Dual Programmed Death Receptor-1 and Vascular Endothelial Growth Factor Receptor-2 Blockade Promotes Vascular Normalization and Enhances Antitumor Immune Responses in Hepatocellular Carcinoma
Abstract
Background and Aims
Activation of the antitumor immune response using programmed death receptor-1 (PD-1) blockade showed benefit only in a fraction of patients with hepatocellular carcinoma (HCC). Combining PD-1 blockade with antiangiogenesis has shown promise in substantially increasing the fraction of patients with HCC who respond to treatment, but the mechanism of this interaction is unknown.
Approach and Results
We recapitulated these clinical outcomes using orthotopic—grafted or induced—murine models of HCC. Specific blockade of vascular endothelial receptor 2 (VEGFR-2) using a murine antibody significantly delayed primary tumor growth but failed to prolong survival, while anti-PD-1 antibody treatment alone conferred a minor survival advantage in one model. However, dual anti-PD-1/VEGFR-2 therapy significantly inhibited primary tumor growth and doubled survival in both models. Combination therapy reprogrammed the immune microenvironment by increasing cluster of differentiation 8–positive (CD8+) cytotoxic T cell infiltration and activation, shifting the M1/M2 ratio of tumor-associated macrophages and reducing T regulatory cell (Treg) and chemokine (C-C motif) receptor 2–positive monocyte infiltration in HCC tissue. In these models, VEGFR-2 was selectively expressed in tumor endothelial cells. Using spheroid cultures of HCC tissue, we found that PD-ligand 1 expression in HCC cells was induced in a paracrine manner upon anti-VEGFR-2 blockade in endothelial cells in part through interferon-gamma expression. Moreover, we found that VEGFR-2 blockade increased PD-1 expression in tumor-infiltrating CD4+ cells. We also found that under anti-PD-1 therapy, CD4+ cells promote normalized vessel formation in the face of antiangiogenic therapy with anti-VEGFR-2 antibody.
Conclusions
We show that dual anti-PD-1/VEGFR-2 therapy has a durable vessel fortification effect in HCC and can overcome treatment resistance to either treatment alone and increase overall survival in both anti-PD-1 therapy–resistant and anti-PD-1 therapy–responsive HCC models.
Abbreviations
-
- AA
-
- antiangiogenesis
-
- CA-IX
-
- carbonic anhydrase IX
-
- CCR2
-
- chemokine (C-C motif) receptor 2
-
- CD
-
- cluster of differentiation
-
- CI
-
- confidence interval
-
- CTL
-
- cytotoxic T lymphocyte
-
- DAPI
-
- 4′,6-diamidino-2-phenylindole
-
- DMEM
-
- Dulbecco’s modified essential medium
-
- FBS
-
- fetal bovine serum
-
- FDA
-
- US Food and Drug Administration
-
- FoxP3
-
- forkhead box P3
-
- HBSS
-
- Hank’s balanced salt solution
-
- HCC
-
- hepatocellular carcinoma
-
- HR
-
- hazard ratio
-
- ICB
-
- immune checkpoint blockade
-
- IF
-
- immunofluorescence
-
- IFN
-
- interferon
-
- IgG
-
- immunoglobulin G
-
- IHC
-
- immunohistochemistry
-
- IL
-
- interleukin
-
- Mst1
-
- macrophage-stimulating 1
-
- MVD
-
- microvessel density
-
- OS
-
- overall survival
-
- PD-1
-
- programmed death receptor-1
-
- PD-L1
-
- PD ligand 1
-
- α-SMA
-
- α-smooth muscle actin
-
- TAM
-
- tumor-infiltrating macrophage
-
- TKI
-
- tyrosine kinase inhibitor
-
- Treg
-
- T regulatory cell
-
- VEGF
-
- vascular endothelial growth factor
-
- VEGFR
-
- VEGF receptor
Hepatocellular carcinoma (HCC) is the second most common cause of cancer-related death worldwide.1 HCC is a malignant disease that develops predominantly in patients with underlying chronic liver disease and cirrhosis.2, 3 Therefore, inflammation and angiogenesis are critical during the process of hepatocarcinogenesis, when immune evasive mechanisms are established. These mechanisms include up-regulation of immune checkpoints on HCC cells as well as cluster of differentiation 8–positive (CD8+) cytotoxic T lymphocytes (CTLs), antigen-presenting cells (macrophages and dendritic cells), and immunosuppressive cells such as T regulatory cells (Tregs) or myeloid-derived suppressor cells.4-7 Dysregulation of immune checkpoints is critical for immune response evasion in HCC.5
The programmed death receptor-1 (PD-1)–PD ligand 1 (PD-L1) pathway has been the main target for immune checkpoint blockade (ICB) in HCC as both the receptor and the ligand are often expressed in human HCC.5, 6 PD-L1 up-regulation in the tumor is reported to correlate with alpha-fetoprotein (AFP), a well-known oncofetoprotein in HCC.8 High PD-1 expression is a marker of immune cell activation but also of the “exhausted” phenotype of effector lymphocytes. PD-L1 expression on immune, endothelial, and cancer cells is largely viewed as a marker of immune suppression, which could be induced by microenvironmental factors (for example, hypoxia or interferon gamma [IFN-γ).9-11 PD-1 and PD-L1 expression correlate with CTL infiltration in HCC, and these parameters are associated with survival in patients undergoing surgical resection.12, 13 ICB, such as using anti-PD-1 antibodies, has shown promise in HCC.5, 6 Clinical data showed a response in approximately 15%-20% of patients with HCC, with some long-term survivors.14, 15 As PD-1 blockade is being tested in phase 3 trials, it is clear that the current challenge will be to address the resistance to ICB seen in the majority of patients with HCC.
A critical question is whether combining anti-PD-1/PD-L1 therapy with other treatments could enhance its effectiveness in HCC.16 HCCs are highly vascularized tumors,17 and clinical successes indicated a key role for targeting vascular endothelial growth factor (VEGF) receptor-2 (VEGFR-2)–driven angiogenesis in HCC. Unfortunately, the responses to anti-VEGFR-2 drugs are rare and often short-lived.18 This may be because antiangiogenesis (AA) can excessively prune vessels and increase hypoxia.4, 9 Interestingly, a recent study has shown that anti-PD-1 therapy can increase blood perfusion of tumors by normalizing vessels in breast and colorectal cancer models.19 However, the effect of anti-PD-1 therapy—alone or with anti-VEGF/R therapy—on HCC vasculature is unknown. Understanding this effect is critically important as combination therapy may yield outcomes superior to either monotherapy.20, 21 Importantly, a recent study demonstrated the reciprocal regulation of vascular normalization and immune responses, including in HCC. Tian et al. reported that CD4+ type 1 helper T cells activated with ICB play a pivotal role in tumor vessel normalization.22
We have shown that there is a “window” of normalization depending on the time and dose of AA treatment.23 For example, titration of anti-VEGFR-2 treatment extended the window, which enhanced the efficacy of vaccine therapy in mouse models of breast cancer.24 Here, we hypothesized that combining anti-PD-1 treatment with VEGFR-2 blockade extends the window of normalization and enhances the antitumor immune response. The hypothesis that dual PD-1/VEGFR-2 blockade can be effective in HCC is supported by early clinical data on combinations of lenvatinib and pembrolizumab and of bevacizumab with atezolizumab showing unexpectedly high overall response rates.25, 26 This has led to a breakthrough designation by the US Food and Drug Administration (FDA) for these combination therapies and to three ongoing phase 3 trials of these combinations.
Materials and Methods
Cells and Culture Conditions
We used two murine cell lines: HCA-1, established in our laboratory,27, 28 and RIL-175 (a p53/Hras mutant HCC cell from C57Bl/6 mice, a kind gift from Dr. Tim Greten, National Institutes of Health).29 HCA-1 was maintained in Dulbecco’s modified essential medium (DMEM) with 10% fetal bovine serum (FBS) and pyruvic acid. RIL-175 was maintained in DMEM with 20% FBS with pyruvic acid.
Hypoxic condition cell culture was performed in a Brunswick Galaxy incubator at 1% for 48 hours. Organoid culture was performed using primary cells from tumor tissue. Tumor tissues were resected and minced with a scalpel. Minced tissue fragments were incubated in Hank’s balanced salt solution (HBSS) with 15 µg/mL of collagenase and 1.5 mg/mL of hyaluronidase for 30 minutes at 37°C. Digested tissues were passed through a 70-µm cell strainer and washed twice with HBSS. After counting the cell concentration, cells were diluted to 1 million cells/mL with DMEM with 10% FBS. The cell suspension was poured into a Nano culture plate (MH type; SCIVAX, Organogenix, Japan) for 72 hours to form organoids at 37°C with 5% CO2. After the confirmation of organoid formation by light microscopy, 50 µg/mL of DC101 and/or 20 µg/mL of anti-IFN-γ blocking antibody was added to the medium, and organoids were placed in the specific experimental culture condition. Primary endothelial cells were obtained from a single-cell suspension of RIL-175 tumors. CD31+ cells were sorted using biotin-conjugated CD31 antibody (clone MEC13.3; Biolegend) and antibiotin microbeads (Miltenyi, Germany) by magnetic sorting. Endothelial cells were cultured on the type I collagen–coated cell culture dishes, using Endothelial Cell Growth Medium MV2 (Promo Cell).
HCC Models
The orthotopic HCC mouse models were described elsewhere.30 The HCC cells (1 million 1:1 in Matrigel; Mediatech/Corning, Manassas, VA) were grafted into mice matching their genetic background—HCA-1 in C3H mice and RIL-175 in C57Bl/6 mice. Tumor growth was monitored by high-frequency ultrasonography twice a week. In the macrophage-stimulating 1 (Mst1–/–)Mst2f/– model31 adeno–cyclization recombination at a concentration of 107 pfu was injected through the tail vein in 3-week-old mice. To induce liver damage, 100 µL of 20% CCl4 (Sigma-Aldrich, St. Louis, MO) was administered orally from 8 to 12 weeks.30 When tumors reached ~5 mm in diameter, mice were randomly assigned to a treatment group (n = 6-8). Treatments were administered intraperitoneally thrice per week for 14 days at doses of 10 mg/kg (anti-PD-1 antibody, anti-CD4 antibody, and immunoglobulin G [IgG] control) and 10 mg/kg (low) or 40 mg/kg (standard) (mouse antibody DC101). All animal experiments were performed after approval by the Institutional Animal Care and Use Committee of the Massachusetts General Hospital.
Reagents
DC101 antibody was kindly provided by Eli Lilly and Company (Indianapolis, IN). Mouse anti-PD-1 antibodies (clone RMP-014) and anti-CD4 antibodies were purchased from BioXcel (West Lebanon, NH). Control rat IgG was purchased from Jackson ImmunoResearch (West Grove, PA). Anti-IFN-γ antibody (clone XMG1.2) was purchased from Thermo Fisher Scientific (Waltham, MA).
Flow Cytometry
Tumor tissues were resected and minced, and fragments were incubated in HBSS with 15 µg/mL of collagenase and 1.5 mg/mL of hyaluronidase for 30 minutes at 37°C. Digested tissues were passed through a 70-µm cell strainer and washed twice with phosphate-buffered saline (PBS)/0.5% bovine serum albumin. Single-cell suspensions were incubated with antimouse CD16/32 antibody (clone 93; Biolegend, San Diego, CA) for 5 minutes prior to staining for immune cell markers for 15 minutes at room temperature. For the intracellular markers, cells were fixed and permeabilized with either the FoxP3/Transcription Factor Staining Buffer Set (eBioscience/Thermo Fisher Scientific, Waltham, MA) or BD Cytofix/Cytoperm Kit (BD Biosciences, San Jose, CA), according to the manufacturers’ protocols. For cytokine staining, harvested cells were incubated in Roswell Park Memorial Institute medium with cell activation cocktail with brefeldin A (Biolegend) for 6 hours at 37°C, and stimulated cells were stained as described above. Monoclonal antibodies used for flow-cytometric analysis were CD45 (30-F11), CD3e (145-2C11), CD4 (RM4-5), CD8 (53-6,7), PD-1 (29F.1A12), IFN-γ (XMG1.2), and forkhead box P3 (FoxP3; FJK-165).
Cell Sorting
For the magnetic bead sorting of endothelial cells or myeloid cells, cells were first stained with either biotin-conjugated anti-CD31 (clone MEC13.3) or anti-CD11b (clone M1/70) and anti-CD11c antibodies (clone N418) (all Biolegend). Biotin-labeled cells were separated by magnetic sorting using antibiotin microbeads (Miltenyi). For macrophage collection, the CD45+CD11b+Ly6C-F4/80+ population was sorted using a FACS Aria cell sorter.
Immunohistochemistry and Immunofluorescence
Resected tumor tissues were fixed in 4% paraformaldehyde and embedded either in paraffin or in optimal cutting temperature compound and frozen. Vascular endothelial staining was performed using anti-CD31 antibody (clone DIA-310; Dianova, Germany, for immunohistochemistry [IHC], and Millipore, St. Louis, MO, for immunofluorescence [IF]), pericytes were identified with antibodies to α-smooth muscle actin (SMA) (Sigma-Aldrich), PD-L1 expression was detected using anti-PD-L1 antibodies (eBioscience), and hypoxia was detected with anti–carbonic anhydrase IX (CA-IX) antibody (Abcam, Cambridge, UK). Specimens were incubated with fluorescence (cyanine 3 and Alexa 647)–conjugated antihamster or antirabbit secondary antibodies, as appropriate (Jackson ImmunoResearch). Cell nuclei were identified with 4′,6-diamidino-2-phenylindole (DAPI; ProLong Gold Antifade Mountant with DAPI; Thermo Fisher Scientific). The total number of vessels and pericyte-covered vessels and the fraction of hypoxic tumor area were counted in five random fields using a ×400 magnification lens. These data were analyzed using ImageJ (US National Institutes of Health) and PhotoShop (Adobe Systems Inc., San Jose, CA) software. Organoids were fixed in 0.4% formaldehyde for 15 minutes. Then, organoids were washed 3 times with PBS and immunostained. IF images were analyzed using a confocal microscope (Fluoview FV100; Olympus, Center Valley, PA).
RNA Sequencing Analysis
When tumors reached 200 mm3, three to five samples per group were collected from mice treated with control IgG, anti-PD-1 antibody alone, anti-PD-1 antibody in combination with DC101, and anti-PD-1 antibody in combination with DC101 and anti-CD4 antibodies. Mice were sacrificed, and total RNA was isolated from the freshly isolated tumor tissues using Qiagen kits. Total RNA was sequenced at Molecular Biology Core Facilities, Dana Farber Cancer Institute (Boston, MA), and used for Gene Set Enrichment Analysis.
Cytokine Analysis
Culture supernatants were collected after the specific experimental treatment. Samples were assayed in duplicate using the MSD proinflammatory Panel I, a highly sensitive multiplex enzyme-linked immunosorbent assay (ELISA) for quantitatively measuring 10 cytokines—IFN-γ, interleukin (IL)-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-13, and tumor necrosis factor alpha—from a single small sample volume (25 μL) using electrochemiluminescence-based detection (MesoScale Discovery, Gaithersburg, MD).
Statistical Analysis
All statistical analyses were performed using Stata software, version 14.1 (Stata Corp, College Station, TX). Error bars indicate standard error of the mean. Differences were considered significant at P < 0.05. Quantitative variables were compared using the Student t test. Analysis of experiments with more than two groups was performed using one-way analysis of variance with Scheffe’s correction for multiple comparisons. Median overall survival (OS) was estimated using the Kaplan-Meier method. Statistical analyses in the survival experiments were performed by the Cox proportional hazards model, and hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated.
Results
Dual VEGFR-2/PD-1 Blockade is Effective in Orthotopic HCC Models in Mice
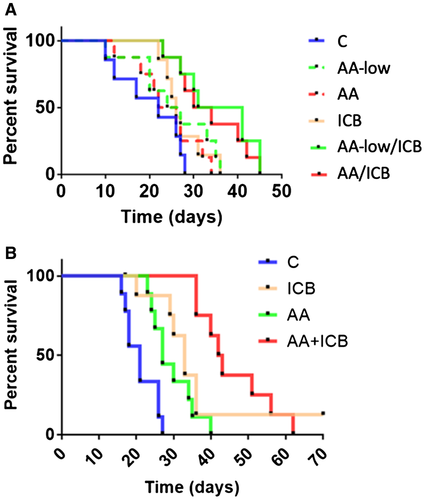
We tested combination therapy in orthotopic models of HCC in mice with (chemically induced) underlying liver damage and examined the impact of the dose of the AA agent. We treated mice with established HCC (5-6 mm in diameter) with the anti-VEGFR-2 antibody DC101 at two different doses (AA-low, 10 mg/kg, and standard AA, 40 mg/kg, both thrice a week), anti-PD-1 antibody (ICB), or their combination. Using HCA-1 in C3H mice, we found that in the single-agent treatment groups there was a significant growth delay only in the AA group compared to control and nonsignificant delays in the others; however, combinations of AA and ICB therapy—irrespective of the dose—induced comparable significant growth delays (Supporting Fig. S1A). In addition, there was a significant increase in median OS in the combination therapy groups, which was not seen in any of the monotherapy arms (Fig. 1A). The lack of survival advantage in the AA group was due to lack of control of metastatic disease, despite delay of the primary tumor growth; the metastatic burden was reduced in all the other groups at the experimental endpoint (Supporting Fig. S1B). Tumor growth inhibition was also seen in the genetically engineered RIL-175 (p53/Hras) and Mst-mutant HCC models (Supporting Fig. S1C,D). Next, we tested the impact of dual AA/ICB in C57Bl/6 mice bearing RIL-175 HCCs—sensitive to anti-PD-1 therapy. We found that the combination therapy produced the longest median OS (Fig. 1B).
Dual VEGFR-2/PD-1 Blockade Reprograms the Immune Microenvironment and Promotes Antitumor Immunity in HCC
Having reproduced the clinical benefit seen with combination immunotherapy/AA in mouse models, we first examined how therapy impacted lymphocyte infiltration. We first analyzed the number of tumor-infiltrating leukocytes as a fraction of total CD45+ immune cells by flow cytometry. The number of tumor-infiltrating CTLs was not affected by treatment with ICB alone or AA-low/ICB (Supporting Fig. S2). AA decreased the proportion of infiltrating CTLs, even when combined with ICB in RIL-175 HCCs (Fig. 2A; Supporting Fig. S2A,B). However, the overall fraction of infiltrating CD4+ T cells and the CD8+/CD4+ T-cell ratio were not affected in any of the combination groups (Supporting Fig. S2C,D). Moreover, the fraction of FoxP3+ Tregs was significantly decreased in all treatment groups, which translated into increased CTL/Treg ratios in the ICB-containing groups but not in the AA alone groups (Fig. 2B,C; Supporting Fig. S2E,F). These findings were confirmed in the Mst-mutant model of HCC, where we found a decrease in Tregs and an increase in CD8+/CD4+ and CTL/Treg ratios (Supporting Fig. S2G-I). Furthermore, when evaluating CTL function by evaluating IFN-γ expression, we found it to be significantly increased in all ICB-containing groups (Supporting Fig. S2J). Finally, using IHC, we found that the number of tumor-infiltrating CTLs was significantly increased in the combination treatment groups compared to AA treatments alone (Fig. S2L).
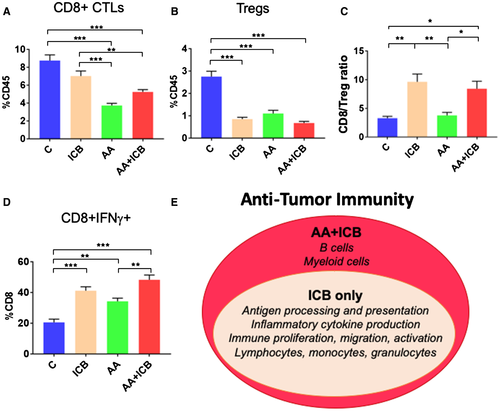
We also evaluated the changes in myeloid cell populations posttreatment using flow cytometry. AA-low treatment increased the fraction of F4/80+CD80+ and/or CD86+ antitumor “M1-type activated” tumor-infiltrating macrophages (TAMs) (Supporting Fig. S3). ICB treatment alone did not change the fraction in these populations; however, AA/ICB combinations increased the fraction of M1-TAMs (Supporting Fig. S3A). Moreover, AA/ICB combinations also decreased the fraction of both protumor F4/80+CD206+ “M2-type activated” TAMs (Supporting Fig. S3B) and CD11b+–chemokine (C-C motif) receptor 2–positive (CCR2+) monocytic cells (Supporting Fig. S4C); these effects appeared to be primarily mediated by AA (Supporting Fig. S3D-F).
To examine more broadly the immune effects of AA/IBC therapy, we performed RNA sequencing and found that ICB (alone or with AA) significantly up-regulated immune pathways (including proliferation, function/activity, and migration) compared to control-treated HCC tissues. Moreover, AA/IBC therapy selectively up-regulated pathways related to myeloid cells and B cells that were not enhanced by ICB alone (Fig. 2E; Supporting Table S1).
PD-L1 and PD-1 Expression is Increased After VEGFR-2 Blockade in HCC In Vivo
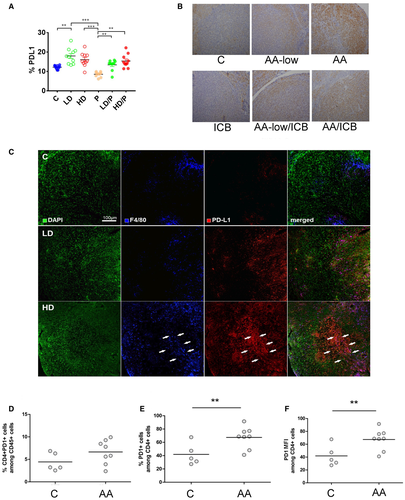
We have reported that VEGFR2 inhibition with the multitargeted tyrosine kinase inhibitor (TKI) sorafenib increases PD-L1 expression in these HCC models.9 Thus, we examined whether specific VEGFR-2 blockade has any impact on PD-L1 expression in HCC tissue in vivo. Using flow cytometry, we found that the number of nonimmune (CD45–) cells expressing PD-L1 was increased in tumors after VEGFR-2 blockade alone or in combination with ICB (Fig. 3A; Supporting Fig. S4A). The high expression of PD-L1 in both cancer and stromal cells was confirmed by IHC (Fig. 3B; Supporting Fig. S4B). Of note, double staining using anti-PD-L1 and anti-F4/80 antibodies showed that PD-L1 was predominantly expressed in TAMs in control and AA-low treated tumors, while AA-treated tumors exhibited PD-L1 expression in both TAMs and nonimmune cells (Fig. 3C). Interestingly, AA significantly increased the fraction of CD4+ cells expressing PD-1 and the PD-1 expression level (Fig. 3D-F).
PD-L1 Expression in HCC Cells is Up-Regulated Upon VEGFR-2 Blockade in Endothelial Cells in Part Through IFN-γ Production
To gain insight into the mechanism of PD-L1 up-regulation after DC101 treatment, we used organoid three-dimensional cultures in vitro. Single suspension of cells was obtained after enzymatic digestion of tumor tissues and then incubated in Nano-cultured plates under normoxic conditions. The cells formed heterotypic tumor organoids consisting of multiple cell types, including endothelial cells, after 3 days; and then organoids were cultured in hypoxic conditions for 48 hours. While PD-L1 expression was detectable, it was only slightly increased in hypoxic conditions (Supporting Fig. S4C,D). However, when we treated the organoids with blocking doses of DC101 (50 μg/mL),32 PD-L1 expression was substantially up-regulated in tumor and stromal cells (Fig. 4A,B). In this HCC model, VEGFR-2 expression was restricted to endothelial cells and to a subset of myeloid cells (Supporting Fig. S5). To elucidate the nature of the paracrine interaction leading to PD-L1 up-regulation after VEGFR-2 blockade, we next depleted certain cell lineages from the original single-cell suspension prior to organoid formation. First, we depleted all VEGFR-2+ cells from the single-cell suspension using magnetic beads and treated the resulting organoids with DC101 under hypoxic conditions. DC101 treatment failed to up-regulate PD-L1 expression in these conditions (Supporting Fig. S4E). Next, we depleted endothelial cells using anti-CD31 antibodies or myeloid cells using anti-CD11b and anti-CD11c antibodies and generated organoids that were treated with DC101 in hypoxic conditions. In these conditions, PD-L1 up-regulation was not seen in organoids where endothelial cells were depleted but was readily detectable in organoids where myeloid cells were depleted (Supporting Fig. S4E). Collectively, these results indicate that up-regulation of PD-L1 in HCC is dependent on VEGFR-2 blockade in endothelial cells, particularly in hypoxic conditions, which mimic the in vivo effects of AA.
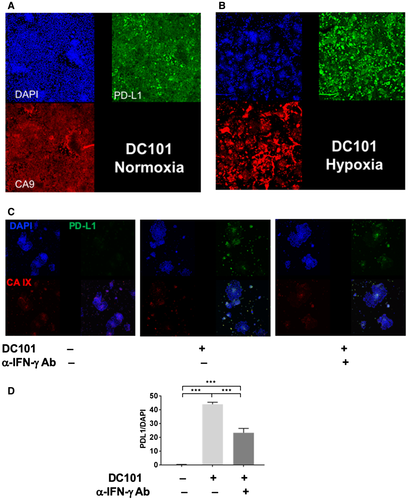
To examine the mechanism of PD-L1 expression regulation in a paracrine manner by endothelial cells, CD31+ cells were sorted from a single-cell suspension of RIL-175 tumors and cultured in vitro. We collected supernatant of endothelial cell culture treated with or without DC101 and evaluated inflammatory cytokine production by multiplexed ELISA. DC101 treatment enhanced most notably IFN-γ production by the endothelial cells (Supporting Fig. S4F). Because IFN-γ is a key inducer of PD-L1 expression,33 we treated the organoids with DC101 and blocked IFN-γ using a blocking antibody. IFN-γ blockade decreased PD-L1 expression by half in HCC endothelial cells (Fig. 4C,D), indicating that the HCC cell PD-L1 up-regulation by DC101 targeting of VEGFR-2 in endothelial cells is in part mediated by IFN-γ expression by endothelial cells.
ICB Therapy Promotes Vascular Normalization When Combined With AA Treatment
Because immunostimulatory reprogramming and vascular normalization may be reciprocally regulated in HCC,22 we next examined the effects of treatment on vascular structure and function in size-matched tumors. We found that ICB increased the total and pericyte-covered microvessel density (MVD) in established HCCs (Fig. 5A-C). Surprisingly, dual AA/ICB treatment further increased total and pericyte-covered MVD and prevented an increase in hypoxia in HCC, in contrast to the effect of AA alone (Fig. 5D,E). These data demonstrate that ICB promotes durable vascular formation and normalization even when used in combination with AA.
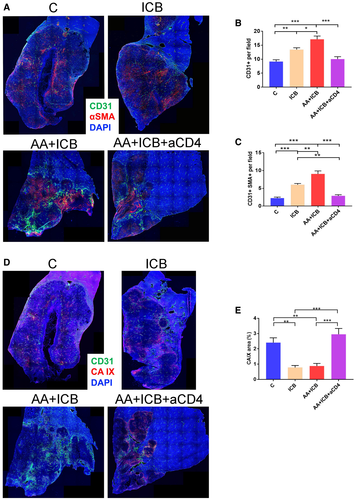
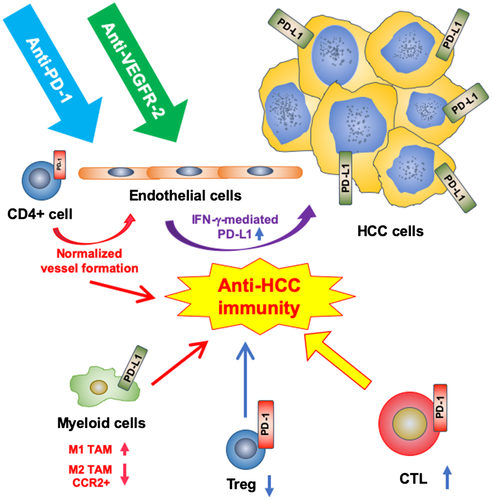
Because combination treatment did not increase CTL infiltration or activation over ICB alone but increased PD-1 expression in CD4+ cells, we next sought to decipher whether CD4+ cells play a role in the modulation of vascular structure and function in HCC. Of note, a recent study showed that CD4+ cells mutually regulate vascular and immune effects in developing tumors in an IFN-γ-dependent manner.22 We found that CD4+ cell depletion abrogated the normalized vessel formation seen after AA/ICB treatment and resulted in increased hypoxia (Fig. 5). Strikingly after CD4+-cell depletion during AA/ICB compared to AA/ICB with intact CD4+ cells, RNA sequencing revealed that 25/235 significantly down-regulated pathways were related to blood vessel formation, maturation, and normalization (Supporting Fig. S6 and Table S1). Thus, CD4+ cells promote vascular formation and remodeling after ICB despite potent AA in HCC (Fig. 6).
Discussion
The effects of AA—a treatment modality widely used in oncology currently—depend on the dose, timing, and specificity of the drugs as well as on the tumor type.4 These effects may involve transient normalization of the vascular structure and function as well as pronounced vascular rarefaction, which have been proven critical for the metastatic progression of tumors as well as for AA interactions with cytotoxic drugs.4 These effects remain unclear in HCC, a disease where several AAs are standard.34 The potential of AAs to enhance antitumor immunity when combined with immunotherapy in patients has been postulated by others and us by diverse mechanisms based on data from preclinical models, which all converged to normalization of tumor vessels and microenvironment as a principle.4, 10, 20, 23, 24 On the other hand, an increasing body of evidence describes the complex effects of immune cell activation on tumor vessel normalization. For example, Tian et al.22 demonstrated that—in a preventative setting—normalized vessel formation and antitumor immune responses were reciprocally regulated by CD4+ and not CD8+ cells in an IFN-γ-dependent manner in breast cancer models. This contrasted with data generated in an interventional setting, where Zheng et al.19 showed that CD8+ T-cell activation by ICB alone mediated the normalization of tumor vessels, albeit also in an IFN-γ-dependent manner.
Other reports showed that the benefit of combinations of AAs with ICB was associated with substantial pruning of the tumor vasculature. Thus, beyond the questions on the specific role of combining AA with ICB for HCC therapy, these reports also raise questions on the roles of passive (pruning of abnormal vessels) versus active (formation of normalized vessels through increased pericyte coverage) normalization and which is more critical for the efficacy of AA/ICB therapy. For example, Schmittnaegel et al.10 showed in the same breast cancer model (mouse mammary tumor virus–polyoma middle tumor antige [MMTV-PyVT]) as well as in pancreatic neuroendocrine tumors (receptor interacting protein 1–T antigen 2 [Rip1-Tag2]) and melanomas that dual blockade of angiopoietin 2 (Ang-2) and VEGF promoted vascular regression, tumor necrosis, and antigen presentation by intratumoral phagocytes. Dual Ang-2/VEGF blockade also structurally normalized the remaining blood vessels and facilitated the extravasation and perivascular accumulation of activated CTLs. This effect was further enhanced by PD-1 blockade, which counteracted the increased endothelial PD-L1 expression induced by IFN-γ. Allen et al.20 showed that the efficacy of AA/ICB treatment with anti-VEGFR-2 and anti-PD-L1 antibodies was associated with significant pruning of vessels and induction of structural normalization and a “high endothelial venule” phenotype in the remaining tumor vessels, with a concomitant increase in T-cell recruitment in MMTV-PyVT, Rip1-Tag2, and glioblastoma models.
Here, using clinically relevant mouse models of HCC, we directly addressed two critical issues. First, we demonstrate that a VEGFR-2-targeted agent can be successfully combined with ICB to increase its efficacy in HCC. For HCC, this question is timely and is in line with promising phase 1 clinical trial data in HCC patients, which showed unexpectedly high response rates and led to randomized phase 3 trials (e.g., NCT03744247, NCT03434379) and the “breakthrough” designation by the FDA. Moreover, dual VEGF/PD-L1 blockade has already shown increased OS in lung cancer patients.35 Second, we unravel an unexpected mechanism of benefit for specific blockade of PD-1 and VEGFR-2 with antibodies in HCC. In size-matched tumor samples, we show here that dual AA/ICB therapy not only does not cause pruning of the abnormal vessels but rather promotes normalized vessel formation mediated by CD4+ cells. These treatment interactions led to reprogramming of the immune microenvironment of HCC (Fig. 6). This is in contrast to pruning of vessels in these tumors after AA alone, which increases the expression of PD-1 on CD4+ cells.
It remains unknown whether titrating the dose of an anti-VEGFR TKI could also induce changes in the tumor microenvironment of HCC that will lead to benefits when combined with ICB. This will be critical in this disease, where several multitargeted TKIs are FDA-approved as monotherapy. In addition to potential effects on the microenvironment, reducing the dose of the TKIs may mitigate concerns related to the toxicity of such combinations in patients with HCC, whooften have poor liver function.15 Also, other mechanisms may play a role in this treatment interaction as VEGF/VEGFR-2 signaling blockade not only affects tumor angiogenesis but also regulates key antitumor immune responses through mechanisms such as suppression of CTL activity through inhibition of dendritic cell maturation,36 inactivation of signal transducer and activator of transcription 3,37, 38 or induction of exhaustion in intratumoral CTLs.39 Terme et al. showed that VEGF pathway inhibition decreased Treg infiltration in a mouse model of colorectal cancer.40 These findings highlight the complexity and the intricate mechanisms of interaction between these treatments in different tumors. They also underscore the potential for dual AA/ICB therapy across tumor types, including in subsets known to be refractory to either one or both treatment types.
While the predictive value of PD-L1 expression for response to anti-PD-1/PD-L1 therapy is being debated, a consistent finding emerging from multiple reports is the up-regulation of PD-L1 in cancer and stromal cells after AA treatment.9, 10, 20, 41 This up-regulation should be examined as a “dynamic biomarker” of response to AA/ICB. The mechanisms of this up-regulation appear to be tumor-dependent, AA agent–dependent, dose-dependent, and context-dependent. PD-L1 up-regulation by hypoxia has been inconsistent between studies10, 20, 39; we found that hypoxia may lead to a modest increase in PD-L1 expression in HCC but that agents such as sorafenib could increase PD-L1 expression even in normoxic conditions through mitogen-activated protein kinase and nuclear factor kappa B activation.41 Here, we used an HCC organoid culture model to demonstrate that PD-L1 up-regulation is mediated by VEGFR-2 blockade in endothelial cells through paracrine effects on cancer and stromal cells, mediated at least in part by IFN-γ production.
Future studies should establish the extent to which immune cell activation improves the cytostatic effect of VEGFR2 blockade or VEGFR2 blockade improves immune cell trafficking and activation; they should also validate our findings using human tumor specimens—if available posttreatment. However, the available clinical evidence supports the further development of dual AA/ICB therapy. High circulating plasma levels of VEGF are associated with treatment resistance in patients with melanoma treated with anti–CTL antigen 4 (CTLA-4) antibodies.42 Hodi et al. reported promising efficacy signals with a combination of anti-VEGF and anti-CTLA-4 therapy in patients with melanoma.43 Moreover, the anti-VEGFR-2 human antibody ramucirumab has shown improved survival in patients with gastric, colorectal, and lung cancers. In HCC, ramucirumab initially failed to increase survival despite showing favorable safety and tolerability profiles and inducing a significant delay in tumor progression compared with placebo among patients previously treated with sorafenib (REACH trial, NCT01140347).44 However, a follow-up biomarker-driven randomized phase 3 clinical trial showed significant survival benefit in HCC patients with high baseline serum AFP level or preserved liver function (REACH-2 trial, NCT02435433).45 Moreover, a recent study of ramucirumab with pembrolizumab showed a manageable safety profile with favorable antitumor activity in patients with previously treated advanced gastric or gastroesophageal junction adenocarcinoma, non-small-cell lung cancer, and urothelial carcinoma.46
In conclusion, we show here that dual PD-1/VEGFR-2 antibody blockade is effective in HCC models. The efficacy was seen both when using “vascular normalizing” and antivascular doses of AA and was mediated by the vascular normalizing effects of anti-PD-1 therapy itself mediated by CD4+ cells. AA/ICB therapy counteracted the immunosuppressive cues in the HCC microenvironment: PD-L1 up-regulation in HCC induced by VEGFR-2 blockade in tumor endothelial cells and Treg and CCR2+ monocyte infiltration. Because of ICB-induced vascular normalization, the dose of anti-VEGFR-2 antibody may not be as critical as when it is given alone. However, our results also indicate that AA-low therapy—seldom tested in preclinical or clinical studies—may be sufficient to appropriately reprogram the immune microenvironment and enhance ICB efficacy. These findings are important for future testing of these clinically relevant drugs in HCC and other cancers.
Acknowledgment
We thank Sylvie Roberge, Anna Khachatryan, Mark Duquette, and Carolyn Smith for outstanding technical support and Drs. Gregory Y. Lauwers, Sergey Kozin, Yunching Chen, and Yoshinori Hoshino for useful advice.
Author Contributions
K.S., M.D., T.H., and S.K. designed and performed experiments, analyzed the data, and wrote the manuscript. I.X.C., E.M., A.M., R.R.R., H.K., S.A., and H.O. performed experiments and analyzed the data. N.B. and P.H. provided reagents and analyzed the data. M.C. and R.K.J. analyzed the data and edited the manuscript. R.K.J. designed the experiments and edited the manuscript. A.X.Z. analyzed the data and edited the manuscript. D.G.D. designed the experiments, analyzed the data, obtained funding, and wrote the manuscript. All authors approved the final version of the manuscript.




