Liver Injury in Patients With Cholestatic Liver Disease Treated With Obeticholic Acid
Abbreviations
-
- ACLF
-
- acute on chronic liver failure
-
- ALP
-
- alkaline phosphatase
-
- ALT
-
- alanine aminotransferase
-
- AST
-
- aspartate aminotransferase
-
- OCA
-
- obeticholic acid
-
- PBC
-
- primary biliary holangitis
-
- PSC
-
- primary sclerosing cholangitis
-
- ULN
-
- upper limit of normal
Obeticholic acid (OCA) was approved for the treatment of primary biliary cholangitis (PBC) based on studies in patients with an alkaline phosphatase (ALP) level of at least 1.67 times the upper limit of normal (ULN) or elevated total bilirubin less than 2 times the ULN.1 Postmarket reports of serious liver injury in patients receiving OCA prompted a boxed warning by the Food and Drug Administration. Drug-induced liver injury (DILI) in patients with PBC can put them at risk for acute on chronic liver failure (ACLF) and increased mortality.2 We describe in this report worsening of liver disease or ACLF after starting OCA among a cohort of patients with PBC and primary sclerosing cholangitis (PSC).
Clinical Observation
Eight patients (52 ± 14 years, 6 were women, and 6 were Childs-Pugh [CP] class A] from four tertiary care hospitals were suspected of having developed liver failure while on OCA therapy. Among these 8 patients, 6 had PBC and 2 had PSC. Health records were reviewed, and pertinent data were extracted in a standardized fashion (Supporting Information). Competing etiologies were excluded based on the review of health records by the local investigator and a senior investigator (P.S.K.). Patients were stratified based on total bilirubin 2× ULN before initiation of OCA (Table 1).
| Baseline Total Bilirubin <2× ULN (n = 4) | Baseline Total Bilirubin >2× ULN (n = 4) | |||
|---|---|---|---|---|
| Number of patients with PBC/PSC | 2/2 | 4/0 | ||
| Age (years) | 52 ± 21 | 52 ± 9 | ||
| Female | 2 | 4 | ||
| Caucasian | 3 | 4 | ||
| Body mass index (kg/m2) | 31 ± 2 | 22 ± 1 | ||
| Cirrhosis or portal hypertension at baseline | 3 of 4 | 4 of 4 | ||
| OCA starting dose | 5 mg once daily | 5 mg once weekly | ||
| OCA dosages at the time of jaundice | 5 mg daily, 10 mg daily, 10 mg 3 times weekly | Not applicable | ||
| OCA dose at the time of decompensation | 5 mg once weekly (n = 1), 5 mg daily (n = 1), 10 mg daily (n = 2) | |||
| Duration of OCA use (days) | 193 ± 114 | 200 ± 89 | ||
| Liver biochemistries | Onset | Peak | Onset | Peak |
|
88 ± 53 | 156 ± 68 | 147 ± 92 | 216 ± 86 |
|
|
100 ± 52 | 156 ± 64 | 164 ± 60 | 205 ± 37 |
|
|
464 ± 121 | 476 ± 121 | 699 ± 364 | 981 ± 508 |
|
|
7.6 ± 5.1 | 13.6 ± 5.0 | 7.9 ± 3.4 | 14.7 ± 6.3 |
|
|
1.0 ± 0.6 | 0.9 ± 0.3 | ||
|
|
1.2 ± 0.1 | 1.4 ± 0.5 | ||
| RUCAM score | 6 ± 1 | 2 ± 0 | ||
| DILIN severity score | 4 (n = 3), 5 (n = 1) | 4 (n = 1), 5 (n = 3) | ||
| Decompensating event | Ascites (2) | Ascites (2), variceal hemorrhage (1) | ||
| Liver transplantation | 1 of 4 | 3 of 4 | ||
- Abbreviations: DILIN, Drug-Induced Liver Injury Network; INR, international normalized ratio; RUCAM, Roussel Uclaf Causality Assessment Method.
All but 1 patient was started on the recommended dosage, with subsequent dose increases in 5 patients based on tolerance. One patient with PBC and CP class B cirrhosis was initiated on OCA 5 mg twice weekly, and this was subsequently increased to 5 mg per day. The latency period was long (210 ± 104 days) and ranged from a minimum of 87 days to a maximum of 379 days. The pattern of liver injury was cholestatic in all patients with modest increase in transaminases at 3× ULN, and peak bilirubin increases at approximately 13× ULN. Ascites was the most common decompensating event and occurred in 4 of the 8 patients.
In the 4 patients with baseline bilirubin less than 2× ULN, there were 2 with PSC and 2 with PBC with prior workup suggestive of advanced fibrosis but not cirrhosis. In a patient with PBC (Fig. 1A), a liver biopsy performed at the time of liver injury showed cirrhosis with extensive loss of bile ducts, severe cholestasis, and moderate interface activity. The injury resolved spontaneously. Of the 2 patients with PSC who received OCA, 1 was participating in a clinical trial with open-label arm (Fig. 1B) and developed new-onset ascites that resolved, and the other was initially diagnosed with PBC, overlap with autoimmune hepatitis, and subsequently recognized to have PSC. Interestingly, this patient presented approximately 3 months after OCA initiation with a total bilirubin of 8.2 mg/dL, ALP of 520 U/L, AST of 157 U/L, and ALT of 136 U/L. By comparison, this patient’s labs at the time of OCA initiation showed a total bilirubin of 1.0 mg/dL, ALP of 372 U/L, AST of 44 U/L, and ALT of 35 U/L. Magnetic resonance and endoscopic cholangiogram both revealed diffuse intrahepatic and extrahepatic dilatation and structuring suggestive of PSC.
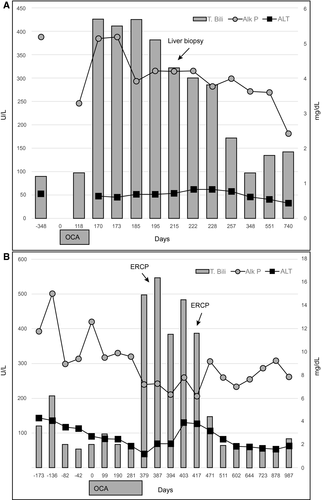
All patients were alive at 6 months, with 4 undergoing liver transplantation for ACLF. In those with normal bilirubin levels at baseline, all but 1 patient recovered with 1 patient’s liver tests returning to baseline levels at 13.2 months.
Discussion
The goal of this paper is to raise awareness of the potential hepatotoxicity of OCA and add a note of caution to the use of OCA in patients with decompensated disease. Patients with PBC/PSC cirrhosis with deterioration in liver function following OCA use appear to develop jaundice without an impressive increase in liver enzymes. About half of the patients progressed to liver failure, requiring liver transplantation due to ACLF. The long latency period presumably related to the dosing strategy raises the possibility that this event may be dose-related. The current case series does not allow us to estimate the incidence of DILI from OCA in comparison to age, gender, and severity-matched PBC patients, but we anticipate that such information would be available from the ongoing phase 4 COBALT study (https://clinicaltrials.gov:/ct2/show/NCT02308111). Additional safety, efficacy, and pharmacology data are needed before OCA is used in cirrhosis from PBC or PSC.




