given the high mortality and a per-pregnancy repeat occurrence rate of 95%, accurate diagnosis of GALD in the first poor outcome is imperative to allow initiation of gestational IVIG in subsequent pregnancies, which prevents clinical liver disease in 94% of cases.20
Maternally Transmitted Chronic VH
MTCT is the primary route of transmission of chronic hepatitis B (HBV) and C virus (HCV) to children. Viral transmission may occur during gestation, delivery, or after delivery.21
Hepatitis B
Women of reproductive age constitute around 25% of the world’s population. The World Health Organization estimates that given global HBV prevalence, approximately 65 million women can potentially transmit HBV to newborns. Universal neonatal HBV vaccination has not been fully instituted. Approximately 40% of newborns worldwide received HBV vaccine at birth in 2014, and 82% of infants received three HBV vaccine doses by age 12 months.22 Risk of HBV acquisition in unvaccinated newborns exposed during pregnancy and delivery is 5%-25%, and up to 90% of these infections become chronic. Risk of MTCT of HBV is increased with maternal positive hepatitis B e antigen (HBeAg) status and circulating HBV-DNA levels >200,000 IU/mL, obstetric procedures, and fetal or neonatal injury. Delay or lack of immunoprophylaxis is probably the most critical factor, given that most HBV infections are acquired around the time of delivery. Society guidelines and expert panels have recommended that pregnant women with HBV-DNA levels >200,000 IU/mL receive tenofovir during the third trimester and for some weeks postpartum.23, 24 In most studies, this regimen has reduced the MTCT of HBV in immunized infants from around 10% to approximately 1%-2%. There are no data to support elective C-section delivery or avoidance of breastfeeding for women with chronic HBV.
HBV acquired by MTCT is usually asymptomatic. The natural history is complex and nonlinear, with several phases of infection. Most experts characterize the immune tolerant phase by normal alanine aminotransferase (ALT), HBeAg positivity, and HBV-DNA levels >200,000 IU/mL, the immune active phase by abnormal ALT and variable HBV DNA, immune control or clearance after HBeAg seroconversion and normalization of ALT, and reactivation or immune escape as the emergence of HBeAg-negative variants with abnormal ALT values. Most MTCT-infected children have normal or near-normal ALT and do not require treatment.23, 24 Most studies use 30 IU/mL as the ALT upper limit of normal, whereas the 95th percentiles for ALT in healthy boys and girls are 25.8 and 22.1 U/L, respectively.25 The pathogenesis of this putative “immune tolerant” infection is still poorly understood as an HBV-specific T-cell response that is present in these children.26 A subset of children, however, will develop an active liver injury, manifested by persistently abnormal ALT and development of fibrosis, and should be considered for antiviral therapy during childhood, although individual recommendations vary in available guidelines.27
Hepatitis C
Incidence of HCV doubled between 2010 and 2014, secondary to injection drug use in young persons, including women of childbearing potential. Approximately 40,000 children are born each year to HCV-infected women in the United States, with a 5% MTCT rate and subsequent chronic HCV infection rate of around 3%.22 Treating HCV infection before pregnancy is recommended. To date, there are no clearly proven methods of HCV MTCT prevention. Most infected newborns become viremic several weeks after delivery, suggesting that transmission around delivery is the dominant process.
Programmatic universal testing of all pregnant women for HCV does not exist, although this has recently been recommended in the shared HCV guidance published by the American Association for the Study of Liver Diseases and the Infectious Disease Society of America.28
Risk factors associated with HCV MTCT include maternal viral load and human immunodeficiency virus (HIV) infection status. Other potential risk factors include prolonged rupture of membranes and fetal scalp monitor use during labor. It has been postulated that the final pathway for several putative risk factors is infection of maternal peripheral blood mononuclear cells.29 Treatment of maternal HIV infection to the point of undetectable viral load abrogates the potentially negative effect of this coinfection. Currently, there are no antiviral strategies to lower maternal HCV viremia, but at least two clinical trials of direct-acting antiviral agents during pregnancy are ongoing.
Exposed children should be tested after age 18 months for anti-HCV antibodies. Although earlier detection (ages 2-4 months) of MTCT can occur by viremia testing, up to 30% of these infections will resolve, and no treatment is expected before age 3 years. Children infected with HCV by MTCT are typically asymptomatic throughout childhood, although cases of advanced liver disease and cirrhosis have been reported in children. Current recommendations include treatment of all children aged ≥3 years, when direct-acting antiviral agents in appropriate formulations become available.28
Hepatitis E Virus
Hepatitis E virus (HEV) is the most common cause of VH in the world. Although acute HEV is typically a self-resolving illness, in pregnant women there is 20% mortality from HEV. In addition, MTCT has been documented when infection occurs during the third trimester, with rates varying from 30% to 100%. Illness in perinatally infected infants can range from mild hepatitis that resolves within 8 weeks to ALF with a high mortality. There are no reported cases of chronic HEV infection after perinatal transmission.30
NAFLD
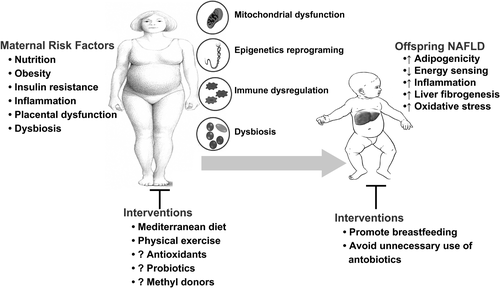
Emerging evidence suggests that maternal nutrition and obesity play a key role in the development of metabolic syndrome, insulin resistance, and NAFLD in offspring.31 Up to 32% of women of reproductive age are obese, which likely contributes to early-onset childhood obesity and associated NAFLD. NAFLD in infants born to obese mothers begins in utero, as demonstrated by up to a 68% increase in intrahepatic lipids in these newborns.32 Steatosis in these infants correlates with their gestational age.33 Furthermore, longitudinal studies have shown an increased risk of NAFLD in adolescents born to obese mothers.34, 35 Multiple pathways are involved in the development of neonatal NAFLD, including mitochondrial dysfunction, nutritional reprograming of the epigenome, dysbiosis, and immune dysregulation. Mechanistic studies performed in nonhuman primates and mice models indicate that exposure to excess maternal macronutrients, independent of diabetes and/or obesity, reduces mitochondrial biogenesis and nutrient sensing, and promotes oxidative stress and triglyceride storage, priming the fetal liver for NAFLD.31
Epigenetics refers to inheritable changes in gene activity and expression in response to developmental and environmental cues, without alterations in DNA sequence. Epigenetic information inherited through the mammalian germline in response to environmental influences may predispose the developing fetus to metabolic derangements and rapidly progressing NAFLD. Epigenetic changes can be linked to transgenerational dietary factors, as suggested by a higher body mass index in the offspring of men exposed to famine in utero.36 Studies using gene-protein enrichment analyses and protein interaction networks suggest that fetal adaptation to an impaired nutritional environment is associated with profound changes in gene expression involving differential DNA methylation and histone acetylation in humans.37 Likewise, mice exposed perinatally to an obesity-inducing diet (OID) exhibit differential hepatic DNA methylation patterns and corresponding profibrogenic and -inflammatory gene signatures. When re-exposed to an OID as adults, these mice develop an accelerated nonalcoholic steatohepatitis (NASH) phenotype with prominent liver fibrosis.38
Delivery method, antibiotic exposure, and neonatal and maternal diets may induce neonatal dysbiosis defined as alterations in composition, diversity, or metabolites of the microbiome. Exposure to an OID in utero and postnatally decreases Bacteroides colonization in the neonate, whereas breastfeeding increases newborn fecal colonization with Bifidobacteria and Lactobacilli. Fecal transplant from human newborns of obese mothers into germ-free mice increased hepatic proinflammatory cytokines, augmented intestinal permeability, and impaired macrophage function. Furthermore, when fed an OID, these mice had accelerated weight gain and NAFLD development,39 suggesting a causal relationship between maternal obesity-associated offspring dysbiosis and development of obesity and NAFLD.
Immune dysregulation is increasingly recognized in obese pregnant women and may predispose the newborn to a proinflammatory state, as suggested by an elevated C-reactive protein in nonobese infants with obese parents.40 In experimental mice, maternal OID increased proinflammatory macrophage hepatic infiltration and cytokine expression in offspring, which persisted after switching to a regular diet,38 supporting the role of maternal obesity-induced immune dysregulation in NAFLD development in the offspring.
Primary management of early-onset NAFLD requires maternal lifestyle interventions and promotion of breastfeeding (Fig. 2). The Mediterranean diet in at-risk pregnant women decreased the odds of gestational diabetes by 35% with a 1.2-kg reduction in weight gain during pregnancy.41 Likewise, intensive maternal lifestyle modification counseling reduced the risk of large for gestational age newborns.42 In an observational study, pediatric NASH and fibrosis were less common in breast-fed infants.43 Neither metformin nor probiotic use in nondiabetic obese pregnant women were effective in lowering offspring weight. However, pyrroloquinoline quinone (a natural antioxidant with a beneficial effect on lipid metabolism and glycemic control) and methyl donors (choline, betaine, vitamin B12, and folic acid) were beneficial in murine NASH.44
AFLP
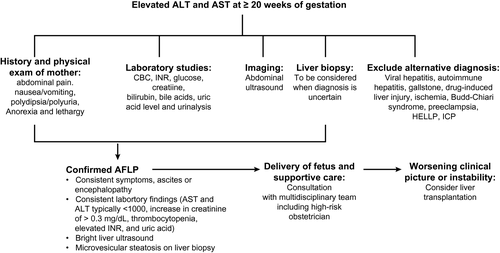
AFLP is an uncommon (1:7,000-15,000) and potentially fatal disease that typically occurs in the third trimester,45 but may present as early as 22 weeks of gestation, and in up to 20% postnatally. Incidence of AFLP is increased in multigravidas, male fetus, first pregnancy, previous AFLP (recurrence rate of 20%), maternal metabolic disorders (e.g., type 2 diabetes), and fetal fatty acid oxidation disorders (FAODs) with deficiencies of specific enzymes involved in mitochondrial metabolism of fatty acids.45 FAODs are autosomal recessive, with the mother a heterozygous carrier. Clinical findings of FAOD range from mild abnormalities, such as hypoglycemia, myopathy, or neuropathy, to multiorgan failure with encephalopathy, and cardiomyopathy.45
AFLP is most commonly caused by a mutation in long-chain 3-hydroxyacyl-coenzyme A (CoA) dehydrogenase (LCHAD), followed by mutations in short-chain 3-hydroxyacyl-CoA dehydrogenase and medium-chain 3-hydroxyacyl-CoA dehydrogenase.45, 46 Physiological changes during fetal and placental development lead to increased fatty acid demands accommodated by increased maternal hormone-sensitive lipase activity and gestational insulin resistance. A concurrent increase in triglyceride lipolysis and free fatty acid (FFA) release makes mothers more vulnerable to FAODs. The placenta contains lipoprotein lipase, fatty acid oxidation (FAO) enzymes (such as LCHAD), and fatty-acid–binding proteins and carnitine transporters that direct the flow of FFA to the fetus.45 Because the fetus and placenta share a uniform genotype, when FAO fails in the fetus, the placenta is similarly unable to proceed with normal metabolic pathways. Intermediate metabolites accumulate, creating toxic effects in the mother.45
Rapid diagnosis and urgent delivery of the fetus is essential to stop liver injury in the mother and is outlined in Fig. 3.45 Maternal mortality is <10% with early recognition and delivery. Time to hepatic recovery depends on severity, with improvement of liver enzymes within 1-2 days, cholesterol and bilirubin within 4-6 days, and acute kidney injury within 7-10 days postpartum. Histological changes may persist for 5 weeks.45 Postpartum AFLP complications include ALF with HE, disseminated intravascular coagulation, acute renal failure, and gastrointestinal bleeding.45 Fetal mortality (as high as 20%) depends on prematurity of the fetus and metabolic acidosis in the mother.45 Newborns of mothers with AFLP should be tested for FAOD, particularly LCHAD.
ICP
ICP is a poorly understood disease, typically of the late second or third trimester of pregnancy, with resolution within 6-8 weeks following delivery. ICP is characterized by pruritus, usually affecting the palms of the hands and soles of the feet, associated with elevated bile acids (BAs), and may be mild (BA 10-39 µmol/L) or severe (BA ≥40 µmol/L).47 Pruritus occurs in 25% of pregnant women attributable to various causes, including dry skin, atopic eruption, pruritic urticarial papules and plaques of pregnancy, and ICP. Asymptomatic hypercholanemia (elevation of BA without associated liver-test abnormalities or impact on mother or fetus) affects 10% and ICP 0.3%-5.6% of pregnant woman.47 Asymptomatic hypercholanemia in the range of 40 µmol/L should be managed as ICP. Transaminases and gamma-glutamyl transferase may be elevated in 30%, with jaundice in 10%-15% of women with ICP. In pregnant women with pruritus, sulfated progesterone metabolites are associated with itch severity and a negative effect on BA uptake and efflux. Furthermore, elevated autotaxin, a mediator of cholestatic pruritus, may be prognostic for ICP.48 Risk of ICP is increased with previous ICP (recurrence, 40%-92%), history of cholestasis from oral contraceptive pills, twin pregnancies (20%), personal and familial history of gallstones, pregnancy by in vitro fertilization, history of HCV infection, low selenium, and a sister with ICP.49, 50 Identifiable gene mutations occur in 20%-25% of women with ICP (Table 1; Fig. 4A).51
| Gene Affected | Encoding Protein | Function | Frequency in ICP Cases | Known Associated Disorder |
|---|---|---|---|---|
| ABCB4 | MDR3 | Phosphatidyl choline flippase | 10%-16% | PFIC3 |
| ABCB11 | BSEP | Bile salt export | 5% | PFIC2 |
| ATP8B1 | FIC1 | Phosphatidyl serine flippase | 3% | PFIC1 |
| TJP2 | ZO-2 | Tight junctions and adherens junctions | Uncommon | PFIC4 |
| ABCC2 | MRP2 | Conjugated bilirubin and organic anions transport | Uncommon | Dubin-Johnson syndrome |
| ABCC3 | MRP3 | Conjugated bilirubin and organic anions transport | Uncommon | Dubin-Johnson syndrome |
| ABCC4 | MRP4 | Conjugated bilirubin and organic anions transport | Uncommon | Dubin-Johnson syndrome |
| NR1I2 | PXR | Nuclear hormone receptor | Uncommon | Cerebrotendinous Xanthomatosis |
| NR1H4 | FXR | Nuclear bile acid receptor | Uncommon | PFIC5 |
- Abbreviations: BSEP, bile salt export pump; FIC1, familial intrahepatic cholestasis; MDR3, multidrug resistant-3 p glycoprotein; MRP, multidrug resistance protein; NR1I2, nuclear receptor subfamily 1, group I, member 2; PXR, pregnane X receptor; TJP2, tight junction protein 2; ZO-2, zona occludens 2.
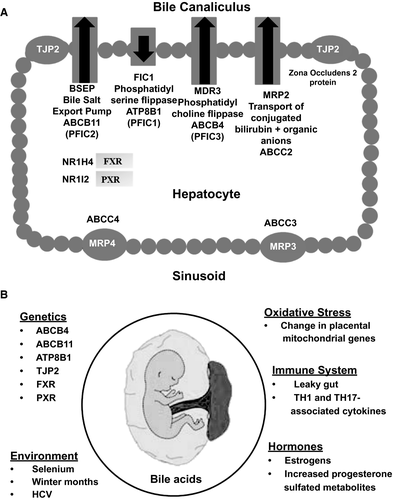
Other ICP contributing factors (Fig. 4B) include reversed fetal-maternal gradient of BA (higher in mother than fetus) with an increase in BA in both mother and umbilical cord blood (cholic acid/chenodeoxycholic acid up to 5:1),52 placental oxidative stress,48 and increased sulfated progesterone metabolites (partial agonists of farnesoid X receptor [FXR]), which inhibit hepatic BA uptake and efflux resulting in cholestasis.49
Treatment with ursodeoxycholic acid (UDCA; 10-15 mg/kg) is safe and often effective for mothers. UDCA decreases pruritus in 77% and total BAs in 35%-77% of mothers, with almost complete correction of BA level in the fetus, and normalization of ALT in 39.5% of mothers.53 However, a recent placebo-controlled, prospective trial in ICP showed minimal benefit of UDCA to the mother or fetus.54 Rifampicin can be used in UDCA-refractory cases, but may cause coagulopathy and should be given with vitamin K.
Fetal complications are common with severe ICP, with the risk increasing by 1%-2% per additional µmole/L of BA >40.50, 52 Fetal complications include spontaneous preterm labor (5%-17%), asphyxia (29%), cardiac arrhythmias, pulmonary dysfunction, and meconium staining of amniotic fluid (10%-58%), and intrauterine death in 1%-3%.50-52 To decrease the risk of maternal-fetal complications, the fetus should be delivered at 37 weeks of gestation and maternal liver enzymes and BAs followed regularly during gestation and up to 6-12 weeks after delivery. Mothers with ICP also have increased risk for gestational diabetes, pre-eclampsia, and hyperlipidemia.55 Long-term fetal risks include diabetes mellitus, metabolic syndrome, obesity, dyslipidemia, and an increased risk for progressive familial intrahepatic cholestasis (PFIC) in 20%-25%.56 Although genetic testing in mothers with ICP or their newborns is not routinely recommended, genetic panels for such testing do exist.
Conclusion
Neonates are susceptible to several unique liver diseases based on complex interactions between maternal-fetal environments. Early recognition and management of these conditions is critical to improve short- and long-term patient morbidity and mortality. For neonatal ALF and maternal AFLP or ICP, progress in next-generation sequencing may allow for enhanced and clinically relevant diagnostic algorithms, which may, in turn, expand available treatment options. Although significant progress has been made in prevention of GALD in subsequent pregnancies, ongoing efforts to further delineate pathogenic mechanisms of GALD may allow for the development of therapeutic strategies that could prevent poor outcome in the first gestation. In contrast, the link between in utero development of NAFLD and maternal obesity and MTCT of VH will require broad public health measures to improve the long-term outcome. Finally, these diseases provide unique opportunities for multidisciplinary collaborative research.
Author Contributions
S.H.I., and S.S.S.: Concept formulation, and review design. All authors participated in drafting the article and revising it critically for important intellectual content, and approved the final version to be published.




