Mammalian Target of Rapamycin Complex 1 Signaling Is Required for the Dedifferentiation From Biliary Cell to Bipotential Progenitor Cell in Zebrafish Liver Regeneration
Abstract
The liver has a high regenerative capacity. Upon two-thirds partial hepatectomy, the hepatocytes proliferate and contribute to liver regeneration. After severe liver injury, when the proliferation of residual hepatocytes is blocked, the biliary epithelial cells (BECs) lose their morphology and express hepatoblast and endoderm markers, dedifferentiate into bipotential progenitor cells (BP-PCs), then proliferate and redifferentiate into mature hepatocytes. Little is known about the mechanisms involved in the formation of BP-PCs after extreme liver injury. Using a zebrafish liver extreme injury model, we found that mammalian target of rapamycin complex 1 (mTORC1) signaling regulated dedifferentiation of BECs and proliferation of BP-PCs. mTORC1 signaling was up-regulated in BECs during extreme hepatocyte ablation and continuously expressed in later liver regeneration. Inhibition of mTORC1 by early chemical treatment before hepatocyte ablation blocked the dedifferentiation from BECs into BP-PCs. Late mTORC1 inhibition after liver injury reduced the proliferation of BP-PC-derived hepatocytes and BECs but did not affect BP-PC redifferentiation. mTOR and raptor mutants exhibited defects in BEC transdifferentiation including dedifferentiation, BP-PC proliferation, and redifferentiation, similar to the chemical inhibition. Conclusion: mTORC1 signaling governs BEC-driven liver regeneration by regulating the dedifferentiation of BECs and the proliferation of BP-PC-derived hepatocytes and BECs.
Abbreviations
-
- BEC
-
- biliary epithelial cell
-
- Bhmt
-
- betaine-homocysteine S-methyltransferase
-
- BP-PC
-
- bipotential progenitor cell
-
- BT
-
- before Mtz treatment
-
- Ccnd1
-
- cyclin D1
-
- cp
-
- ceruloplasmin
-
- Den
-
- Dendra2
-
- DMSO
-
- dimethyl sulfoxide
-
- dpf
-
- days postfertilization
-
- DsRed2
-
- Discosoma sp. red fluorescent protein 2
-
- FISH
-
- fluorescent in situ hybridization
-
- foxa3
-
- forkhead box a3
-
- gc
-
- vitamin D binding protein
-
- GFP
-
- green fluorescent protein
-
- γH2AX
-
- gamma-histone H2AX
-
- hhex
-
- hematopoietically expressed homeobox
-
- Hnf4a
-
- hepatocyte nuclear factor 4a
-
- lfabp
-
- liver-type fatty acid binding protein
-
- mTOR
-
- mammalian target of rapamycin
-
- mTORC1
-
- mTOR complex 1
-
- Mtz
-
- metronidazole
-
- NTR
-
- bacterial nitroreductase
-
- PCNA
-
- proliferating cell nuclear antigen
-
- PH
-
- partial hepatectomy
-
- prox1a
-
- prospero homeobox 1a
-
- p-RS6
-
- phosphorylated ribosomal S6 protein
-
- R0h
-
- regeneration 0 hour
-
- sox9b
-
- SRY (sex determining region Y)-box 9b
-
- Tg
-
- transgenic
-
- Urb2
-
- URB2 ribosome biogenesis homolog
-
- WISH
-
- whole-mount in situ hybridization
The liver is a unique organ with high regenerative capacities in vertebrates. Upon acute and mild injury, hepatocytes mainly originate from self-renewal.1, 2 In chronic and severe injury, particularly when the proliferation of remaining hepatocytes is blocked, there will be a kind of progenitor which can differentiate into hepatocytes and cholangiocytes.3, 4 Whether these progenitors come from hepatocytes or biliary epithelial cells (BECs) has been the subject of much debate.5-9 However, recent studies in zebrafish10, 11 and mice12-15 demonstrated that BECs extensively contribute to liver regeneration through bipotential progenitor cells (BP-PCs) after severe loss of hepatocytes. BECs first dedifferentiate into bipotential progenitors, in which early developmental markers are reactivated, then proliferate and differentiate to hepatocytes.10, 11
Progenitor cells are hardly detected in normal liver and are activated when the liver is injured with hepatocyte proliferation inhibited.16 Many developmental regulators such as Wnt,17-21 Notch,18, 22, 23 fibroblast growth factor (FGF),24 and bone morphogenetic protein (BMP)25 have been shown to be involved in progenitor regulation. Tumor necrosis factor–like weak inducer of apoptosis26 and FGF724 play roles in inducing de novo activation of progenitors. Although many factors involved in cell proliferation have been identified, regulators of progenitor cell activation in vivo remain largely unknown. Zebrafish becomes a useful animal model to study basic mechanisms of liver diseases27 based on the transparency of embryos and quick liver regeneration after severe hepatocyte ablation, making this model suitable to study mechanisms underlying progenitor activation.
Mammalian target of rapamycin (mTOR) is a protein kinase that regulates cell growth, proliferation, and survival, as well as transcription and protein synthesis. mTOR is found in two distinct protein complexes with specific binding partners, including raptor in mTOR complex 1 (mTORC1) and rictor in mTORC2. Importantly, rapamycin has a striking specificity of effect on mTORC1.28 mTORC1 inhibition will down-regulate the protein expression, proliferation, and cell cycle at the G1 to S phase transition in hepatocytes.29 Phosphoinositide 3-kinase/AKT activates mTOR to regulate the cell cycle and cell proliferation during liver regeneration.30 Cyclin D1 is a downstream effector of rapamycin-mediated cell cycle arrest.29, 31, 32 Phosphorylation and activation of ribosomal S6 protein kinase 1 (S6K) are dependent on mTOR activation, which plays a dominant role in regulating the cell cycle during liver regeneration.33 Rapamycin treatment inhibits hepatocyte proliferation during liver regeneration by reducing the expression of proliferating cell nuclear antigen (PCNA).34, 35 Although mTORC1 plays an important part in liver regeneration after partial hepatectomy (PH), its role in BEC-driven hepatocyte regeneration has not been addressed.
We set out to study mTORC1 signaling during BEC-driven liver regeneration. We combined chemical treatment and genetic inactivation of mTOR and raptor to address the dedifferentiation of BECs and proliferation of BP-PCs. We reveal that mTORC1 signaling is essential for the dedifferentiation from BECs to BP-PCs. mTORC1 activity is the prerequisite to activate the hepatoblast and hepatocyte markers in BECs after liver injury. Besides, mTORC1 also regulates the proliferation of BP-PC-derived hepatocytes and BECs during liver regeneration. This study suggests essential roles of mTORC1 in BP-PC activation and cell proliferation during liver regeneration in vivo.
Materials and Methods
Zebrafish Strains
Zebrafish strains were raised and maintained under standard laboratory conditions according to Institutional Animal Care and Use Committee protocols. The transgenic zebrafish lines Tg(liver-type fatty acid binding protein [lfabp]:Dendra2-bacterial nitroreductase)cq1, abbreviated as Tg(lfabp:DenNTR)cq1; Tg(hsp70l:URB2 ribosome biogenesis homolog [Urb2]-Flag)cq73; Tg(β-actin:loxP–Discosoma sp. red fluorescent protein 2 [DsRed]-loxP–green fluorescent protein [GFP])s928; Tg(lfabp:loxP-STOP-loxP-DsRed)cq4; and Tg(keratin 18 [Krt18]:CreER)cq74 were used. The mutant mTORcq69, raptorcq70, urb2cq42, and rictorcq72 were used. Fish embryos were treated with 0.003% 1-phenyl-2-thiourea (Sigma-Aldrich, Darmstadt, Germany) from 24 hours postfertilization to inhibit pigmentation.
Rapamycin and Metronidazole Treatment
The Tg(lfabp:DenNTR)cq1 transgenic larvae at 5 days postfertilization (dpf) was incubated with 10 mM metronidazole (Mtz; Sigma-Aldrich) in 0.2% dimethyl sulfoxide (DMSO) for 24 hours. Then, larvae were washed 3 times and recovered in egg water, marking the regeneration 0 hour (R0h). For rapamycin treatment, larvae were treated with 2 µM rapamycin (Selleck) in 0.2% DMSO for 24 hours. Then, the larvae were added to newly prepared rapamycin solution or washed 3 times and recovered in egg water.
Antibody Staining
For whole mounts, antibody staining was as described.36-38 Briefly, the skin of larvae was manually removed and incubated with the following antibodies: Dendra2 (1:1,000; AB821; Evrogen, Moscow, Russia), GFP (1:1,000; sc-9996; Santa Cruz Biotechnology, Santa Cruz, CA), GFP (1:1,000; ab6658; Abcam, Cambridge, MA), 2F11 (1:1,000; ab71826; Abcam), PCNA (1:500; SAB2701819; Sigma, St. Louis, MO), PCNA (1:500; ab29; Abcam), phosphorylated ribosomal S6 protein (p-RS6; 1:200; no. 2215; New England Biolabs, Hitchin, UK), betaine-homocysteine S-methyltransferase (Bhmt; 1:500, a kind gift from J. Peng, Zhejiang University, China), gamma-histone H2AX (γH2AX; 1:500; GTX127342; Gene Tex), activated leukocyte cell adhesion molecule (Alcam; 1:50; zn5; ZIRC, Eugene, OR), and DsRed2 (1:1,000; sc-101526, Santa Cruz, Dallas, TX). The primary antibodies were diluted in the blocking solution and incubated at 4°C overnight. Then, larvae were washed with phosphate-buffered saline +1% Triton X-100 5 times and incubated with Alexa fluorescent-conjugated secondary antibodies (1:1,000; Invitrogen, Grand Island, NY) diluted in the blocking solution at 4°C overnight. After five washes, larvae proceeded to mounting and imaging.
Additional methods are available in the Supporting Information.
Results
mTORC1 Signaling is Required for BEC-Driven Liver Regeneration
mTORC1 signaling is essential for hepatocyte proliferation during liver regeneration after PH,30, 31, 35, 39, 40 but its roles in the other liver injury model, in particular after extensive hepatocyte ablation, are unknown. To explore the roles of mTORC1 signaling in BEC-driven hepatocyte regeneration after extreme hepatocyte loss, we used the NTR-Mtz zebrafish liver injury model.10, 11 After extreme loss of hepatocytes, the BECs steadily lost their tubular morphology and dedifferentiated into BP-PCs, which express hepatoblast and hepatocyte markers. The BP-PCs then proliferate and redifferentiate into mature hepatocytes and BECs to accomplish liver regeneration.10, 11 Rapamycin, which specifically inhibits mTORC1 signaling,28 was applied to treat the larvae at different stages of liver injury and regeneration (Fig. 1A). Without rapamycin, the liver of the larvae treated with 10 mM Mtz from 5 to 6 dpf regenerated to half the size after regeneration for 48 hours (R48h) (Fig. 1B). After incubation with 2 µM rapamycin from 4 to 5 dpf, 4 to 6 dpf, and 5 to 6 dpf, respectively, the liver did not regenerate at R48h (Fig. 1A,B). Larvae treated with 2 µM rapamycin from 6 dpf/R0h to R48h regenerated a smaller liver (Fig. 1A,B). These data indicate that mTORC1 inhibition affects mainly the early stage and less so the later stage of liver regeneration.
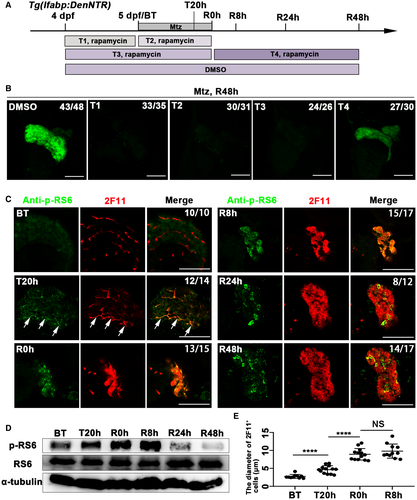
To evaluate the activity of mTORC1 signaling during liver regeneration, we analyzed the level of p-RS6, a well-established downstream effector of mTORC1 signaling.41 Although p-RS6 was only weakly expressed in liver before Mtz treatment (BT) (Fig. 1C), its expression was up-regulated in BECs when the larvae had been incubated with Mtz for 20 hours (T20h) (Fig. 1C, arrowheads). The p-RS6 expression remained up-regulated in all of the 2F11+ cells at R0h and R8h but became heterogeneous at R24h and R48h (Fig. 1C,D). Along with the activation of p-RS6, the diameter of 2F11+ cells enlarged (Fig. 1E). Validated by the BEC-specific promoter krt18 and the hepatocyte-specific promoter lfabp, 2F11 was shown to label the BECs in the control (Supporting Fig. S1A) and the BEC-derived hepatocytes during regeneration (Supporting Fig. S1B). High expression levels of p-RS6 in 2F11+ cells at T20h, R0h, and R8h indicate that mTORC1 signaling has important roles in the initiation of liver regeneration.
Early mTORC1 Inhibition Blocks the Dedifferentiation of BECs
To avoid the delayed effects of rapamycin treatment from 5 to 6 dpf/R0h on the late stage of liver regeneration, we used the protocol of rapamycin incubation from 4 to 5 dpf (early mTORC1 inhibition) before Mtz treatment to explore the roles of mTORC1 in the early stage of liver regeneration (Fig. 1A; Supporting Fig. S2A). Treatment with rapamycin before Mtz did not affect liver development (Supporting Fig. S2B) or the morphology of the biliary duct system (Supporting Fig. S2C) at BT. Body shape remained unaffected at R48h (Supporting Fig. S2B). Transdifferentiation from BECs to hepatocytes was significantly repressed by the early mTORC1 inhibition (Supporting Fig. S2C). Blockade of p-RS6 expression at BT by the early mTORC1 inhibition (Supporting Fig. S3A,B) validated the efficient inhibitory effects of rapamycin on the mTORC1 signaling. The up-regulation of p-RS6 expression was constantly inhibited from T20h to R48h after rapamycin treatment (Supporting Fig. S3C-G). These results indicate that rapamycin treatment reduces the transdifferentiation of BECs to hepatocytes, suggesting important roles of mTORC1 signaling in BEC-driven liver regeneration.
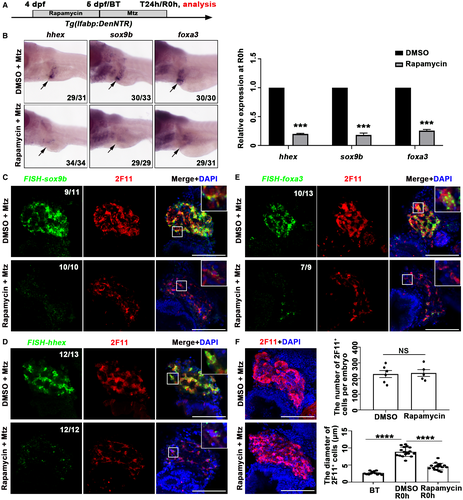
Given that dedifferentiation from BECs to BP-PCs is the first step of regeneration after liver injury, during which the endoderm and hepatoblast developmental markers like forkhead box a3 (foxa3), hematopoietically expressed homeobox (hhex), and SRY (sex determining region Y)-box 9b (sox9b) are activated,10 we analyzed whether mTORC1 signaling is required for the dedifferentiation of BECs at R0h (Fig. 2A). The transcriptional activation of hhex, sox9b, and foxa3 was repressed at R0h after early mTORC1 inhibition (Fig. 2B). The repressed expression of hhex, sox9b, and foxa3 in BECs was further confirmed by the combination of fluorescent in situ hybridization (FISH) and antibody staining (Fig. 2C-E). BECs lost their morphology during dedifferentiation in the control, whereas they maintained their tubular structure to some extent after rapamycin treatment (Fig. 2C-F). These data indicate that early mTORC1 inhibition blocks the dedifferentiation of BECs after extreme liver injury.
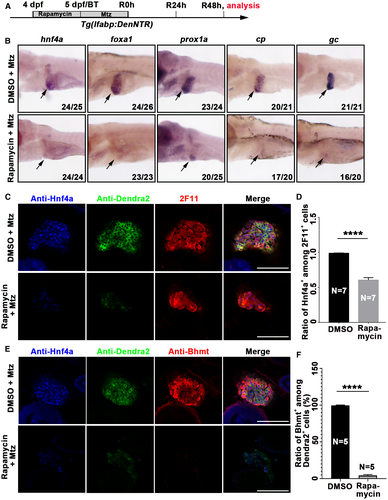
Given that early mTORC1 inhibition blocks dedifferentiation from BECs to BP-PCs (Fig. 2), the first step of the BEC-driven liver regeneration, the later BP-PC redifferentiation process, should also be defective. To validate that, early mTORC1 inhibition was carried out (Fig. 3A), and the subsequent expression of hepatoblast/hepatocyte marker hepatocyte nuclear factor 4a (Hnf4a),42 the hepatocyte/BEC marker prospero homeobox 1a (Prox1a),43 the liver pioneer factor Foxa1,44 and functional hepatocyte markers ceruloplasmin (cp) and vitamin D binding protein (gc) was analyzed. Early mTORC1 inhibition caused loss of hnf4a, foxa1, cp, and gc expression as well as reduced prox1a expression in the regenerating livers at R48h (Fig. 3B). Quantitatively, at R48h after early rapamycin treatment, the proportion of hepatocytes/hepatoblasts marked by Hnf4a among 2F11+ cells was reduced, while the proportion of mature hepatocytes marked by Bhmt among Dendra2+ cells became even more significantly reduced (Fig. 3C-F). These results validate that the blockade of BP-PC formation by early mTORC1 inhibition leads to defects in subsequent redifferentiation of hepatocytes.
Early mTORC1 Inhibition Represses Proliferation of The 2F11+ Cells but does not Cause Excessive Apoptosis
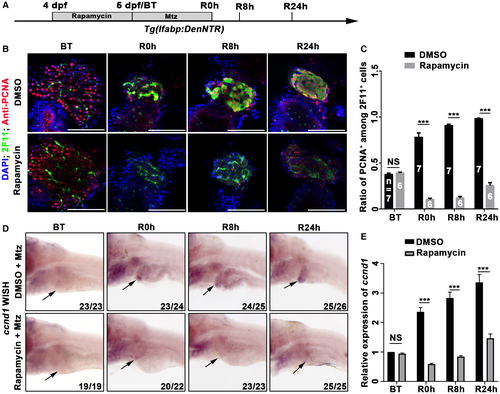
To assess the effects of early mTORC1 inhibition on cell proliferation during liver regeneration, the expression of PCNA, which shows individual cells outside of the G0 phase of the cell cycle, was analyzed. Rapamycin without Mtz treatment did not affect the proliferation of hepatocytes (Supporting Fig. S4A-C). However, cell proliferation significantly increased in BP-PCs at R0h after Mtz treatment and reached the maximal level at R24h (Fig. 4A-C). In contrast, after early rapamycin treatment, 2F11+ cells rarely proliferated at R0h and R8h and only slightly proliferated at R24h (Fig. 4B,C). In mammalian hepatocytes, rapamycin inhibits the expression of cyclin D1 (Ccnd1), which regulates G1 progression.32 Thus, we examined the effects of rapamycin on ccnd1 expression. The expression of ccnd1 in the liver from R0h to R24h was significantly down-regulated by early rapamycin treatment (Fig. 4D,E). All of these data demonstrate that early mTORC1 inhibition represses proliferation of 2F11+ cells.
To explore whether early mTORC1 inhibition causes excessive apoptosis, we used the terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) assays (Supporting Fig. S5A,B). Cells double-positive for TUNEL and 2F11 were hardly detected in the regenerating livers after early mTORC1 inhibition (Supporting Fig. S5B). We next examined the expression of γH2AX, a rapid and sensitive cellular response factor to the presence of DNA double-strand breaks.45 γH2AX-positive signals were undetectable in 2F11+ cells after liver injury (Supporting Fig. S5C). These results show that early mTORC1 inhibition does not cause excessive apoptosis of 2F11+ cells.
mTOR and raptor Mutants Display Similar Liver Regeneration Phenotypes as Early mTORC1 Inhibition
mTOR is the core mediator of mTORC1 signaling.46 To confirm the roles of mTORC1 signaling in liver regeneration, we generated mTORcq69 mutants containing a 16-bp deletion in the fourth exon using clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated 9 (Cas9)–mediated genome editing technology (Supporting Fig. S6A). The mTOR mutants exhibited normal body morphologies except for smaller eyes and defective swim bladder at 5 dpf (Supporting Fig. S6B). As reported,47 the volume of the liver was reduced to half the size in mTOR mutants at 5 dpf, but the biliary duct system developed normally compared to siblings (Supporting Fig. S6C). Less proliferation but not excessive apoptosis accounted for the smaller number of hepatocytes in the mTOR mutant (Supporting Fig. S6D,E). mTOR mutants surviving from Mtz treatment exhibited relatively normal body shape, but their liver regeneration was defective (Fig. 5A). The dedifferentiation from BECs to BP-PCs arrested in the mTOR mutant, as assessed by the expression of hhex, sox9b, and foxa3 at R0h (Fig. 5B). In the following stage of BP-PC redifferentiation, expression of hepatocyte markers Hnf4a, cp, and gc was nondetectable in the mTOR mutant (Fig. 5C,D), indicating defective hepatocyte redifferentiation. Furthermore, cell proliferation was silent in 2F11+ cells in the mTOR mutant at R24h (Fig. 5E).
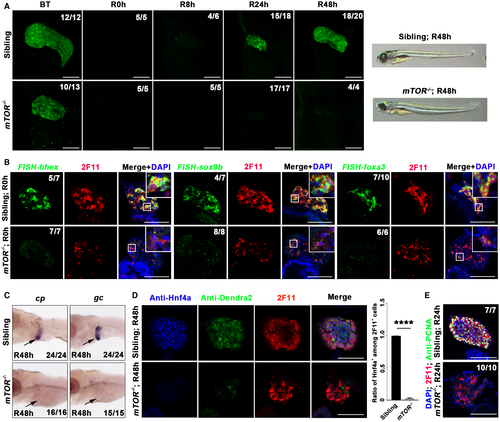
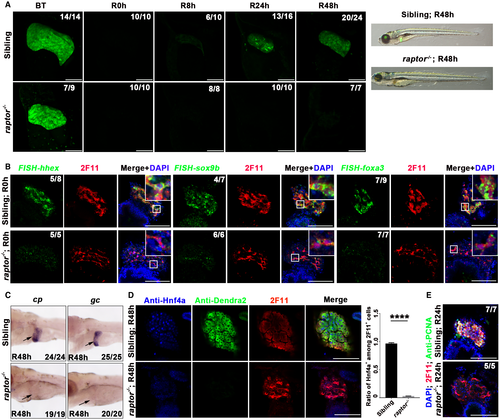
Raptor facilitates substrate recruitment to mTORC1 through binding to the TOR signaling motif on canonical mTORC1 substrates.41 A raptorcq70 mutant line containing a 14-bp deletion in the seventh exon was generated using CRISPR/Cas9-mediated genome editing (Supporting Fig. S7A). The liver appeared to be smaller in the raptor mutant at 5 dpf compared to siblings, whereas the biliary duct system and the body morphology were relatively normal (Supporting Fig. S7B,C). Less proliferation but not excessive apoptosis of hepatocytes accounted for the smaller liver of the raptor mutant (Supporting Fig. S7D,E). The mutants surviving from Mtz treatment exhibited severe defects in liver regeneration (Fig. 6A). Similar to the mTOR mutant, expression of hhex, sox9b, and foxa3 at R0h (Fig. 6B), as well as of cp, gc, and Hnf4a at R48h (Fig. 6C,D), was significantly repressed in the raptor mutant. Furthermore, we could hardly detect cell proliferation in 2F11+ cells in the raptor mutant at R24h (Fig. 6E). Taken together, defects in liver regeneration in the mTOR and raptor mutants validate the loss of the BEC dedifferentiation process after early mTORC1 inhibition.
Late mTORC1 Inhibition Reduces the Proliferation of BP-PC-Derived Hepatocytes and BECs
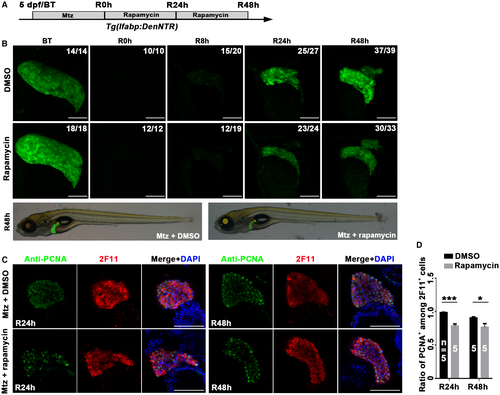
To evaluate the roles of mTORC1 signaling in the late phase of BEC-driven liver regeneration, we used the protocol of rapamycin incubation from R0h to R48h (late mTORC1 inhibition) to bypass its earlier effects on BEC dedifferentiation (Fig. 7A; Supporting Fig. S8A). Late mTORC1 inhibition was confirmed by the loss of p-RS6 at R24h and R48h (Supporting Fig. S8B). Although late mTORC1 inhibition did not affect the redifferentiation of BP-PCs to hepatocytes, as assessed by the expression of Hnf4a, Dendra2, cp, and gc (Supporting Fig. S8C,D), the regenerating liver was smaller than the control at R48h (Fig. 7B). The proportion of PCNA+ cells among all of the 2F11+ cells reduced after late rapamycin treatment (Fig. 7C,D), whereas apoptotic cells were not detected in regenerating livers at R24h and R48h (Supporting Fig. S8E,F). Furthermore, late mTORC1 inhibition affected the proliferation of newly formed hepatocytes and BECs marked by Bhmt and Alcam, respectively (Supporting Fig. S9A-E). These data demonstrate that late mTORC1 inhibition suppresses the proliferation of BP-PC-derived hepatocytes and BECs without affecting BP-PC redifferentiation or inducing apoptosis, thus leading to the smaller liver at R48h.
Defects in BEC Dedifferentiation Caused by Rapamycin Treatment are Partially Rescued by Urb2
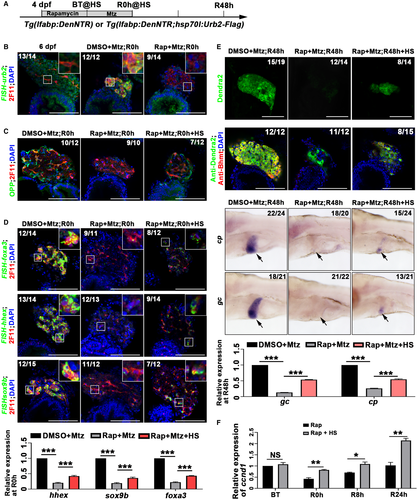
To understand the potential mechanisms involved in mTORC1-regulated BEC dedifferentiation, expression of factors that have been reported to regulate liver regeneration was analyzed. Many well-known regulatory factors of liver regeneration, including urb2 and bromodomain containing 2a, exhibited transcriptional down-regulation after rapamycin treatment (Supporting Fig. S10A), suggesting that mTORC1 activity induced a transcriptional program that is required for changes in cell fate. Because mTORC1 signaling plays important roles in protein synthesis41 and Urb is required for protein synthesis downstream of mTORC1,47 we further explored whether Urb2 mediates the regulation of liver regeneration by mTORC1. The transcriptional level of urb2 was up-regulated in BECs after Mtz treatment but significantly reduced after early mTORC1 inhibition (Fig. 8A,B). Furthermore, liver regeneration exhibited defects in the urb2 mutant (Supporting Fig. S11A-C). To carry out rescue experiments, we used the Tg(lfabp:DenNTR; hsp70l:Urb2-Flag) line to overexpress Urb2 upon heat shock (Fig. 8A). Overexpression of Urb2-Flag could partially rescue protein synthesis defects in liver caused by rapamycin treatment (Fig. 8C). Consequently, gene expression repressed by rapamycin treatment, including hepatoblast markers foxa3, hhex, and sox9b; hepatocyte markers Dendra2, Bhmt, cp, and gc; as well as the cell cycle marker ccnd1, was partially rescued by Urb2 overexpression (Fig. 8D-F). These results demonstrate that after extreme liver injury, mTORC1 regulates dedifferentiation of BECs partially through Urb2-mediated protein synthesis.
Discussion
The kinetics of the zebrafish extensive hepatocyte ablation and the mouse severe liver injury models allow evaluation in vivo of BEC dedifferentiation, BP-PC proliferation, redifferentiation, and the mechanisms involved in these cellular responses.10-14, 25 The mTORC1 inhibitor rapamycin is known to significantly delay S-phase entry and liver regeneration after PH, indicating important roles of mTORC1 signaling.32, 48 Here, we describe that early mTORC1 inhibition, mTOR, and raptor mutants severely block the dedifferentiation of BECs to BP-PCs and the proliferation of BEC-derived cells after liver injury. Moreover, we describe that late mTORC1 inhibition delays the proliferation of BP-PC-derived hepatocytes and BECs but not cell differentiation and apoptosis. Regulation of the dedifferentiation of BECs by mTORC1 signaling is partially mediated by Urb2.
mTOR inhibition has received wide interest in the treatment of liver cancer.49 mTORC1 is a key regulator of cell proliferation and is reported to be involved in liver regeneration after PH.34, 35 AKT through tuberous sclerosis complex subunits 1 and 2/mTOR signaling regulates cell proliferation during rat liver regeneration after liver PH.30 The downstream effectors of mTORC1 signaling such as S6K and 4e binding protein 1 also modulate hepatocyte proliferation after liver PH in mouse.33, 48 So, a role of mTORC1 signaling in cell proliferation during liver regeneration after PH is relatively clear. In cases of BEC-driven hepatocyte regeneration, the primary effects of early mTORC1 inhibition consist of blockade of BEC dedifferentiation. In turn, cell proliferation and redifferentiation are arrested because there are no BP-PCs available for further proliferation and redifferentiation. When early effects of rapamycin on BEC dedifferentiation are bypassed in the case of late mTORC1 inhibition, abnormal cell proliferation becomes visible without affecting redifferentiation or inducing apoptosis. Thus, mTORC1 signaling plays multiple roles in different stages of liver injury and regeneration.
mTORC1 signaling moderates digestive development in zebrafish.47 Although the liver was smaller in mTOR and raptor mutants, the intrahepatic duct system has relatively normal morphology. Besides, many developmental factors and mechanisms can be reactivated to regulate liver regeneration such as Wnt, Bmp, and Notch, which have been reported to regulate BEC dedifferentiation, BP-PC proliferation, and redifferentiation in zebrafish.10, 11, 25, 50 Consistent with chemical inhibition, the dedifferentiation of BECs and redifferentiation were defective after liver injury in the mTOR and raptor mutants. The mTOR mutant affects both mTORC1 and mTORC2 signaling. Although the regenerating liver of the rictor mutant was approximately 20% smaller than the wild type (Supporting Fig. S12A-C), expression of hepatoblast and hepatocyte markers showed normal BEC dedifferentiation and BP-PC redifferentiation in the rictor mutant (Supporting Fig. S12D,E). Therefore, control of BEC dedifferentiation by mTOR is mainly mediated by mTORC1 signaling, not by mTORC2 signaling.
In summary, this study explored the roles of mTORC1 signaling in BEC-driven liver regeneration. Early mTORC1 inhibition resulted in defective BEC dedifferentiation as well as later proliferation and redifferentiation. Late mTORC1 inhibition resulted in inhibited cell proliferation without affecting the redifferentiation or inducing apoptosis. mTOR and raptor mutants validated the roles of mTORC1 signaling in BEC dedifferentiation and BP-PC proliferation. This study indicates the future clinical potential of mTORC1 signaling in therapeutics after severe liver injuries.
Acknowledgment
We thank X. Tang, Q. Yang, W. Zhang, X. Tan, and D. Wang for technical assistance, and thank J. Peng for the antibodies.




