Letter to the Editor: The Kynurenine Pathway in Hepatic Cirrhosis
To the Editor:
Clària et al.1 reported that cirrhosis activates the kynurenine (Kyn) pathway (KP) of tryptophan (Trp) degradation (Fig. 1) by a systemic inflammation–dependent induction of indoleamine 2,3-dioxygenase (IDO), leading to increased plasma levels of Kyn and its metabolites kynurenic acid (KA) and quinolinic acid (QA). I propose additional and alternative interpretations of these data.
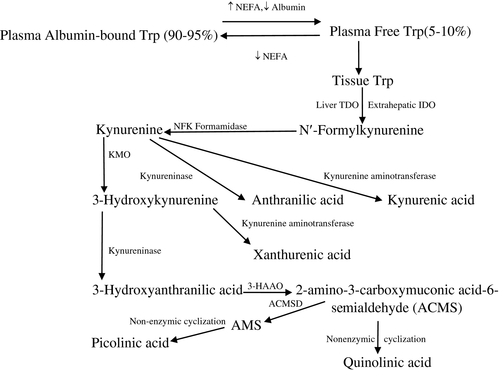
Because the authors determined IDO mRNA expression in only 4 control subjects and 6 patients with acute decompensated cirrhosis, we cannot be sure of the IDO catalytic activity in this and the other patient subgroups, especially those with acute-on-chronic liver failure (ACLF) and ACLF-KF (ACLF with kidney failure). Although the authors1 conclude that the greater plasma levels of Kyn, KA, and QA in ACLF-KF versus ACLF implies greater KP activation in kidney disease, a more likely explanation is that, in kidney disease, these differences are the result of enhancement of tryptophan 2,3-dioxygenase (TDO2) and kynurenine aminotransferase, and inhibition of kynureninase and 2-amino-3-carboxymuconic acid-semialdehyde decarboxylase, with IDO being unchanged.2, 3
I also suggest that an increased flux of plasma-free Trp through TDO24 is the major mechanism of the increased KP activity in cirrhosis, mediated by decreased circulating albumin and increased non-esterified fatty acids (NEFAs), both of which are established features of liver cirrhosis. The combination of these two changes leads to maximum elevation of plasma-free Trp (for example, in fulminant liver failure, free and total Trp can rise to values of 60-112 µM).5 Although TDO has a much greater capacity for Trp, IDO has a much reduced capacity and is inhibited by Trp of at least 50 µM.4 The increase or the absence of a decrease in total Trp in cirrhosis is due to TDO inhibition.
I believe that future development of the important platform provided by Clària et al.1 can be strengthened by extending observations to include measurements of plasma-free (as well as total) Trp and determinants of Trp binding (albumin and NEFA), direct measurements of IDO activity in peripheral blood cells across patient groups, and whenever possible, liver TDO activity.




